Abstract
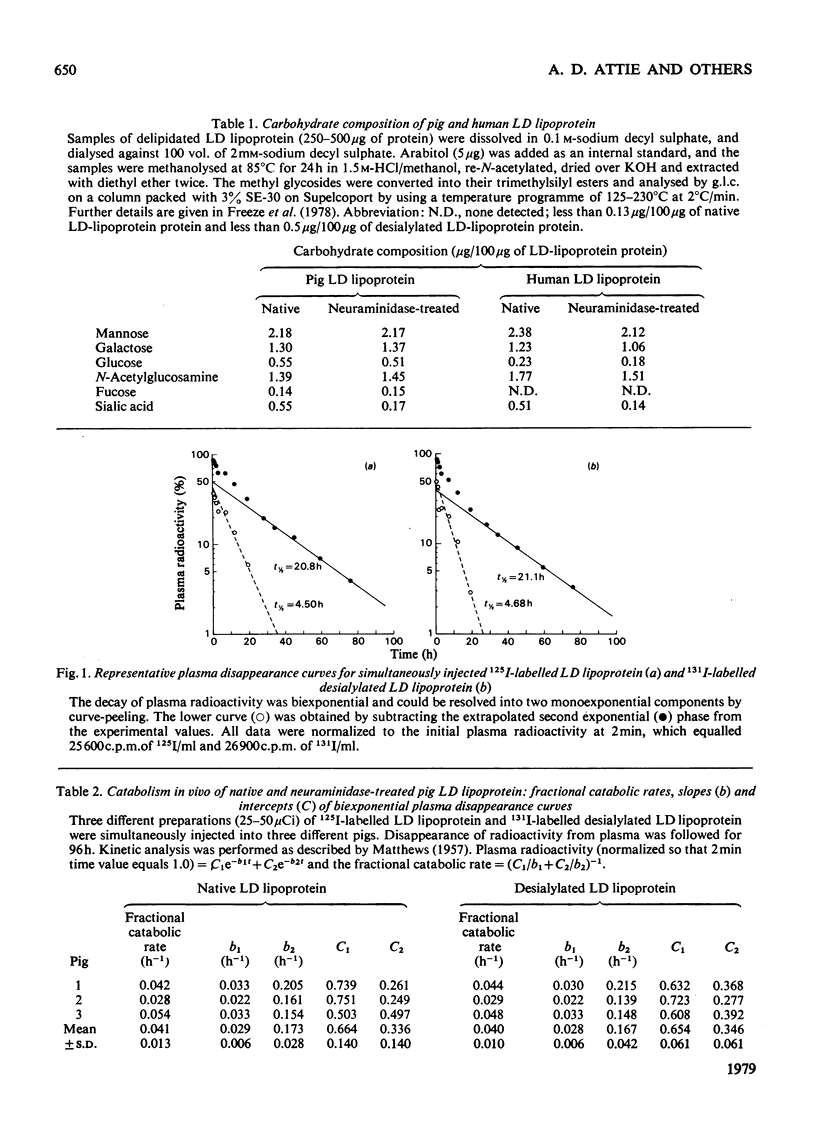
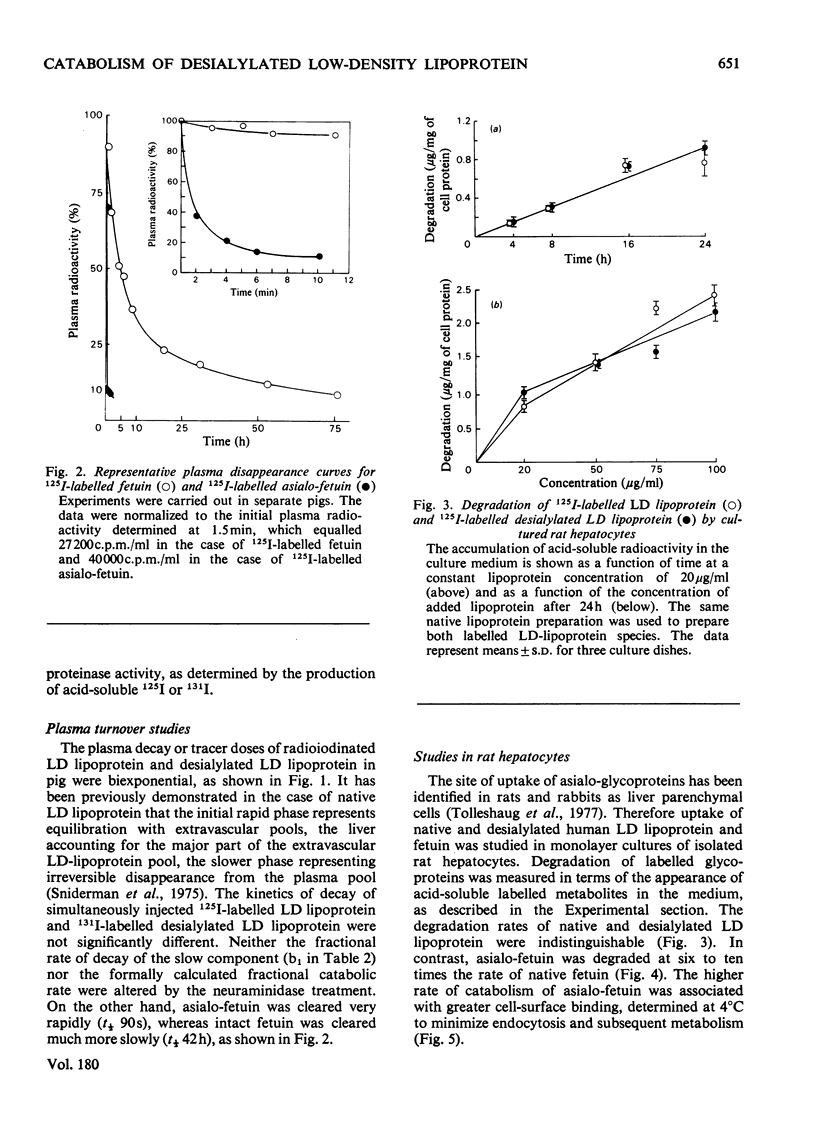
Removal of the terminal sialic acid residues from many serum glycoproteins results in exposure of their penultimate galactose residues and rapid clearance from circulation by the liver. Low-density lipoprotein is a glycoprotein containing 21 galactose and 9 sialic acid residues per particle. Studies in this laboratory and others have shown that both the liver and extrahepatic tissues contribute to the degradation of low-density lipoprotein. This study was undertaken to determine whether desialylation of pig low-density lipoprotein alters its removal from circulation. Low-density lipoprotein was incubated at 37 degrees C with an agarose-bound neuraminidase, proteinase-free, from Clostridium perfringens. After 18 h at pH 5.0, 70% of the sialic acid residues were removed. The desialylated 131I-labelled and native 125I-labelled low-density lipoproteins were simultaneously injected into a pig, and their disappearance from plasma was followed for 96 h. The turnovers of the two were identical. In contrast, neuraminidase-treated fetuin was cleared about 200-fold faster than native fetuin. Studies were also performed in cultured rat hepatocytes. Rates of degradation of native and neuraminidase-treated low-density lipoprotein were similar, whereas asialo-fetuin was degraded at six to ten times the rate of native fetuin. Thus desialylation does not appear to alter low-density-lipoprotein catabolism by hepatic or extrahepatic cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Bierman E. L. The effect of hypoxia on uptake and degradation of low density lipoproteins by cultured human arterial smooth muscle cells. Biochim Biophys Acta. 1976 Mar 26;424(3):422–429. doi: 10.1016/0005-2760(76)90031-x. [DOI] [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Azuma J., Kashimura N., Komano T. Studies on pig serum lipoproteins. IV. Isolation and characterization of glycopeptides from pig serum low density lipoprotein. J Biochem. 1977 Jun;81(6):1613–1619. doi: 10.1093/oxfordjournals.jbchem.a131620. [DOI] [PubMed] [Google Scholar]
