Abstract
Purpose of Review
Positron emission tomography (PET) combined with computed tomography (CT) has proven useful as a cancer screening technique in patients with inflammatory myopathy, mainly dermatomyositis. In this review, we focus on advances in this direction and other potential applications of PET/CT in patients with inflammatory myopathy.
Recent Findings
Cancer screening by PET/CT seems suitable and cost-effective in patients with myositis. It has also shown value as a hybrid technique for diagnosing myositis versus controls and could be of interest for differentiating between polymyositis and sporadic inclusion body myositis. Quantification of muscle activity by PET/CT seems reliable. Preliminary data suggest that it could also be used to diagnose and measure the activity of the disease in the lung.
Summary
PET/CT should be in the toolbox of physicians managing patients with myositis. The multiple applications of PET/CT include its value for cancer screening, measuring the activity of the disease in muscle, and helping to differentiate between myositis phenotypes. The possibility to diagnose and monitor inflammatory lung activity remains to be demonstrated in well-designed studies.
Keywords: PET/CT, Myositis, Malignancy, Activity, Dermatomyositis, Sporadic inclusion body myositis
Introduction
Idiopathic inflammatory myopathies, generally referred to as myositis, are a group of heterogeneous disorders of autoimmune origin. They are considered systemic in nature and display several clinical manifestations. In addition to muscle inflammation, the hallmark of the disease, there may be arthritis, interstitial lung disease, and characteristic skin involvement [1]. These disorders can be categorized into five phenotypes: dermatomyositis, polymyositis, sporadic inclusion myositis, immune-mediated necrotizing myopathy, and antisynthetase syndrome [2]. Some of these phenotypes, mainly dermatomyositis, are considered paraneoplastic, as nearly one third of patients with dermatomyositis harbour cancer at the time of the diagnosis [3].
Over the last decade, positron emission tomography (PET) combined with computed tomography (CT) has proven to be a useful, non-invasive, hybrid technique (combining nuclear and CT imaging) for a myriad of purposes [4]. It can be used to detect morphologic changes through imaging, as well as functional changes related to the avidity of tissues for isotope uptake. The isotope most commonly used in the examinations is [18F] fluorodeoxyglucose ([18F] FDG), but other isotopes have been incorporated recently, such as florbetapir for amyloid diagnosis and quantification. It is relevant to differentiate between [18F]-FDG PET as an isotopic technique alone and the mixed [18F]-FDG PET nuclear medicine and CT radiology technique (PET/CT), which enables better anatomic resolution than [18F]-FDG PET alone [5]. Nowadays, most studies are performed with the PET/CT hybrid technique, and [18F] FDG is the main isotope used in myositis patients.
PET/CT findings are usually evaluated by visual assessment. Any lesion showing isotope uptake higher than background activity should be considered abnormal (pathological), while always taking into consideration the morphological changes detected by CT. The visual assessment should be accompanied by a semiquantitative evaluation involving the standardized uptake value (SUV). The SUV is a measure of the uptake of a given lesion in relation to the isotope dose administered. It is an indirect indication of the uptake intensity of the lesion in comparison with the normal uptake or the uptake of other lesions found in different organs or tissues. SUVmax is defined as the highest uptake in a single pixel, SUVmean as the mean uptake of all pixels in a selected area, and SUV ratio as the ratio between the SUV in a given lesion and a reference tissue, usually the liver. The software currently used to read PET/CT studies incorporates semiautomatic tools to calculate the SUV [6].
In this review, we will discuss available data on the usefulness of PET/CT in myositis patients, including screening for associated cancer, diagnostic purposes, measuring the activity of the disease, and determining the myositis phenotype.
Cancer Screening
It is well recognized that cancer can be associated with some myositis phenotypes. This is the case of dermatomyositis and, to a lesser extent, polymyositis and immune-mediated necrotizing myositis [7–10]. The potential presence of an occult malignancy in a patient with dermatomyositis is a cause of concern for the treating clinician. In addition to laboratory tests such as the immune profile, which includes autoantibodies (e.g. TIF1γ [11] and NXP-2) [12], a good strategy to detect occult malignancy in this scenario is imaging screening. [18F]-FDG PET/CT seems to be a suitable option for this purpose [12] (Fig. 1). One study performed a decade ago prospectively analysed a cohort of 55 dermatomyositis patients over a 3-year period, comparing the use of conventional broad cancer screening (thoracoabdominal CT, mammography, gynaecologic examination, ultrasonography, and tumour marker analysis) with a single [18F]-FDG PET/CT study [13]. The authors found that the predictive value for detecting occult malignancy was similar between the two approaches. Although it seems clear from the patient’s perspective that a single test is better than a battery of tests involving several hospital appointments, issues have been raised about the cost burden and higher radiation exposure of PET/CT relative to plain PET without concurrent CT. In a retrospective cohort study [14•] (2005–2014), the total mean cost to insurance companies and the mean out-of-pocket cost to patients were analysed. Researchers found that the cost of a single PET/CT scan compared to broad cancer screening was higher for the companies but lower for patients, both men and women. These results opened the door to more generalized implementation of PET/CT for dermatomyositis cancer screening in countries where this test is not available through publically funded health resources.
Fig. 1.
