ABSTRACT
Eosinophilia can be due to both infectious and non-infectious causes, many of which may be clinically indistinguishable. Filariasis, a tropical and subtropical infection, is caused by Wuchereria bancrofti, Brugia timori (B. timori), and Brugia malayi. Filariasis is conventionally diagnosed by demonstration of microfilaria in the peripheral blood smear. The disease may be missed if one is not aware of the possibility. We report two cases in two individuals with eosinophilia due to filaria resulting in tropical pulmonary eosinophilia and deep vein thrombosis (DVT). They had complete symptomatic improvement after a 3-week course of diethylcarbamazine (DEC) +/- ivermectin.
Keywords: Eosinophilia, filariasis, tropical pulmonary eosinophilia
Introduction
Peripheral blood eosinophilia may be caused by numerous conditions, including allergic, infectious, inflammatory, and neoplastic disorders. Among individuals with eosinophilia, the likelihood of an infectious cause is highest. In general, helminths are the most commonly identified infectious causes of eosinophilia.[1] Categories of helminths include flukes (trematodes), tapeworms (cestodes), and roundworms (nematodes). Filariasis is caused by slender threadlike nematodes belonging to superfamily Filarioidea. Wuchereria bancrofti, Brugia timori (B. timori), and Brugia malayi are the most common organisms causing filariasis in humans. Filariasis is endemic in tropical countries, especially India, China, Indonesia, and parts of Africa, and is a huge public health problem of the tropics. Filariasis and its consequences are a major health problem in tropical countries, such as India. Wuchereria bancrofti, Brugia malayi, and Brugia timori are the three nematodes responsible for the filariasis. Of these, only W. bancrofti and B. malayi are found in India. Both parasites produce essentially similar clinical presentation in man, related mainly to the pathology of the lymphatic system. The most widespread infection is due to W. bancrofti (98%) and the remaining by B. malayi (2%). In India, W. bancrofti is transmitted by the ubiquitous mosquito, Culex quinquefasciatus, and B. malayi is transmitted by Mansonia mosquitoes.[2] Eosinophilia and microfilaremia are commonly seen in the acute phase of infection. The chronic stage is generally characterized by lymphadenopathy, lymphedema, hydrocele, and elephantiasis. We report two cases of filariasis causing eosinophilia and presenting as tropical pulmonary eosinophilia and deep vein thrombosis (DVT) of the lower limb.
This case series sheds light on the diverse clinical manifestations of filarial infection, which often masquerade as other conditions, leading to diagnostic challenges. By presenting cases of tropical pulmonary eosinophilia and DVT associated with filarial eosinophilia, this study underscores the importance of considering filariasis in the differential diagnosis of eosinophilia, particularly in tropical regions. Moreover, the discussion on treatment modalities and long-term management highlights the significance of early recognition and intervention in preventing complications and improving patient outcomes.
Case 1
We report a case of 14-year-old boy who was admitted with recurrent episodes of fever, nocturnal cough, and dyspnea for 1-year duration. He denied any history of chest pain and hemoptysis. The past medical history was not significant. The general physical examination was normal. The respiratory system examination revealed wheeze bilaterally. Cardiovascular system and abdomen examination were normal. Laboratory investigations revealed elevated white blood cell (WBC) count (55,700), normal hemoglobin, and normal platelet count. The erythrocyte sedimentation rate was 72 mm in 1 hour. His absolute eosinophil count was 33,700, and immunoglobin E (IgE) levels were more than 1000. Peripheral smear showed increased eosinophils with normocytic normochromic red blood cells (RBCs) and normal platelet count. Chest X-ray at admission showed bilateral lung infiltrates [Figure 1]. Ultrasonography (USG) abdominal ultrasound was normal. High-resolution computed tomography (HRCT) thorax [Figure 2] showed tiny miliary nodules scattered throughout the entire lung fields. He also had normal perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) and cytoplasmic anti-neutrophil cytoplasmic antibody (c-ANCA) levels. His stool routine and microscopy were negative for any parasite ova and cyst. Examination of sputum was also normal. Bone marrow study was conducted to rule out the possibility of hematological malignancies. Bone marrow aspirate and bone marrow trephine showed predominantly mature eosinophils without any blasts [Figure 3]. His immunoglobin M (IgM) microfilarial antibody was positive and was started on oral diethylcarbamazine (DEC) 300 mg in three divided doses daily for 3 weeks. During follow-up after 3 weeks, his symptoms resolved, his WBC counts normalized, and his chest X-ray showed clearing of lung fields.
Figure 1.
