Abstract
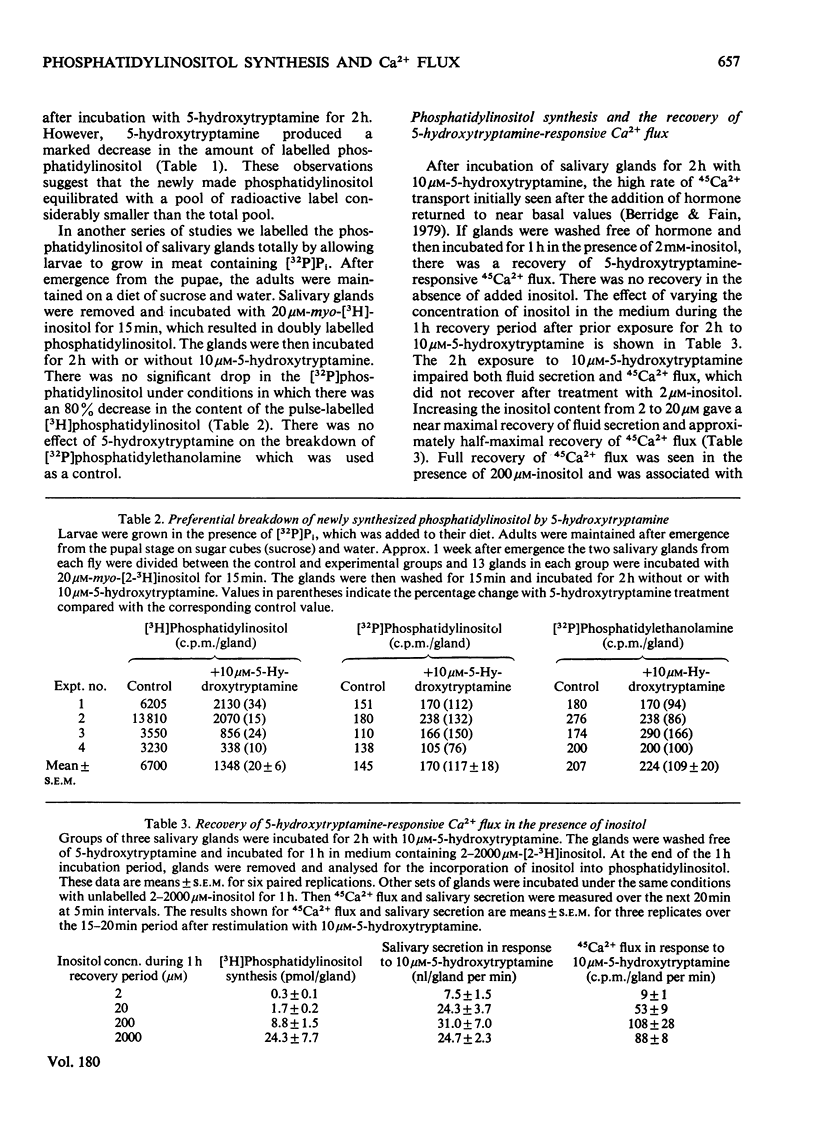
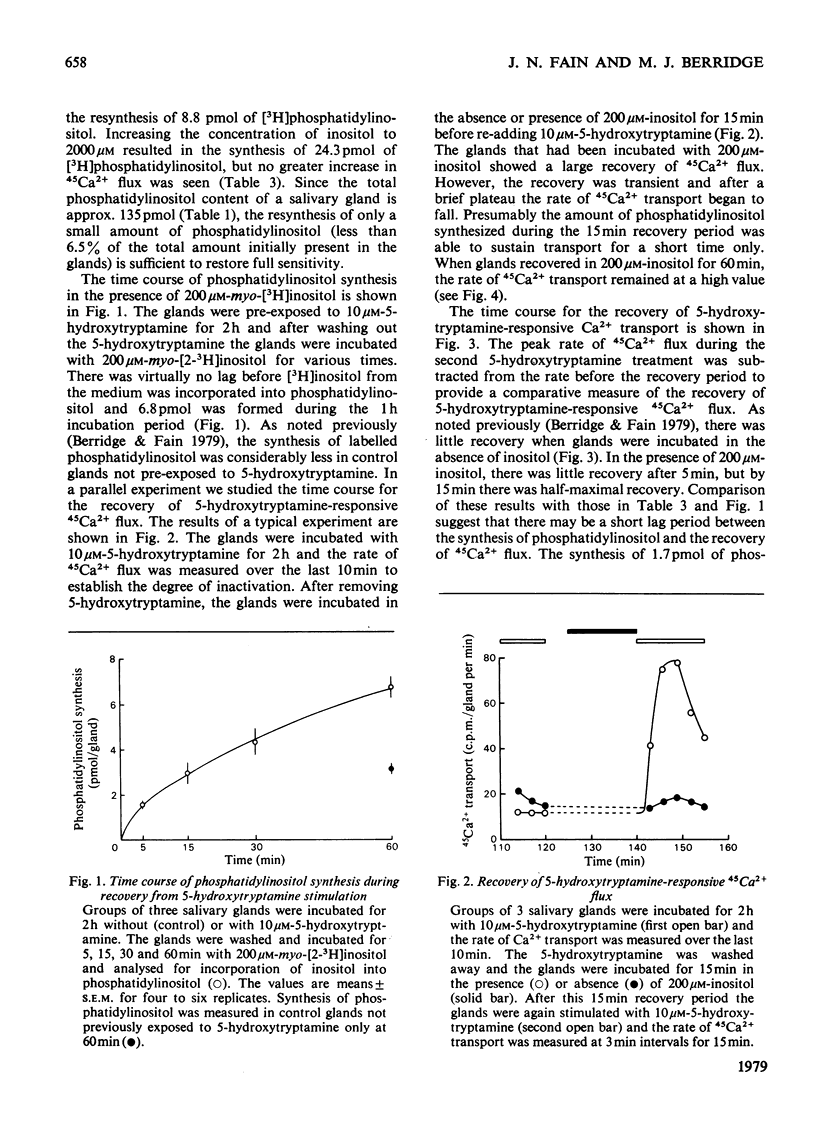
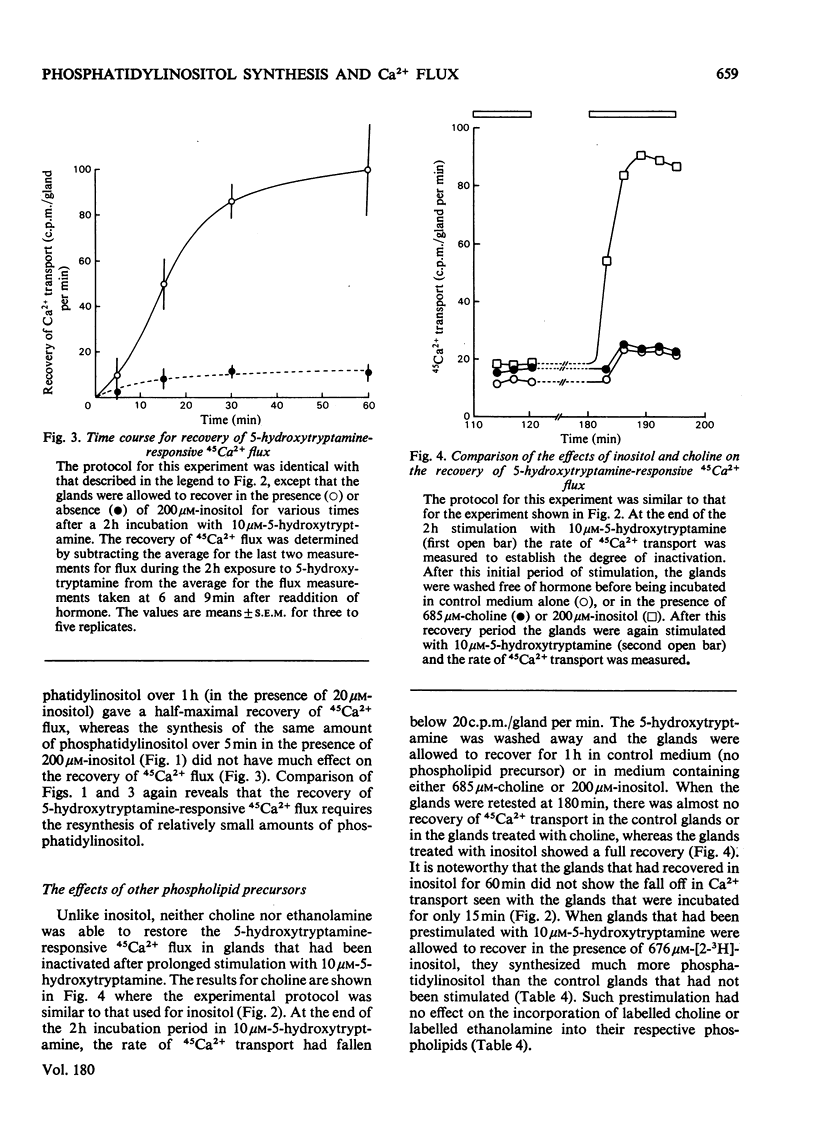
Each salivary gland contains about 135 pmol of phosphatidylinositol. In glands prelabelled by incubation for 1 h with [32P]Pi or [3H]inositol there was a subsequent breakdown of 80% of the labelled phosphatidylinositol over a 2 h incubation period with 10 micrometer-5-hydroxytryptamine. However, there was no detectable decrease either in total phosphatidylinositol based on phosphorus analysis by chemical estimation or in the radioactivity of [32P]phosphatidylinositol in salivary glands of flies raised from the larval stage on diets containing[32P]Pi and whose phospholipids were uniformly labelled. These results suggest that the pool of phosphatidylinositol involved with Ca2+ gating is a small fraction of the total phosphatidylinositol content. Furthermore it is this