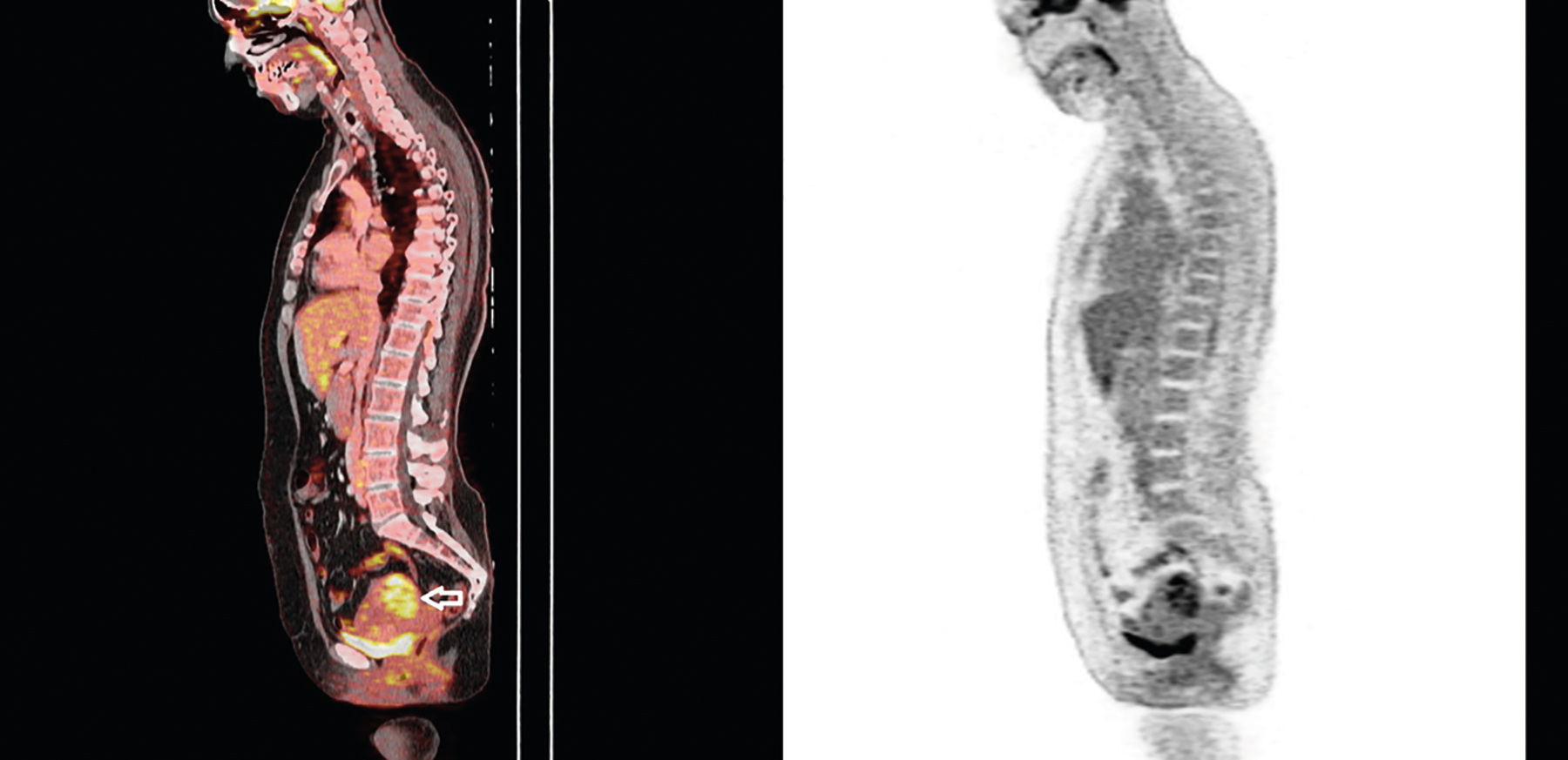
A 49-year-old woman presented with the characteristic Gottron sign and heliotrope rash. She reported progressive muscle weakness during the last 2 months. Blind screening with [18F]-FDG PET/CT (left panel) disclosed a uterine mass yielding a high standardized uptake value (SUV max = 12.8) (arrow). Hysterectomy revealed an undifferentiated uterine sarcoma. The right panel shows [18F]-FDG PET image
Nevertheless, the idea that PET/CT should be carried out in all patients diagnosed with dermatomyositis has been challenged, at least at the onset of the autoimmune disease. In a retrospective cohort study performed between 2005 and 2018 in Montreal, [18F]-FDG PET/CT results were investigated in 63 patients diagnosed with various myositis phenotypes, nearly half of them (31 of 63) with dermatomyositis [15]. Only 3 cases of cancer-associated myositis were found (all in dermatomyositis patients, 10%), and all were diagnosed by conventional screening. PET/CT did not identify any occult malignancy, and the findings led to additional biopsies, which ultimately tested negative. The authors concluded that PET/CT did not seem useful for cancer screening as compared to conventional methods and warned about possible iatrogenic consequences. However, some factors, such as the retrospective nature of the study, the small number of cancer patients analysed, and the fact that most patients did not undergo whole-body PET/CT advise caution with these conclusions. Prospective studies comparing the two approaches and studies analysing the cumulative experience of years of PET/CT use will help to define the reliability of these tests for diagnosing occult malignancy in dermatomyositis patients. In any case, cancer screening strategies should not only involve imaging techniques such as PET/CT, but also epidemiological, phenotypical, and immunological assessment [16].
