Abstract
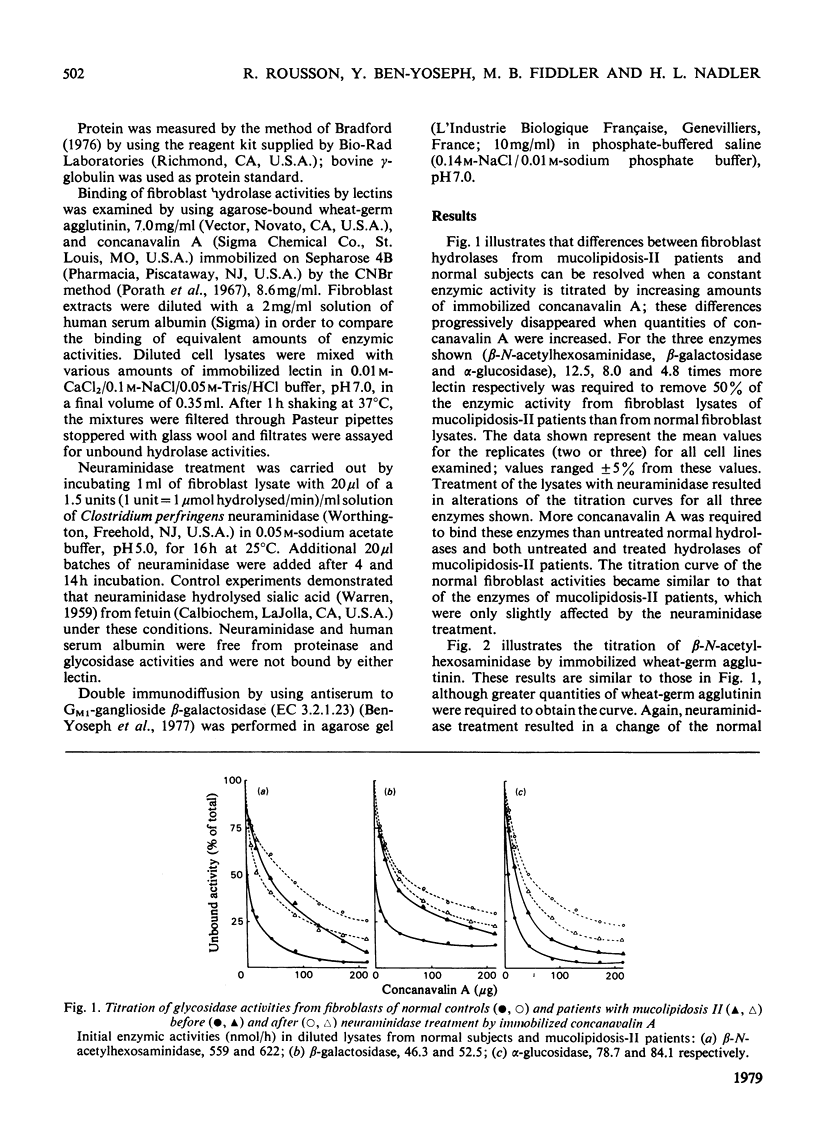
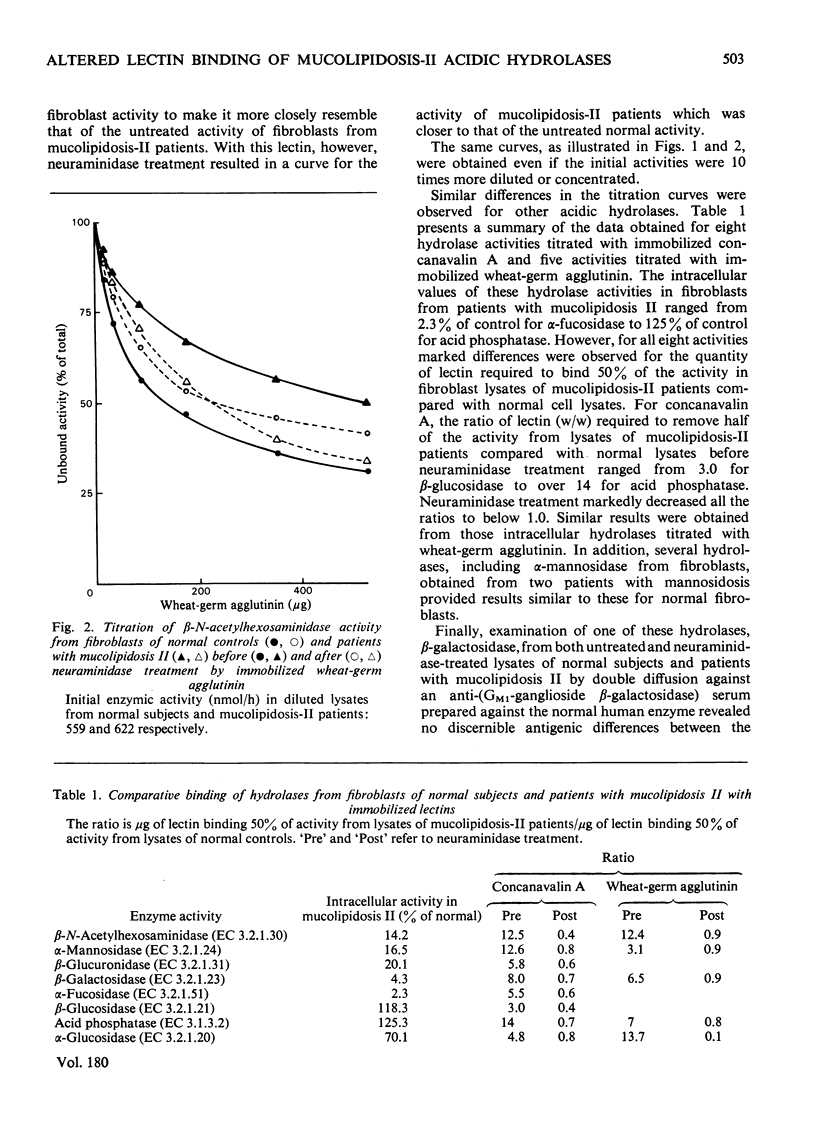
Decreased binding by the lectins concanavalin A and wheat-germ agglutinin was found for a number of acidic hydrolases from skin fibroblasts of three unrelated patients with mucolipidosis II. This decreased binding as compared with normal controls was demonstrated by titration of hydrolase activities with increasing amounts of immobilized lectins. Neuraminidase treatment slightly improved the binding of enzymes from mucolipidosis-II patients, in contrast with the diminished binding found or hydrolases from control cell lines. The abnormality in binding by lectins of hydrolases of mucolipidosis-II patients was observed for enzymes with various degrees of intracellular deficiency as well as for enzymes with normal intracellular activities. These findings suggest a generalized alteration of fibroblast acidic hydrolase molecules in mucolipidosis II.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Yoseph Y., Burton B. K., Nadler H. L. Quantitation of the enzymically deficient cross reacting material in GM1 gangliosidoses. Am J Hum Genet. 1977 Nov;29(6):575–580. [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Hungerford M., Nadler H. L. The nature of mutation in Krabbe disease. Am J Hum Genet. 1978 Nov;30(6):644–652. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Champion M. J., Shows T. B. Electrophoretic abnormalities of lysosomal enzymes in mucolipidosis fibroblast lines. Am J Hum Genet. 1977 Mar;29(2):149–163. [PMC free article] [PubMed] [Google Scholar]