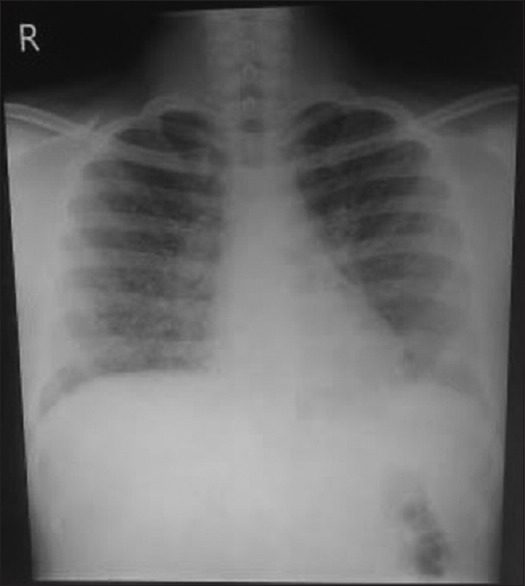
Chest X-ray at the time of admission
Figure 2.
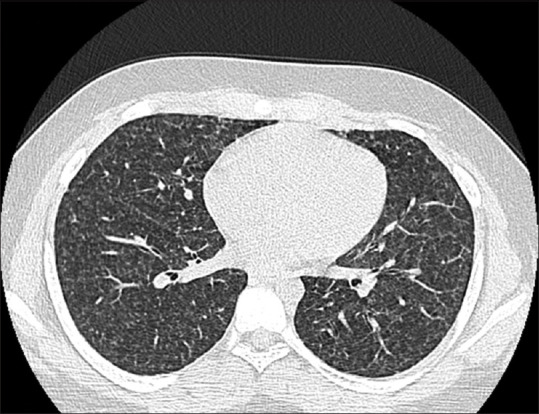
HRCT thorax
Figure 3.
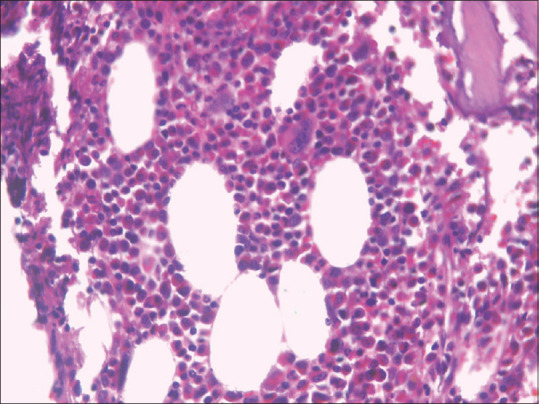
Bone marrow trephine showing eosinophilic infiltrates
Case 2
An 18-year-old boy presented with fever, pain, and swelling of the left thigh for a 1-week duration. He was diagnosed to have extensive DVT in the left lower limb involving the common femoral vein, Sapheno-femoral vein, popliteal vein, and deep calf veins and was admitted for the evaluation and management of the same. He denied any history of long travel/recent surgeries/other comorbid illnesses. His general examination and detailed systemic examination were unremarkable except for the left lower limb DVT. Blood routine investigations were striking for an elevated leukocyte count, erythrocyte sedimentation rate (ESR), and thrombocytosis (Tc-23,300 DC-P39L30M31, Hb—10.5, MCV—85, platelet—6.67 Lakhs). Leukocyte mixed fraction was high; hence, peripheral smear examination was conducted, which showed moderate anemia, microcytic hypochromic, WBC count with predominant eosinophils, and adequate platelet count. The absolute eosinophil count was 10520. Differential diagnoses considered were hematological malignancy, collagen vascular diseases, and vasculitis, and work-up was planned accordingly to evaluate the cause of eosinophilia. To rule out clonal eosinophilia, bone marrow biopsy was conducted which was normal, except for marked increase in mature eosinophilic precursors, with no increase in blasts or atypical cells. FIP1L1-PDGFR fusion gene mutation was not present. Then, he was evaluated for reactive eosinophilia. Stool microscopy showed no ova/cyst of parasites, and c-ANCA and p-ANCA were negative. Filarial antigen (ELISA) was positive. This test detects Wuchereria bancrofti circulating filarial antigen released by adult worm in blood, serum, or plasma with a sensitivity of 100% and specificity of 94%. The event appeared as an unprovoked DVT; hence, a full screening of potential underlying cause of thrombosis was ruled out. Inherited thrombophilia was looked for, including protein C, protein S, and factor 5-laden mutation, and tested negative. Acquired thrombophilia—Antiphospholipid antibody (APLA) panel—was negative. Occult malignancy screening based on chest X-ray, computed tomography (CT) thorax, and abdomen did not reveal any pathology. He was treated with single-dose ivermectin, DEC 6 mg/kg/day in three divided doses for 21 days along with anticoagulation. Over the days, the patient symptomatically improved, counts normalized, and he was discharged.