- Berman M., Hall M., 3rd, Levy R. I., Eisenberg S., Bilheimer D. W., Phair R. D., Goebel R. H. Metabolsim of apoB and apoC lipoproteins in man: kinetic studies in normal and hyperlipoproteininemic subjects. J Lipid Res. 1978 Jan;19(1):38–56. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert G. D., Scott P. J., Sharpe D. N. The plasma and tissue turnover and distribution of two radio-iodine-labelled pig plasma low density lipoproteins. Atherosclerosis. 1975 Nov-Dec;22(3):601–628. doi: 10.1016/0021-9150(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Saik R. P., Johansen K. H., Dennis C. A., Steinberg D. Low density and high density lipoprotein turnover following portacaval shunt in swine. J Lipid Res. 1976 Sep;17(5):441–450. [PubMed] [Google Scholar]
- Dumas P., Maziere B., Autissier N., Michel R. Specificite de l'iodotyrosine desiodase des microsomes thyroïdiens et hepatiques. Biochim Biophys Acta. 1973 Jan 12;293(1):36–47. doi: 10.1016/0005-2744(73)90373-2. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- Freeze H., Kress B. C., Williams J. C., Cerda-Ruiz M., Miller A. L. Carbohydrate composition of purified serum glycoproteins in mucolipidosis II and mucolipidosis III. Mol Cell Biochem. 1978 Oct 13;21(1):17–21. doi: 10.1007/BF00230192. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Lipoprotein receptors, cholesterol metabolism, and atherosclerosis. Arch Pathol. 1975 Apr;99(4):181–184. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Regoeczi E., Kaur H. Bovine transferrin glycopeptide: the relevance of its structure to interaction with the mammalian hepatic lectin that binds asialoglycoproteins. Can J Biochem. 1978 May;56(5):339–344. doi: 10.1139/o78-053. [DOI] [PubMed] [Google Scholar]
- Hay R. V., Pottenger L. A., Reingold A. L., Getz G. S., Wissler R. W. Degradation of I 125 -labelled serum low density lipoprotein in normal and estrogen-treated male rats. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1471–1477. doi: 10.1016/s0006-291x(71)80251-6. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Kayden H. J., Goldstein J. L. Binding, internalization, and hydrolysis of low density lipoprotein in long-term lymphoid cell lines from a normal subject and a patient with homozygous familial hypercholesterolemia. J Exp Med. 1976 Aug 1;144(2):444–455. doi: 10.1084/jem.144.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Murthy V. K., Monchesky T. C., Steiner G. In vitro labeling of beta-apolipoprotein with 3H or 14C and preliminary application to turnover studies. J Lipid Res. 1975 Jan;16(1):1–6. [PubMed] [Google Scholar]
