Abstract
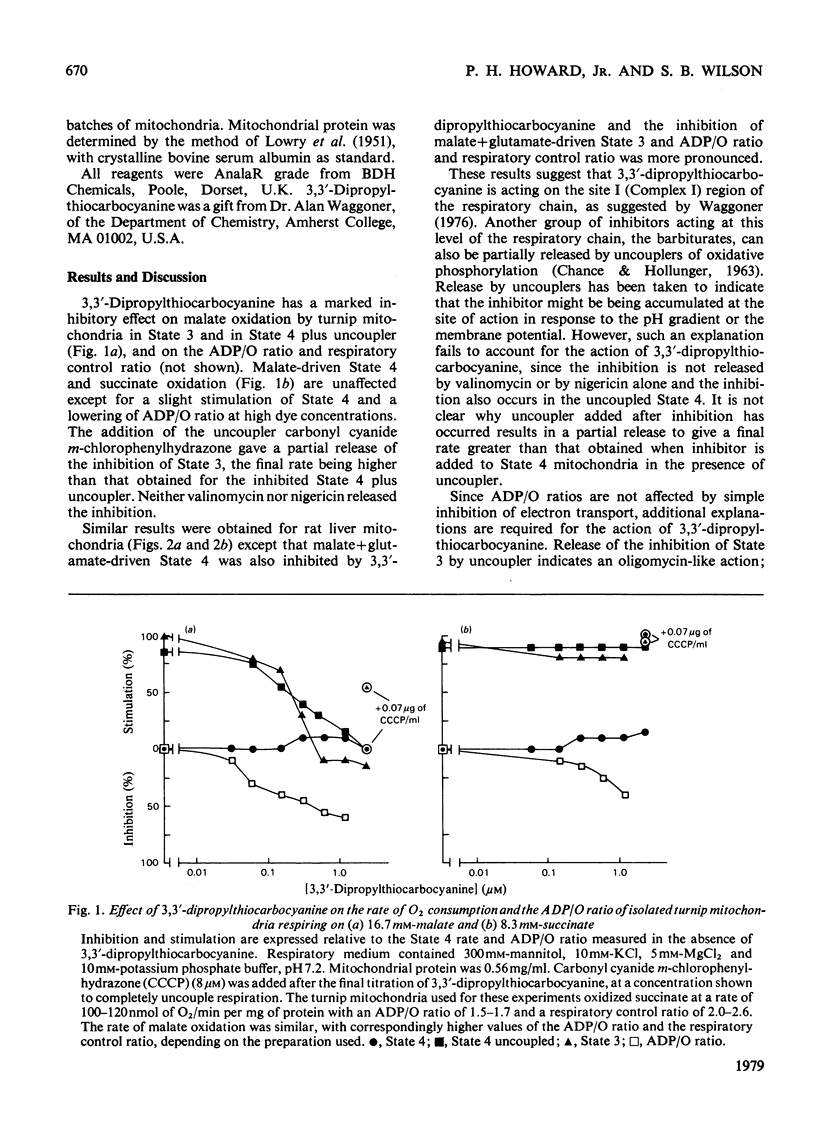
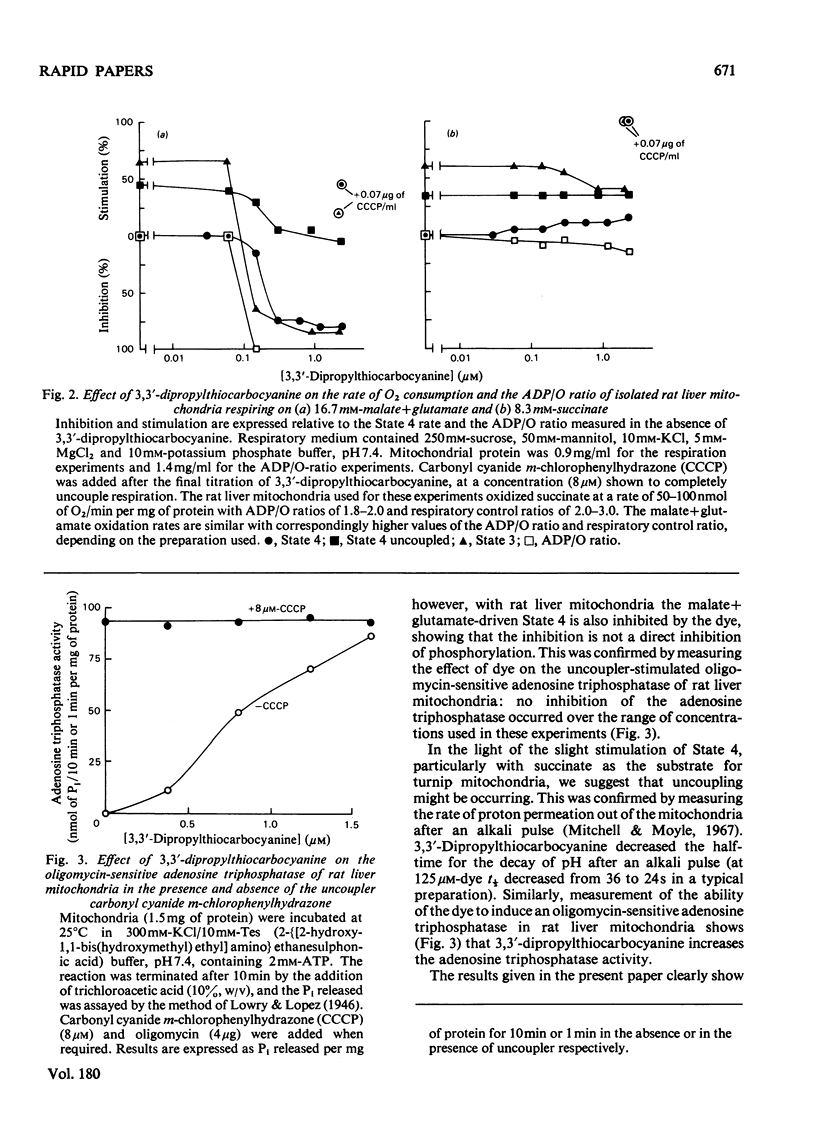
Mitochondrial respiration and oxidative phosphorylation were inhibited by the membrane potential probe 3,3'-dipropylthiocarbocyanine [diS-C3-(5)]. Evidence is presented that suggests that the dye acts as both an inhibitor of electron transport and an uncoupler of oxidative phosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R., HOLLUNGER G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963 Jan;238:418–431. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Tedeschi H. Metabolic effects of some electrofluorimetric dyes. Biochim Biophys Acta. 1978 Aug 8;503(2):380–388. doi: 10.1016/0005-2728(78)90195-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laris P. C., Bahr D. P., Chaffee R. R. Membrane potentials in mitochondrial preparations as measured by means of a cyanine dye. Biochim Biophys Acta. 1975 Mar 20;376(3):415–425. doi: 10.1016/0005-2728(75)90163-2. [DOI] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A., Johnstone R. M. Monitoring membrane potentials in Ehrlich ascites tumor cells by means of a fluorescent dye. Biochim Biophys Acta. 1976 Jun 17;436(2):475–488. doi: 10.1016/0005-2736(76)90209-1. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Effects of cyanine dye membrane probes on cellular properties. Nature. 1978 Mar 2;272(5648):83–84. doi: 10.1038/272083a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Avron M. Measurement of transmembrane potentials in Rhodospirillum rubrum chromatophores with an oxacarbocyanine dye. Biochim Biophys Acta. 1976 Jul 9;440(1):189–204. doi: 10.1016/0005-2728(76)90123-7. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y. Respiration-department uncoupler-stimulated ATPase activity in castor bean endosperm mitochondria and submitochondrial particles. Biochim Biophys Acta. 1975 Mar 20;376(3):505–518. doi: 10.1016/0005-2728(75)90171-1. [DOI] [PubMed] [Google Scholar]
- Tedeschi H. Mitochondrial membrane potential: evidence from studies with a fluorescent probe. Proc Natl Acad Sci U S A. 1974 Feb;71(2):583–585. doi: 10.1073/pnas.71.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Wilson S. B., Moore A. L. The effects of protein synthesis inhibitors on oxidative phosphorylation by plant mitochondria. Biochim Biophys Acta. 1973 Apr 5;292(3):603–610. doi: 10.1016/0005-2728(73)90008-x. [DOI] [PubMed] [Google Scholar]


