Abstract
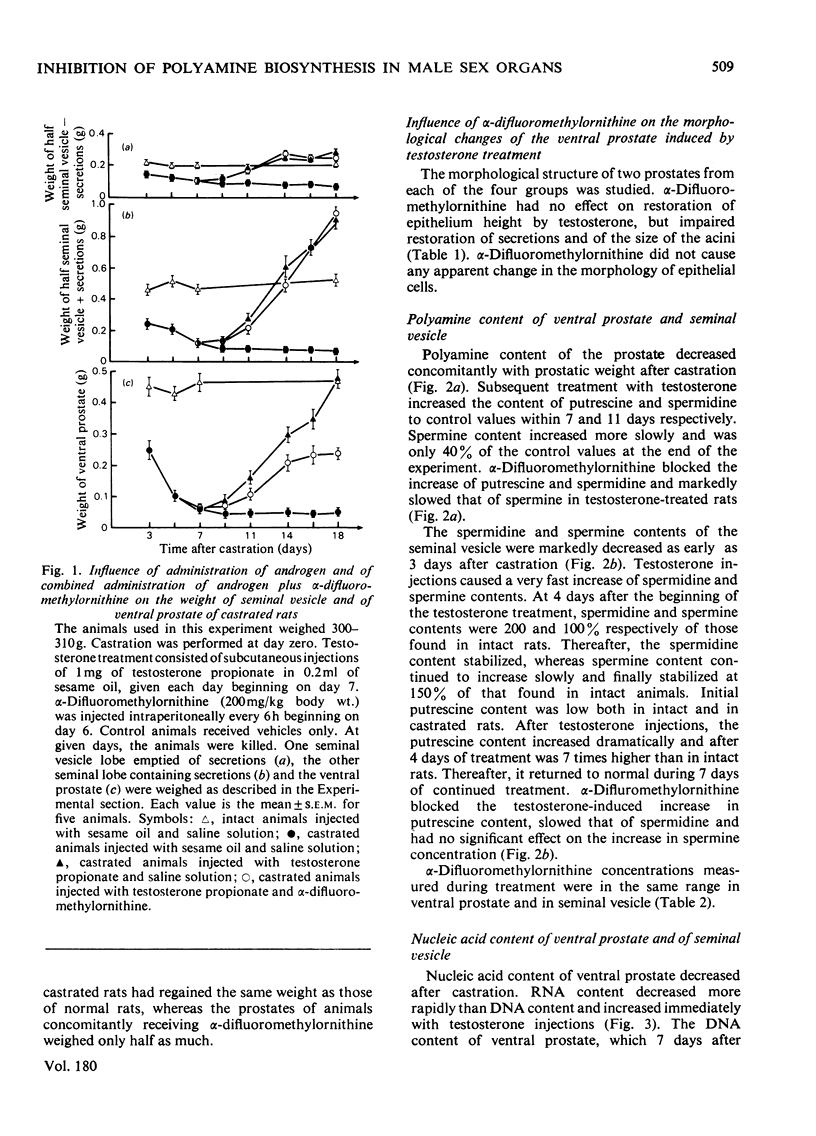
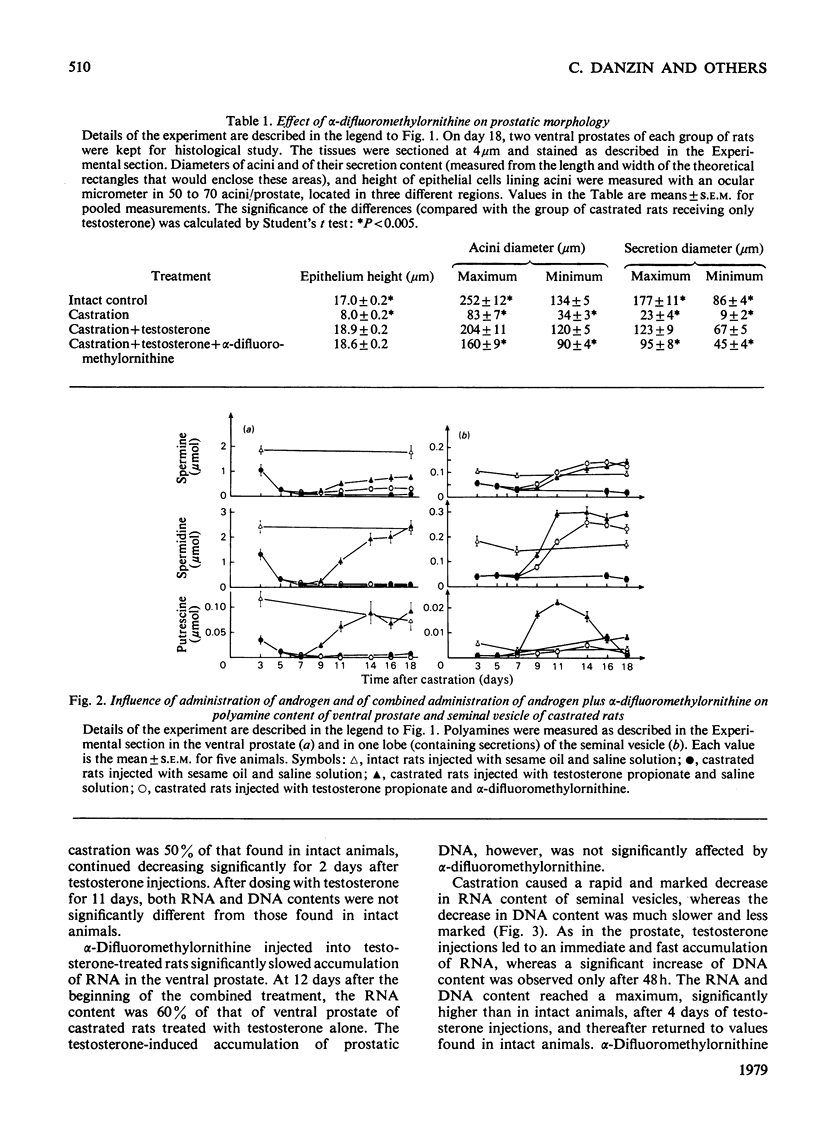
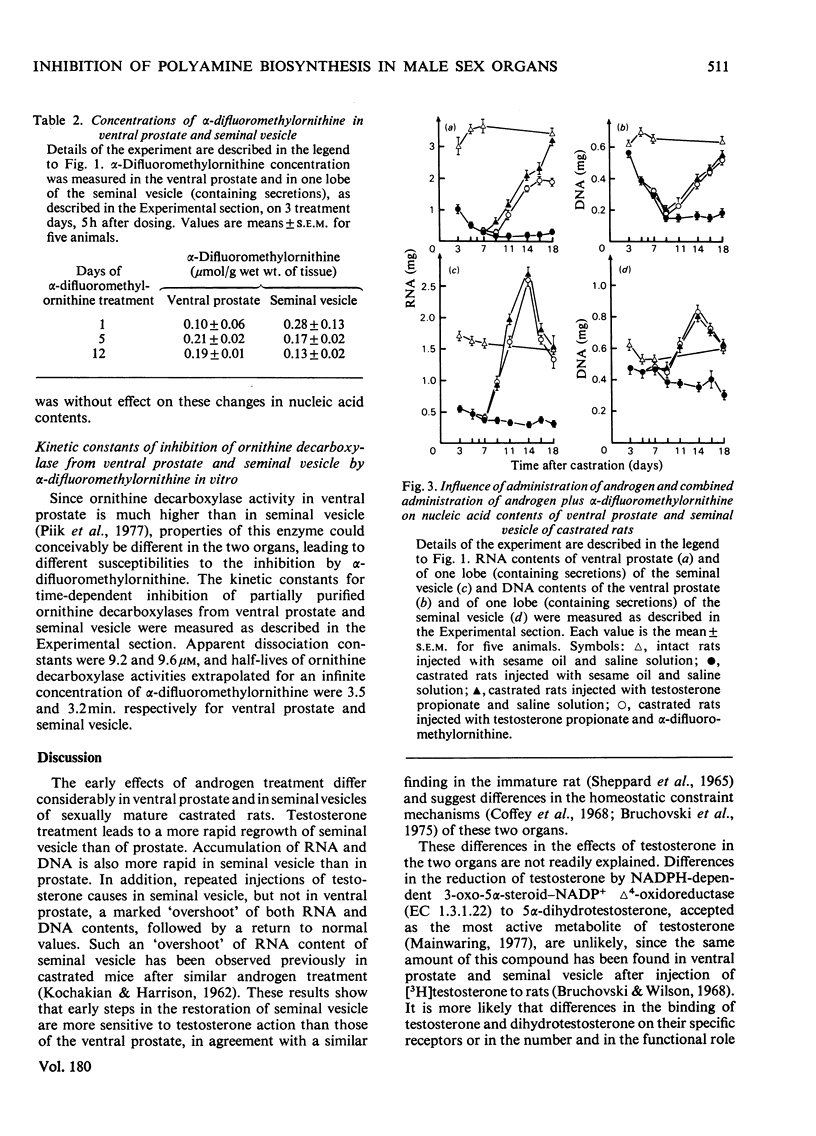
1. Castration of adult rats markedly decreases the amounts of polyamines (putrescine, spermidine and spermine) and of RNA and DNA in the ventral prostate and the seminal vesicle. 2. Daily injections of testosterone propionate to rats castrated 7 days previously increase polyamine and nucleic acid contents more rapidly in the seminal vesicle than in the ventral prostate. 3. After 7 days of androgen treatment, polyamine and nucleic acid contents of the seminal vesicle are significantly higher than those of intact animals. Nucleic acid, but not polyamine, contents return to normal values during the next 4 days of continued treatment. In the prostate, androgen treatment increases polyamine and nucleic acid contents to, but not above, normal values. 4. Repeated doses of α-difluoromethylornithine, a potent enzyme-activated irreversible inhibitor of ornithine decarboxylase, totally blocked the testosterone-induced increase of putrescine and spermidine in the ventral prostate and of putrescine in the seminal vesicle. They slowed significantly the accumulation of spermine in the ventral prostate and of spermidine in the seminal vesicle. α-Difluoromethylornithine also retarded the testosterone-induced accumulation of RNA in the ventral prostate. However, no clear correlation was apparent between accumulation of polyamines and of nucleic acids in the two organs. 5. α-Difluoromethylornithine markedly slows the testosterone-induced weight gain of the prostate, but not of the seminal vesicle. Cytological studies suggest that this effect on the prostate is due to inhibition of the androgen-induced restoration of the secretion content of prostatic acini.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDES D., GROTH D. P. FUNCTIONAL ULTRASTRUCTURE OF RAT PROSTATIC EPITHELIUM. Natl Cancer Inst Monogr. 1963 Oct;12:47–62. [PubMed] [Google Scholar]
