Abstract
The human antibody immunoglobulin G1 (IgG1) b12 neutralizes a broad range of human immunodeficiency virus-type 1 (HIV-1) isolates in vitro and is able to protect against viral challenge in animal models. Neutralization of free virus, which is an antiviral activity of antibody that generally does not require the antibody Fc fragment, likely plays an important role in the protection observed. The role of Fc-mediated effector functions, which may reduce infection by inducing phagocytosis and lysis of virions and infected cells, however, is less clear. To investigate this role, we constructed a panel of IgG1 b12 mutants with point mutations in the second domain of the antibody heavy chain constant region (CH2). These mutations, as expected, did not affect gp120 binding or HIV-1 neutralization. IgG1 b12 mediated strong antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of HIV-1-infected cells, but these activities were reduced or abrogated for the antibody mutants. Two mutants were of particular interest. K322A showed a twofold reduction in FcγR binding affinity and ADCC, while C1q binding and CDC were abolished. A double mutant (L234A, L235A) did not bind either FcγR or C1q, and both ADCC and CDC functions were abolished. In this study, we confirmed that K322 forms part of the C1q binding site in human IgG1 and plays an important role in the molecular interactions leading to complement activation. Less expectedly, we demonstrate that the lower hinge region in human IgG1 has a strong modulating effect on C1q binding and CDC. The b12 mutants K322A and L234A, L235A are useful tools for dissecting the in vivo roles of ADCC and CDC in the anti-HIV-1 activity of neutralizing antibodies.
The broadly neutralizing antibody immunoglobulin G1 (IgG1) b12, directed to an epitope overlapping the CD4 binding site of gp120, was originally isolated from a human immunodeficiency virus type 1 (HIV-1)-infected individual by means of phage-display library cloning (11). This antibody neutralizes T-cell-line-adapted (TCLA) viruses and a broad range of primary viruses in various in vitro assays (13, 30, 41, 52). Several studies demonstrated that IgG1 b12 completely protects severe combined immunodeficiency (SCID) mice populated with human peripheral blood lymphocytes (hu-PBL-SCID mice) from infection with both TCLA and primary viruses (21, 41). The protection against primary viruses is apparent even if the antibody is given several hours after viral challenge (21). Furthermore, IgG1 b12 also protected against vaginal challenge with a pathogenic R5 SHIV (simian immunodeficiency virus [SIV]/HIV chimera expressing HIV-1 envelope) in rhesus macaques (43). In addition to IgG1 b12, a second broadly neutralizing monoclonal antibody (MAb) to gp120 (2G12 [53]) and three broadly neutralizing MAbs to gp41 (2F5, Z13, and 4E10 [9, 39, 60]) have been described. Recent studies demonstrated that 2G12 and 2F5, alone or in combination with one another, can protect against intravenous and/or mucosal SHIV challenge in macaques (3, 35, 36). Sterile protection typically requires that high antibody serum concentrations be achieved (e.g., in vitro neutralization titers of 1:100 or greater) (40, 42, 43), although some exceptions have been noted. In vaginal challenge studies with SHIV89.6PD in rhesus macaques, for example, MAb 2G12 protected at antibody serum concentrations close to the 90% virus neutralization titer (36).
The antiviral activity of antibodies can be mediated by the neutralization of free virions or by binding to virus-specific proteins expressed on the surface of infected cells and the recruitment of Fc-mediated effector function (40).The importance of Fc-mediated effector function in protection against HIV-1 infection, however, is unclear. In a recent study, Binley and colleagues infused serum immunoglobulins purified from SIVmac251-infected macaques (SIVIG) into other SIVmac251-infected macaques and measured the impact on plasma viremia of infected animals. The effects on viral load observed were very modest and transient, with kinetics which seemed inconsistent with the neutralization of free viruses as the mechanism driving the effect, and a role of Fc-mediated effector mechanisms was therefore suggested. An experiment using SIVIG F(ab′)2 fragments to address this hypothesis, however, was inconclusive (7).
Fc receptors expressed on human peripheral blood cells play an important role in stimulating a variety of cytotoxic, phagocytic, and inflammatory functions. Once a virus-infected cell is opsonized by IgG, it may cross-link FcγR on the cell surface of an effector cell and mediate a cytotoxic response (antibody-dependent cellular cytotoxicity [ADCC]). Arrays of antibody Fc's presented on the surface of an infected cell may also activate the classical pathway of complement activation ultimately leading to cell lysis (complement-dependent cytotoxicity [CDC]).