Inflammatory Activity in the Muscle
Muscle activity in myositis is usually measured on clinical grounds with the help of well-recognized instruments (e.g. manual muscle testing) [17] or by using magnetic resonance imaging (MRI) of the muscle [18]. Differentiation between autoimmune disease activity and muscle damage based only on clinical factors can be difficult in some cases. Thus, as a complementary examination, [18F]-FDG PET/CT can help to define the muscle activity (Fig. 2).
Fig. 2.
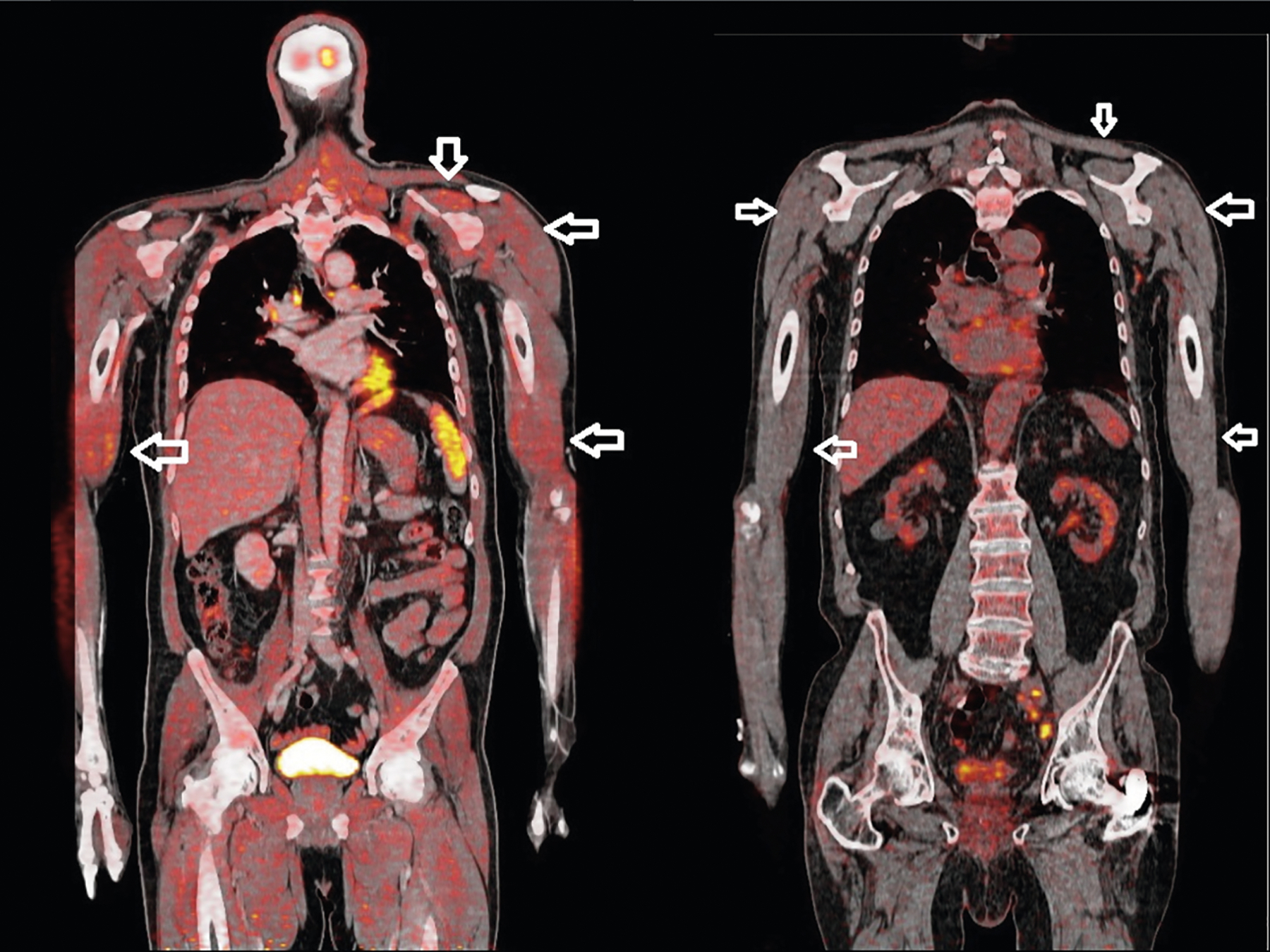
A 58-year-old man was diagnosed with statin-associated immune-mediated necrotizing myopathy, with positive anti-HMGCR antibodies. Cancer screening with [18F]-FDG PET/CT (left panel) detected no malignancy but documented high uptake (SUVmax = 3.4) in the proximal muscles (arrows). At the time PET/CT was performed, the creatine kinase value was 49,010 IU/L (normal value, < 195 IU/L). The patient improved after immunosuppressive therapy. The right panel shows normal muscle uptake (arrows) in a patient without myositis
One of the first studies assessing [18F]-FDG PET/CT for determining muscle inflammation activity was reported in 2014 by Tateyama et al. [19]. The authors used the standardized uptake value (SUVmax) measure and simple visual evaluation in a retrospective study of 33 patients diagnosed with dermatomyositis or polymyositis. All patients had undergone PET, and 25 of them had concurrent CT. A good correlation was found between the SUVmax and the serum creatine kinase value, muscle biopsy findings, and visual evaluation. Muscle involvement was quite symmetrical and significantly higher in myositis patients than in the 22 patients with amyotrophic lateral sclerosis included as controls.