small compartment that is preferentially radioactively labelled during short-term incubations with radioactively labelled precursors. In salivary glands incubated for 2 h with 10 micrometer-5-hydroxytryptamine there was a marked decrease in the flux of 45Ca2+ across the gland. After removal of the hormone, incubation of salivary glands for 1 h in the presence of 2mM-inositol, but not choline or ethanolamine, resulted in a recovery of hormone-responsive 45Ca2+ flux. Quantitative studies revealed that less than 9 pmol of phosphatidylinositol must be formed to fully restoret he 5-hydroxytryptamine-responsive 45Ca2+ flux.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berridge M. J., Fain J. N. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J. 1979 Jan 15;178(1):59–69. doi: 10.1042/bj1780059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Acetylcholine causes a net decrease in phosphatidylinositol and a net increase in phosphatidic acid in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):763–768. doi: 10.1016/s0006-291x(74)80483-3. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Metabolism and role of phosphatidylinositol in acetylcholine-stimulated membrane function. Adv Exp Med Biol. 1977;83:429–446. doi: 10.1007/978-1-4684-3276-3_40. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Breakdown of phosphatidylinositol provoked by muscarinic cholinergic stimulation of rat parotid-gland fragments. Biochem J. 1974 Sep;142(3):583–590. doi: 10.1042/bj1420583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rossignol B. Lanthanum as a tool to study the role of phosphatidylinositol in the calcium transport in rat parotid glands upon cholinergic stimulation. Eur J Biochem. 1978 Apr;85(1):77–83. doi: 10.1111/j.1432-1033.1978.tb12213.x. [DOI] [PubMed] [Google Scholar]