- BUTLER W. W., 3rd, SCHADE A. L. The effects of castration and androgen replacement on the nucleic acid composition, metabolism, and enzymatic capacities of the rat ventral prostate. Endocrinology. 1958 Sep;63(3):271–279. doi: 10.1210/endo-63-3-271. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Corti A., Caldarera C. M. RNA Polymerase activity in purified nuclei from rat prostate gland in the presence of polyamines. FEBS Lett. 1971 Mar 5;13(3):169–172. doi: 10.1016/0014-5793(71)80227-2. [DOI] [PubMed] [Google Scholar]
- Bartos F., Bartos D., Grettie D. P., Campbell R. A. Polyamine levels in normal human serum. Comparison of analytical methods. Biochem Biophys Res Commun. 1977 Apr 25;75(4):915–919. doi: 10.1016/0006-291x(77)91469-3. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Lesser B., Van Doorn E., Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/s0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Caldarera C. M., Moruzzi M. S., Barbiroli B., Moruzzi G. Spermine and spermidine of the prostate gland of orchiectomized rats and their effect on RNA polymerase activity. Biochem Biophys Res Commun. 1968 Oct 24;33(2):266–271. doi: 10.1016/0006-291x(68)90779-1. [DOI] [PubMed] [Google Scholar]
- Coffey D. S., Shimazaki J., Williams-Ashman H. G. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968 Mar 20;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Donaldson L. J., Thomas G. H. Ornithine decarboxylase activity and: [125I]iododeoxyuridine incorporation in rat prostate. Biochem J. 1975 Sep;150(3):557–559. doi: 10.1042/bj1500557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- KOCHAKIAN C. D., HARRISON D. G. Regulation of nucleic acid synthesis by androgens. Endocrinology. 1962 Jan;70:99–108. doi: 10.1210/endo-70-1-99. [DOI] [PubMed] [Google Scholar]