The main difficulty in evaluating myositis activity by [18F]-FDG PET/CT is that normal values and the values denoting activity have not been conclusively defined. This lack of standardization has led to discordant results in the related studies. In a small study including 12 myositis patients (10 dermatomyositis and 2 polymyositis), Pipitone et al. [20] found that increased proximal muscle activity based on [18F]-FDG PET/CT was evident in myositis patients versus controls (sensitivity 75%, specificity 100%), but a good correlation with creatine kinase levels and muscle strength measured by manual muscle testing was lacking. In a retrospective study of 24 patients with myositis (11 polymyositis, 13 dermatomyositis), Owada et al. [21] reported that FDG-PET imaging had lower sensitivity (33%) for the myositis diagnosis than conventional examinations, such as electrophysiological studies (74%), MRI (51%), or muscle biopsy (100%), whereas specificity remained very high (97%). The main difference between the two studies was that FDG uptake was expressed as the SUVmax ratio of the proximal muscle to the liver in the first study, whereas in the second, positive activity was defined as a higher SUVmax in the muscle than the liver, currently the standard way of measuring the activity in the vessels in vasculitis patients [22].
Other studies [23, 24] have also analysed the correlation between creatine kinase levels and FDG uptake (SUVmean and SUVmax, respectively) as an indicator of disease activity in patients with myositis. The correlation with serum creatine kinase and muscle strength seemed good when the proximal muscle was analysed, but it failed when the average SUVmax—that is, the average of SUVmax values calculated for the proximal muscles bilaterally—was used as the measure [24]. An interesting finding emerged in the study by Tanaka et al. [23]: a good correlation was found between the PET/CT SUVmax and the results of muscle biopsy in myositis patients. As has been seen in studies using MRI [25], [18F]-FDG PET/CT may be a good technique for determining the site where the muscle should be biopsied. Finally, a recent article by Matuszac et al. [26••] focused on determining the disease activity in the muscle with [18F]-FDG PET/CT and assessed the validity of the technique. The authors analysed 34 myositis patients who underwent 44 PET/CT studies. Subsequent examinations in 10 patients allowed changes in the muscle activity to be detected over time (AUC 0.96; 95% CI 0.84–1). The method they used to measure the activity was the average of 16 muscle SUVmax values, normalized to the mean liver SUV. The authors concluded that PET/CT may be useful for monitoring the muscle activity in myositis.
In comparisons with other myopathies and controls, current data suggest that [18F]-FDG PET/CT is a good imaging technique for diagnosing myositis activity. Although its usefulness for monitoring muscle activity is still uncertain, at least one article has established that the use of the average muscle SUVmax value normalized to the mean liver SUV is a reliable method for this purpose [26••]. The question that still remains is what are the advantages (if any) of PET/CTcompared to muscle MRI to assess the muscle disease activity, given that MRI does not use ionizing radiation (potential for longitudinal follow-up) and does not have the normalization limitations of PET/CT.
Inflammatory Activity in the Lung
Idiopathic inflammatory myopathies are considered systemic diseases that involve organs other than the muscle, skin, and joints [1, 2]. The lungs are often affected, and the clinical presentation can vary considerably from rapidly progressive interstitial lung disease associated with anti-MDA5 antibodies [27] to a more chronic form, such as antisynthetase syndrome [28]. In this situation, clinicians need to assess the level of disease activity to decide when and how immunosuppressive therapy should be started or withdrawn. At present, the best way to determine and monitor respiratory function is with lung function testing, which includes the forced vital capacity (FVC) and carbon monoxide-diffusing capacity (DLCO) [29].
Interstitial lung disease is an important cause of death in patients with myositis [29]. It mainly develops in the context of antisynthetase syndrome, where it usually accompanies other manifestations, some of the major ones such as arthritis and myositis, and some minor such as Raynaud phenomenon, fever, or “mechanic hands”. However, other dermatomyositis patients, mainly those with anti-MDA5 antibodies, can also develop rapidly progressive interstitial lung disease. Although high-resolution CT (HRCT) and lung function tests (FVC and DLCO) are commonly used for the diagnosis, evaluation, and follow-up of this condition, some authors have evaluated the hybrid technique PET/CT for detecting and quantifying alveolitis in the lungs, based on increased FDG uptake in inflammatory cells (Fig. 3). As the lung is normally filled with air, the metabolic index is null and the SUVmax is usually negligible in this organ. Therefore, the detection of any inflammatory activity in the lung will differ from the physiological uptake, which is near zero.
Fig. 3.