- Fiddler M. B., Ben-Yoseph Y., Nadler H. L. Binding of human liver hydrolases by immobilized lectins. Biochem J. 1979 Jan 1;177(1):175–180. doi: 10.1042/bj1770175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., Miller A. L., Frost R. G., O'Brien J. S. Characterization of beta-D-galactosidase isolated from I-cell disease liver. Am J Hum Genet. 1975 Nov;27(6):719–727. [PMC free article] [PubMed] [Google Scholar]
- Miller A. L. I-Cell disease: isoelectric focusing, concanavalin A-Sepharose 4B binding and kinetic properties of human liver acid beta-D-galactosidases. Biochim Biophys Acta. 1978 Jan 12;522(1):174–186. doi: 10.1016/0005-2744(78)90333-9. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Lagwinska E., Schlesinger S. Enveloped virus acquires membrane defect when passaged in fibroblasts from I-cell disease patients. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2443–2447. doi: 10.1073/pnas.73.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker G., Michalski J. C. Biochemical basis of six different types of sialidosis. FEBS Lett. 1978 Jan 1;85(1):20–24. doi: 10.1016/0014-5793(78)81239-3. [DOI] [PubMed] [Google Scholar]
- Thomas G. H., Tiller G. E., Jr, Reynolds L. W., Miller C. S., Bace J. W. Increased levels of sialic acid associated with a sialidase deficiency in I-cell disease (mucolipidosis II) fibroblasts. Biochem Biophys Res Commun. 1976 Jul 12;71(1):188–195. doi: 10.1016/0006-291x(76)90267-9. [DOI] [PubMed] [Google Scholar]
- Vladutiu G. D., Rattazzi M. C. Cell disease: desialylation of beta-hexosaminidase and its effect on uptake by fibroblasts. Biochim Biophys Acta. 1978 Feb 13;539(1):31–36. doi: 10.1016/0304-4165(78)90118-6. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wiesmann U. N., Vassella F., Herschkowitz N. N. Mucolipidosis II (I-cell disease). A clinical and biochemical study. Acta Paediatr Scand. 1974 Jan;63(1):9–16. doi: 10.1111/j.1651-2227.1974.tb04343.x. [DOI] [PubMed] [Google Scholar]