Discussion
Eosinophilia manifests heterogeneously. Any organs can be infiltrated by eosinophils. Accordingly, patients can be pauci-symptomatic or manifest a rapid fatal course. Hence, a finding of eosinophilia opens up a broad differential diagnosis for clinicians, which include acute or chronic, benign or malignant disorders. We herein described two presentations of reactive eosinophilia secondary to filarial infection one presenting as pulmonary symptoms and the other as DVT. Tropical pulmonary eosinophilia also known as Weingarten’s syndrome is a rare syndrome characterized by marked peripheral eosinophilia and pulmonary interstitial infiltrates. It is a hypersensitivity reaction to microfilariae of Wuchereria bancrofti and Brugia malayi. Studies have shown that < 0.5% of the 130 million people globally who are infected with filariasis apparently develop this condition. The clearance of rapidly opsonized microfilariae from the bloodstream results in a hypersensitive immunological response and abnormal recruitment of eosinophils, as reflected by very high IgE levels of above 1000 IU/ml. It most frequently occurs in the Indian subcontinent and Southeast Asia. Approximately 7% of cases of Tropical Pulmonary Eosinophilia (TPE) show extrapulmonary manifestations.[3] Although TPE typically has a nonspecific presentation and may mimic a number of conditions, it is most often misdiagnosed as asthma because of pulmonary manifestations, such as paroxysmal cough and dyspnea.[4,5] Pulmonary eosinophilia is a heterogeneous group of infectious and non-infectious conditions that involve infiltration of eosinophils into the lung parenchyma and the airways.[6] Uncommon findings are bronchiectasis, air trapping, mediastinal lymphadenopathy, and pleural effusion.[7] It is important to note that chest radiograph findings may be normal in up to 20% of TPE cases.[8] Rapid recognition and treatment with anti-filarial drug is paramount important, as delay in treatment leads to progressive interstitial fibrosis and irreversible damage.[9] The rate of relapse after DEC therapy is estimated to be 20% over a follow-up period of 5 years.[10] Several pathophysiological mechanisms have been described for the hypercoagulable state induced by eosinophilia that may lead to thrombosis. Eosinophilic activation and degranulation increase the release of eosinophilic cationic protein, which binds with heparin. It modulates thrombomodulin, thereby reducing the physiological anticoagulation mechanisms and increasing the prothrombotic state. Furthermore, eosinophilic granules activate the kallikrein system and so the factor 12 activation, thereby switching on the clotting cascade. As eosinophilia is not considered a cause for provoked DVT, the patient was assigned to long-term treatment of 1 year of anticoagulation with directly acting oral anticoagulant, rivaroxaban 15 mg once daily. DEC is the drug of choice for lymphatic filariasis. It is a potent microfilaricidal agent and also kills 50% of adult worm. Single-dose ivermectin reduces microfilaremia by 90% in Wuchereria bancrofti filariasis. It has no effect on adult worm viability.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wilson ME, Weller PF. Eosinophilia. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Philadelphia: Saunders Elsevier; 2011. p. 939. [Google Scholar]
- 2.Das PK, Prasad PS, Krishnamoorthy K. Prospects of elimination of lymphatic filariasis in India. ICMR Bulletin. 2002;32:41–54. [Google Scholar]
- 3.Santeliz JV. Tropical pulmonary eosinophilia: An epidemiological and clinical review. Int J Respir Pulm Med. 2019;6:102. [Google Scholar]
- 4.Obaray A, Khan F, Azueta V, Wollschlager C. Tropical eosinophilia presenting as acute bronchial asthma: Case report with clinical, physiologic, and histologic features before and after treatment. Heart Lung. 1982;11:464–8. [PubMed] [Google Scholar]
- 5.Jiva TM, Israel RH, Poe RH. Tropical pulmonary eosinophilia masquerading as acute bronchial asthma. Respiration. 1996;63:55–8. doi: 10.1159/000196517. [DOI] [PubMed] [Google Scholar]
- 6.Datta A, Chhotray P, Jena B, Sivasankar R. A case of tropical pulmonary eosinophilia with incomplete response to diethylcarbamazine therapy. Cureus. 2023;15:e34359. doi: 10.7759/cureus.34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angirish B, Jankharia B, Sanghavi P. The role of HRCT in tropical pulmonary eosinophilia. Eur J Radiol. 2020;131:109207. doi: 10.1016/j.ejrad.2020.109207. [DOI] [PubMed] [Google Scholar]
- 8.Udwaida FE, Herzog H. Progress in Respiration Research. Vol. 7. Basel, Switzerland: S. Karger; 1975. Tropical eosinophilia, pulmonary eosinophilia; pp. 35–155. [Google Scholar]
- 9.Vijayan VK. Tropical pulmonary eosinophilia: Pathogenesis, diagnosis and management. Curr Opin Pulm Med. 2007;13:428–33. doi: 10.1097/MCP.0b013e3281eb8ec9. [DOI] [PubMed] [Google Scholar]
- 10.Mullerpattan JB, Udwadia ZF, Udwadia FE. Tropical pulmonary eosinophilia-a review. Indian J Med Res. 2013;138:295–302. [PMC free article] [PubMed] [Google Scholar]