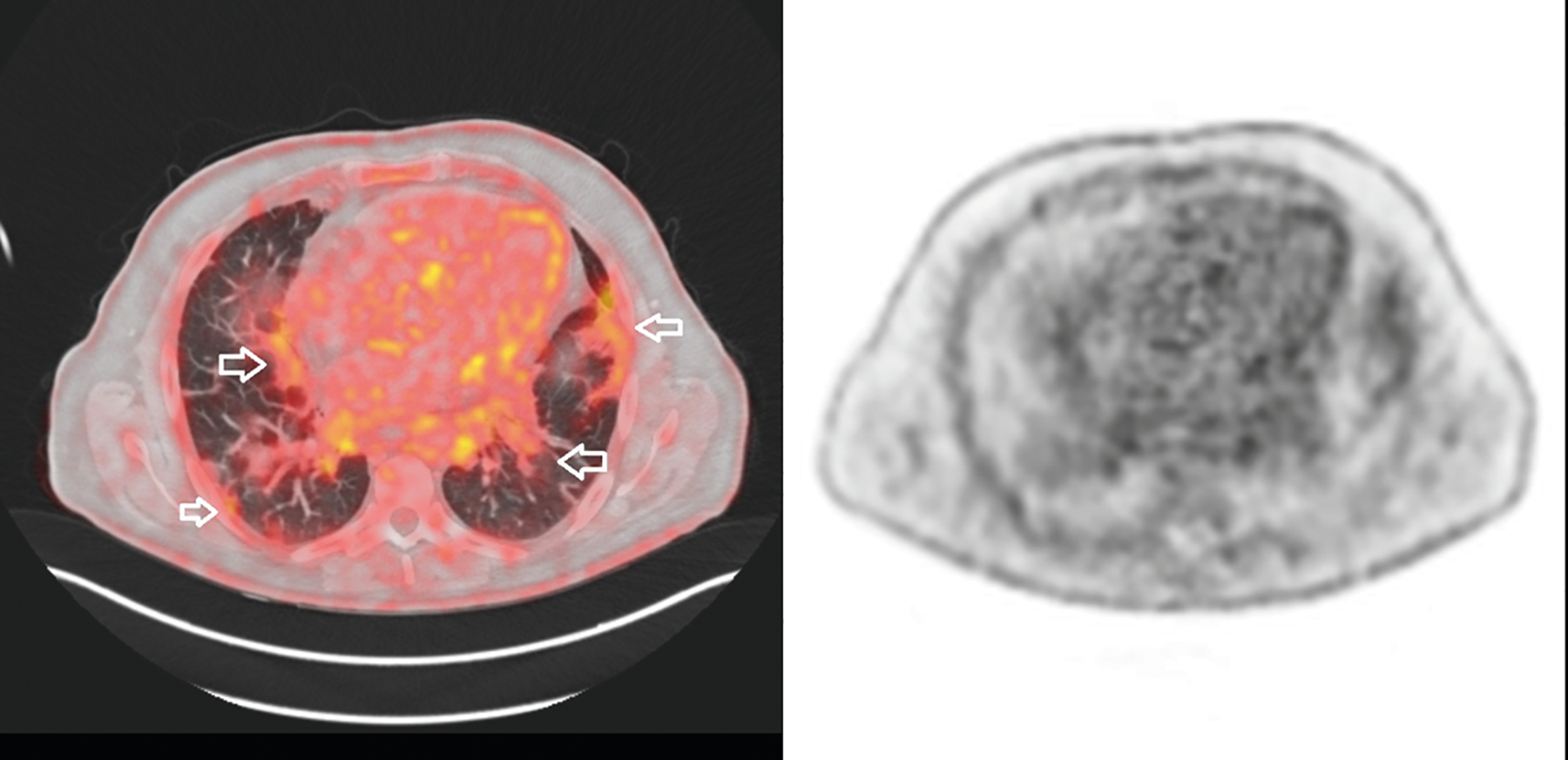
A man aged 55 years consulted for dyspnoea and mild muscle weakness. On physical examination, mechanic’s hands were observed, and lung crackles were noted at auscultation. He was diagnosed with antisynthetase syndrome and received treatment with tacrolimus and prednisone, achieving a mild improvement. [18F]-FDG PET/CT performed for cancer screening (left panel) did not detect cancer, but isotope uptake was high in both lung bases (SUV max = 4.5) (arrows). As lungs are normally filled with air, the metabolic index is null and the SUVmax is usually negligible in physiologic conditions. Infectious complications were ruled out. The right panel shows [18F]-FDG PET image
Investigators from Japan [30] performed a study including 22 patients with antisynthetase syndrome and interstitial lung disease, and what they found was interesting. High [18F]-FDG uptake (SUVmax) was observed in the areas of the lung where inflammatory lesions were seen on HRCT, and a good correlation was observed between the SUVmax and the HRCT scan score, as well as a classic biomarker of activity, blood levels of Krebs von den Lungen-6 (KL-6). Another study [31] including 45 patients with connective tissue disease, 16 of them diagnosed with dermatomyositis/polymyositis, described the value of this hybrid technique for identifying inflammatory lung lesions in patients with associated interstitial lung disease. This research is noteworthy because the authors found that the [18F]-FDG SUVmax changed after treatment and was consistent with the activity of the interstitial lung disease. The possible imaging confounder due to respiratory movements was overcome by using a deep-inspiration breath-hold technique [31, 32].
In a case report [33] by Japanese researchers, [18F]-FDG PET/CT was able to detect inflammatory activity in the lung 1 month before the development of a rapidly progressive interstitial lung disease in an anti-MDA5-positive amyopathic dermatomyositis patient. Although, unfortunately, the patient died despite the initiation of treatment, there is a possibility that early PET/CT examination may improve the outcome of these patients. In a retrospective study [34••], researchers in China reported high sensitivity and specificity for detecting rapidly progressive interstitial lung disease when the [18F]-FDG SUVmax was higher than 2.5 on PET/CT, although the diagnostic yield of this technique did not differ from that of HRCT. Additional studies in larger patient series are needed to know whether PET/CT is warranted for early detection of this severe manifestation.
Although other techniques have been proposed to quantify the activity of interstitial lung disease (e.g. gadolinium-enhanced MRI or high-field MRI) [35, 36], this is still a challenging clinical problem and PET/CT should also be considered a promising tool in this line.