- Lee P. L. Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med. 1974 Jun;10(2):107–121. doi: 10.1016/0006-2944(74)90013-1. [DOI] [PubMed] [Google Scholar]
- Mangan F. R., Pegg A. E., Mainwaring I. P. A reappraisal of the effects of adenosine 3':5'-cyclic monophosphate on the function and morphology of the rat prostate gland. Biochem J. 1973 May;134(1):129–142. doi: 10.1042/bj1340129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton L. J., Heby O., Wilson C. B., Lee P. L. An automated micromethod for the quantitative analysis of di- and polyamines utilizing a sensitive high pressure liquid chromatographic procedure. FEBS Lett. 1974 Apr 15;41(1):99–103. doi: 10.1016/0014-5793(74)80963-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piik K., Rajamäki P., Guha S. K., Jänne J. Regulation of L-ornithine decarboxylase and S-adenosyl-L-methionine decarboxylase in rat ventral prostate and seminal vesicle. Biochem J. 1977 Dec 15;168(3):379–385. doi: 10.1042/bj1680379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPPARD H., TSIEN W. H., MAYER P., HOWIE N. METABOLISM OF THE ACCESSORY SEX ORGANS OF THE IMMATURE MALE RAT: CHANGES IN NUCLEIC ACID COMPOSITION AND UPTAKE OF THYMIDINE-3-H INDUCED BY CASTRATION AND METHANDROSTENOLONE. Biochem Pharmacol. 1965 Jan 1;14:41–51. doi: 10.1016/0006-2952(65)90056-0. [DOI] [PubMed] [Google Scholar]
- Takyi E. E., Fuller D. J., Donaldson L. J., Thomas G. H. Deoxyribonucleic acid and polyamine synthesis in rat ventral prostrate. Effects of age of the intact rat and androgen stimulation of the castrated rat with testosterone, 5 alpha-dihydrotestosterone and 5 alpha-androstane-3 beta, 17 beta-diol. Biochem J. 1977 Jan 15;162(1):87–97. doi: 10.1042/bj1620087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Tadolini B., Wilson J., Corti A. Polynucleotide polymerizations and prostate proliferation. Vitam Horm. 1975;33:39–60. doi: 10.1016/s0083-6729(08)60950-4. [DOI] [PubMed] [Google Scholar]