- PHELPS R. A., CANN J. R. On the modification of conalbumin by acid. II. Effect of pH and salt concentration on the sedimentation behavior, viscosity and osmotic pressure of conalbumin solutions. Arch Biochem Biophys. 1956 Mar;61(1):51–71. doi: 10.1016/0003-9861(56)90316-2. [DOI] [PubMed] [Google Scholar]
- Pottenger L. A., Frazier L. E., DuBien L. H., Getz G. S., Wissler R. W. Carbohydrate composition of lipoprotein apoproteins isolated from rat plasma and from the livers of rats fed orotic acid. Biochem Biophys Res Commun. 1973 Sep 18;54(2):770–776. doi: 10.1016/0006-291x(73)91490-3. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. Subcellular distribution of a mammalian hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Dec 10;251(23):7539–7544. [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckless J. P., Weinstein D. B., Steinberg D. Lipoprotein and cholesterol metabolism in rabbit arterial endothelial cells in culture. Biochim Biophys Acta. 1978 Jun 23;529(3):475–487. doi: 10.1016/0005-2760(78)90091-7. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W. Studies of the metabolism of asialotransferrins: the mechanism for the hypercatabolism of human asialotransferrin in the rabbit. Can J Biochem. 1974 Jul;52(7):645–651. doi: 10.1139/o74-092. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Eisenberg S., Levy R. I. Lipoprotein apoprotein metabolism. J Lipid Res. 1978 Aug;19(6):667–687. [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Sniderman A. D., Carew T. E., Chandler J. G., Steinberg D. Paradoxical increase in rate of catabolism of low-density lipoproteins after hepatectomy. Science. 1974 Feb 8;183(4124):526–528. doi: 10.1126/science.183.4124.526. [DOI] [PubMed] [Google Scholar]
- Sniderman A. D., Carew T. E., Steinberg D. Turnover and tissue distribution of 125-I-labeled low density lipoprotein in swine and dogs. J Lipid Res. 1975 Jul;16(4):293–299. [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Swaminathan N., Aladjem F. The monosaccharide composition and sequence of the carbohydrate moiety of human serum low density lipoproteins. Biochemistry. 1976 Apr 6;15(7):1516–1522. doi: 10.1021/bi00652a024. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]