Sporadic Inclusion Body Myositis, Focal Myositis, and Rare Organ-Specific Involvement
Most of the data mentioned up to now have been focused on dermatomyositis, polymyositis, and alveolitis detection in myositis-associated interstitial lung disease. However, other myositis phenotypes may benefit from PET/CT, such as sporadic inclusion body myositis (sIBM). Although several criteria have been proposed for diagnosing sIBM, it may be difficult to reach a diagnosis in some patients, and misdiagnoses (e.g. polymyositis) can occur, particularly at the onset of the disease [37]. Autoantibodies to cytosolic 5′-nucleotidase 1A (anti-cN1A) can contribute to the diagnosis, although they can also test positive in other myositis subsets [38, 39]. The rimmed vacuoles detected in muscle biopsy of sIBM patients are rich in neurodegenerative proteins, including β-amyloid [40]. Hence, the use of PET with [18F] florbetapir, a good marker for detecting amyloid disease, could be an interesting approach. Lilleker et al. from Manchester have explored this strategy [41••]. In a small study, these authors performed [18F] florbetapir PET in 10 sIBM patients and compared the findings with those of 6 polymyositis patients. The florbetapir SUV was significantly higher in those with sIBM, yielding a sensitivity of 80% and specificity of 100% for the sIBM diagnosis. The passage of time and additional studies in larger patient series will determine whether [18F] florbetapir PET is a useful complementary technique for the diagnosis of sIBM, although the initial results are encouraging [42].
Focal myositis, a benign condition that sometimes requires treatment with immunosuppressive therapy, can be identified using [18F]-FDG PET/CT. In this scenario, the role of the technique is not related to detecting occult malignancy or assessing muscle activity, but it has proven useful for diagnosing focal myositis as an unexpected finding in patients undergoing this test for non-specific signs and symptoms (e.g. fever of unknown origin, a localized mass, pain, and swelling) and other complaints [43–45].
The reported rates of cardiac involvement in patients with myositis range from 9 to 70%, and true myocarditis seems to be rare [46]. Nonetheless, one study in patients with antisynthetase syndrome reported a myocarditis rate of 42% [47]. In addition, necrotizing myositis and myocarditis are among the complications described in relation to cancer immunotherapy [48]. The diagnosis of cardiac involvement mainly relies on clinical criteria, MRI findings, and high levels of troponin I. However, there is some evidence that [18F]-FDG PET/CT may be of help as a complementary tool to diagnose myocarditis in myositis patients [49].
A Comprehensive Approach for the Use of PET/CT in Patients with Myositis
In the heterogeneous group of autoimmune systemic diseases referred to as myositis, the muscle, skin, lung, and even the heart can be affected, and malignancies are common in some disease phenotypes. Thus, it makes sense to use a hybrid technique such as PET/CT to assess these various manifestations. Only 1 study has focussed on investigating the value of [18F]-FDG PET/CT for this multifaceted task. Li et al. [34••] retrospectively analysed a cohort of 38 patients with various myositis phenotypes and sought the presence of malignancy, muscle activity, and interstitial lung disease. The technique correctly detected 7 malignancies and found higher muscle FDG uptake in myositis patients than in controls. Furthermore, PET/CT findings showed a good correlation with creatine kinase values, muscle weakness, and interstitial lung disease detected by HRCT, which was acquired simultaneously with PET/CT. In addition, FDG uptake in the lung was higher in patients with rapidly progressive interstitial lung disease. The data from this first evaluation suggest that PET/CT could be a useful complementary examination to be performed systematically at the diagnosis of patients with inflammatory myopathy.
Conclusions and Future Directions
As has been highlighted in this review, the main interest of PET/CT in myositis patients centres on cancer screening and objective measures of muscle activity. Other potential applications, such as quantifying alveolitis over time in interstitial lung disease, achieving an early diagnosis in rapidly progressive lung disease, and establishing the differential diagnosis between sIBM and polymyositis require further investigation in well-designed studies in large patient samples.
Beyond the above-mentioned usefulness of PET/CT in myositis, ongoing advances in this technique may lead to further applications. Assessment of coronary inflammation [50] by PET/CT using FDG or new tracers such as 68Ga-DOTATATE, a good marker of coronary inflammation; choline, a biomarker of macrophage infiltration; or 18F-sodium fluoride for coronary calcification, among others, will undoubtedly expand the value of PET/CT for myositis and some of its associated complications. Finally, techniques that combine PET and MRI instead of CT may lead to a better resolution for visualizing the vessels, soft tissue, skin, and muscle, while avoiding cumulative radiation exposure if repeated imaging is needed over time [51, 52].
Funding
This work was supported by the Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) (grant number PI15/02100).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734–47. [DOI] [PubMed] [Google Scholar]
- 2.Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17:816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of malignancy in dermatomyositis and polymyositis. J Cutan Med Surg. 2017;21:131–6. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49:17N–21N 37N. [PubMed] [Google Scholar]
- 5.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124–45. [DOI] [PubMed] [Google Scholar]
- 6.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134:1087–95. [DOI] [PubMed] [Google Scholar]
- 8.Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–7. [DOI] [PubMed] [Google Scholar]
- 9.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. [DOI] [PubMed] [Google Scholar]
- 10.Allenbach Y, Keraen J, Bouvier AM, Jooste V, Champtiaux N, Hervier B, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139:2131–5. [DOI] [PubMed] [Google Scholar]
- 11.Trallero-Araguás E, Rodrigo-Pendás JA, Selva-O’Callaghan A, et al. Usefulness of antip155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64:523–32. [DOI] [PubMed] [Google Scholar]
- 12.Albayda J, Pinal-Fernandez I, Huang W, Parks C, Paik J, Casciola-Rosen L, et al. Antinuclear matrix protein 2 autoantibodies and edema, muscle disease, and malignancy risk in dermatomyositis patients. Arthritis Care Res (Hoboken). 2017;69:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, Vidaller-Palacín A, Martínez-Gómez X, Trallero-Araguás E, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558–62. [DOI] [PubMed] [Google Scholar]
- 14.•.Kundrick A, Kirby J, Ba D, Leslie D, Olsen N, Foulke G. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2018;49:140–4. 10.1016/j.semarthrit.2018.10.021. [DOI] [PubMed] [Google Scholar]; This study states that PET/CT whole body may be expensive for insurance companies but not for the patients, opening the door to more generalised implementation of PET/CT for dermatomyositis cancer screening in countries where this test is not available through publically-funded health resources.
- 15.Maliha PG, Hudson M, Abikhzer G, Singerman J, Probst S. 18F-FDG PET/CT versus conventional investigations for cancer screening in autoimmune inflammatory myopathy in the era of novel myopathy classifications. Nucl Med Commun. 2019;40:377–82. [DOI] [PubMed] [Google Scholar]
- 16.Selva-O’Callaghan A, Martínez-Gómez X, Trallero-Araguás E, Pinal-Fernández I. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S118–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinal-Fernandez I, Casal-Dominguez M, Carrino JA, Lahouti AH, Basharat P, Albayda J, et al. Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann Rheum Dis. 2017;76:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tateyama M, Fujihara K, Misu T, Arai A, Kaneta T, Aoki M. Clinical values of FDG PET in polymyositis and dermatomyositis syndromes: imaging of skeletal muscle inflammation. BMJ Open. 2015;5:e006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipitone N, Versari A, Zuccoli G, Levrini G, Macchioni P, Bajocchi G, et al. 18F-Fluorodeoxyglucose positron emission tomography for the assessment of myositis: a case series. Clin Exp Rheumatol. 2012;30:570–3. [PubMed] [Google Scholar]
- 21.Owada T, Maezawa R, Kurasawa K, Okada H, Arai S, Fukuda T. Detection of inflammatory lesions by F-18 fluorodeoxyglucose positron emission tomography in patients with polymyositis and dermatomyositis. J Rheumatol. 2012;39:1659–65. [DOI] [PubMed] [Google Scholar]
- 22.Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche EU. The value of [18F] FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674–81. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Ikeda K, Uchiyama K, Iwamoto T, Sanayama Y, Okubo A, et al. [18F] FDG uptake in proximal muscles assessed by PET/CT reflects both global and local muscular inflammation and provides useful information in the management of patients with polymyositis/dermatomyositis. Rheumatology (Oxford). 2013;52:1271–8. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Dong Y, Zhang N, Lv X, Chen Q, Wei W. [(18)F] Fluorodeoxyglucose positron emission tomography/computed tomography for diagnosing polymyositis/dermatomyositis. Exp Ther Med. 2018;15:5023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van De Vlekkert J, Maas M, Hoogendijk JE, De Visser M, Van Schaik IN. Combining MRI and muscle biopsy improves diagnostic accuracy in subacute-onset idiopathic inflammatory myopathy. Muscle Nerve. 2015;51:253–8. [DOI] [PubMed] [Google Scholar]
- 26.••.Matuszak J, Blondet C, Hubelé F, Gottenberg JE, Sibilia J, Bund C, et al. Muscle fluorodeoxyglucose uptake assessed by positron emission tomography-computed tomography as a biomarker of inflammatory myopathies disease activityRheumatology (Oxford). 2019. 10.1093/rheumatology/kez040 [DOI] [PubMed]; This is a well conducted study which demonstrates that PET/CT could be useful for monitoring muscle disease activity. The method that they used to measure muscle activity showed an excellent reliability and validity.
- 27.Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res (Hoboken). 2012;64:1602–10. [DOI] [PubMed] [Google Scholar]
- 28.Trallero-Araguás E, Grau-Junyent JM, Labirua-Iturburu A, García-Hernández FJ, Monteagudo-Jiménez M, Fraile-Rodriguez G, et al. Clinical manifestations and long-term outcome of anti-Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group. Semin Arthritis Rheum. 2016;46:225–31. [DOI] [PubMed] [Google Scholar]
- 29.Barba T, Mainbourg S, Nasser M, Lega JC, Cottin V. Lung diseases in inflammatory myopathies. Semin Respir Crit Care Med. 2019;40:255–70. [DOI] [PubMed] [Google Scholar]
- 30.Motegi SI, Fujiwara C, Sekiguchi A, Hara K, Yamaguchi K, Maeno T, et al. Clinical value of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for interstitial lung disease and myositis in patients with dermatomyositis. J Dermatol. 2019;46:213–8. [DOI] [PubMed] [Google Scholar]
- 31.Uehara T, Takeno M, Hama M, Yoshimi R, Suda A, Ihata A, et al. Deep-inspiration breath-hold 18F-FDG-PET/CT is useful for assessment of connective tissue disease associated interstitial pneumonia. Mod Rheumatol. 2016;26:121–7. [DOI] [PubMed] [Google Scholar]
- 32.Nehmeh SA, Ertii YE, Meirelles GS, Squire O, Larson SM, Humm JL, et al. Deep-inspiration breath-hold PET/CT of the thorax. J Nucl Med. 2007;48:22–6. [PubMed] [Google Scholar]
- 33.Morita Y, Kuwagata S, Kato N, Tsujimura Y, Mizutani H, Suehiro M, et al. 18F-FDG PET/CT useful for the early detection of rapidly progressive fatal interstitial lung disease in dermatomyositis. Intern Med. 2012;51:1613–8. [DOI] [PubMed] [Google Scholar]
- 34.••.Li Y, Zhou Y, Wang Q. Multiple values of (18)F-FDG PET/CT in idiopathic inflammatory myopathy. Clin Rheumatol. 2017;36:2297–305 [DOI] [PubMed] [Google Scholar]; This study demonstrates the multiple values of PET/TC in patients with myositis by detecting malignancies, assessing muscle activity, determining interstitial lung activity, and predicting rapidly-progressive interstitial lung disease.
- 35.Gaeta M, Blandino A, Scribano E, Minutoli F, Barone M, Andò F, et al. Chronic infiltrative lung diseases: value of gadolinium-enhanced MRI in the evaluation of disease activity–early report. Chest. 2000;117:1173–8. [DOI] [PubMed] [Google Scholar]
- 36.Lutterbey G, Grohé C, Gieseke J, von Falkenhausen M, Morakkabati N, Wattjes MP, et al. Initial experience with lung-MRI at 3.0T: comparison with CT and clinical data in the evaluation of interstitial lung disease activity. Eur J Radiol. 2007;61:256–61. [DOI] [PubMed] [Google Scholar]
- 37.Hilton-Jones D, Brady S. Diagnostic criteria for inclusion body myositis. J Intern Med. 2016;280:52–62. [DOI] [PubMed] [Google Scholar]
- 38.Pluk H, van Hoeve BJ, van Dooren SH, Stammen-Vogelzangs J, van der Heijden A, Schelhaas HJ, et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann Neurol. 2013;73:397–407. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, Tiniakou E, Petri M, Baer A, et al. Cytosolic 5′-nucleotidase 1A as a target of circulating autoantibodies in autoimmune diseases. Arthritis Care Res (Hoboken). 2016;68:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruitt JN 2nd, Showalter CJ, Engel AG. Sporadic inclusion body myositis: counts of different types of abnormal fibers. Ann Neurol. 1996;39:139–43. [DOI] [PubMed] [Google Scholar]
- 41.••.Lilleker JB, Hodgson R, Roberts M, Herholz K, Howard J, Hinz R, et al. [18F] Florbetapir positron emission tomography: identification of muscle amyloid in inclusion body myositis and differentiation from polymyositis. Ann Rheum Dis. 2019. Feb 13. 10.1136/annrheumdis-2018-214644 [DOI] [PMC free article] [PubMed] [Google Scholar]; [18F] florbetapir positron emission tomography can detect tissue deposits of amyloid. In this study the authors suggest that this technique may allow to differentiate inclusion body myositis from polymyositis.
- 42.Pinal-Fernandez I, Mammen AL. Amyloid-PET: a new tool for diagnosing IBM? Nat Rev Rheumatol. 2019;15:321–2. [DOI] [PubMed] [Google Scholar]
- 43.Bennett O, Ravi Kumar AS, Agnew J. Focal inflammatory myositis on 18F-FDG PET/CT. Clin Nucl Med. 2016;41:469–71. [DOI] [PubMed] [Google Scholar]
- 44.Dong A, Bai Y, Wang Y. Focal myositis of the leg presenting as fever of unknown origin detected by FDG PET/CT. Clin Nucl Med. 2019;44:251–4. [DOI] [PubMed] [Google Scholar]
- 45.Marie I, Sauvêtre G, Becker S, Bedat-Millet AL. Clinical images: focal myositis demonstrated on positron emission tomography. Arthritis Rheumatol. 2014;66:1871. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Wang GC, Ma L, Zu N. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol. 2012;35:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieval C, Deligny C, Meyer A, Cluzel P, Champtiaux N, Lefevre G, et al. Myocarditis in patients with antisynthetase syndrome: prevalence, presentation, and outcomes. Medicine (Baltimore). 2015;94:e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puwanant A, Isfort M, Lacomis D, Živković SA. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord. 2019;29:127–33. [DOI] [PubMed] [Google Scholar]
- 49.Shigematsu T, Okayama H, Hiasa G, Kazatani Y. (18) F-fluorodeoxyglucose positron emission tomography for the diagnosis of myocarditis associated with polymyositis. Circ J. 2016;81:121–2. [DOI] [PubMed] [Google Scholar]
- 50.Pelletier-Galarneau M, Ruddy TD. Molecular imaging of coronary inflammation. Trends Cardiovasc Med. 2019;29:191–7. [DOI] [PubMed] [Google Scholar]
- 51.Wehrl HF, Sauter AW, Divine MR, Pichler BJ. Combined PET/MR: a technology becomes mature. J Nucl Med. 2015;56:165–8. [DOI] [PubMed] [Google Scholar]
- 52.Jadvar H, Colleti PM. Competitive advantage of PET/MRI. Eur J Radiol. 2014;83:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