Multiple sites on IgG have been proposed to interact with FcγR. Mutagenesis studies have shown that the lower hinge region (234-LLGGPS-239) of IgG plays an important role in the binding of IgG Fc receptors (14, 19, 28, 33, 34, 38, 47, 58, 59). High-affinity binding to FcγRI is most notably affected by mutation of L235. Mouse (m)IgG2b, which does not bind FcγRI, has a glutamic acid at this position, and substitution of this residue by leucine was shown to restore the binding affinity of mIgG2b to be comparable to that of hIgG1 (19). The binding affinity of IgG for FcγRII, in contrast, seems more sensitive to mutations of L234 than L235, indicating that the FcγR interaction sites are overlapping but not identical (33). Residues in the lower part of the hinge region itself and the lower CH2 domain in addition may have a modulating effect on FcγR affinity (15, 33).
Complement activation via the classical pathway is activated through binding of C1q to the Fc domain of IgG or IgM, complexed with antigens (12, 25). Duncan and Winter (18) showed alanine substitutions in mIgG2b at positions E318, K320, K322, and N297, the last leading to the removal of carbohydrate, resulting in mutants in which binding to human C1q was strongly reduced compared to the wild type and the ability to mediate CDC was abrogated. Since E318, K320, and K322 are conserved residues in human IgG and IgG of several other species, they were designated the binding site for C1q (18). However, this binding motif has been conserved in all four human IgG subclasses which, in apparent conflict, exhibit large differences in their C1q binding abilities. Several studies have indeed implicated additional residues in C1q binding and have suggested that the binding sites for C1q on mIgG2b and hIgG are not completely identical (8, 27, 38, 51, 59). In a recent study on rituxan, a chimeric MAb with hIgG1 constant domains used in the therapy of non-Hodgkin's B-cell lymphomas, Idusogie and colleagues demonstrated that alanine substitution at positions D270, K322, P329, and P331 but not at positions E318 and K320 significantly reduced the ability of the chimeric MAb to bind C1q and activate complement, suggesting that E318 and K320 are only of minor importance for complement activation by hIgG1 (27). Some studies have furthermore suggested that C1q binding and complement activation may be modulated by residues in the lower hinge region (38). Thus, there are species differences in C1q binding. Finally, it has been shown that C1q binding alone is not sufficient for complement activation and complement-mediated cell lysis (51). Intrinsic factors, such as segmental flexibility of the hinge region (10, 50) but also extrinsic factors such as antigen specificity and density (5, 6), may play an important modulating role.
In the present study, we introduced point mutations in the heavy chain constant domain of IgG1 b12. Amino acid mutations were chosen on the basis of the studies discussed above. However, because of subtle species differences in C1q and FcγR binding and the possible modulation of ADCC and CDC by antigen specificity and density, the impact of these mutations on the biological activity of IgG against HIV-1-infected cells was not immediately clear. The mutants were compared for their ability to bind FcγR and C1q and to mediate ADCC and CDC. These mutants can now be used to examine the role of Fc-mediated effector function in protection against HIV-1 infection in vivo.
MATERIALS AND METHODS
Cell lines, viruses, and MAbs.
Uninfected CEM-NKr and CEM-NKr cells chronically infected with HIV-1MN were obtained from Shermaine Tilley (Public Health Research Institute, New York, N.Y.) (1). To obtain clones of the infected CEM-NKr cells with an increased level of envelope expression, we performed a limiting dilution and characterized for Env expression by flow cytometry. A clone with a higher level of Env expression (75% of cells expressing Env) was selected for our experiments. Adult human elutriated monocytes were obtained from Advanced Biotechnologies, Columbia, Md. HIV-1 primary isolates HIV-1JR-CSF, HIV-1JR-FL (contributed by Irvin Chen) (31), and HIV-189.6 (contributed by Ronald Collman) (16) were obtained from the National Institutes of Health (NIH) AIDS Research and Reagent Reference Program (ARRRP). IgG1-CLB was a purified paraprotein obtained from the CLB, Amsterdam, The Netherlands. Anti-FcγRI MAb 10.1 was provided by Nancy Hogg (Leukocyte Adhesion Laboratory, London, United Kingdom). Fab 10.1 was prepared by papain digestion. Fab fragments of anti-FcγRII MAb IV.3 and F(ab′)2 fragments of anti-FcγRIII MAb 3G8 were provided by Medarex (Anandale, N.J.). Humanized OKT3 (IgG1 and IgG4) antibody was provided by Robert A. Zivin (R.W. Johnson Pharmaceutical Research Institute, Raritan, N.J.).
Mutagenesis of heavy-chain constant domain.
Mutatagenesis was performed on the heavy-chain constant region derived from the IgG1 b12-expression plasmid pDR12 (see below) (13). A SacI-SalI endonuclease restriction fragment from pDR12, containing the CH2 fragment, was subcloned into M13 mp18. Site-directed mutagenesis (32) was performed using the Muta-gene M13 in vitro mutagenesis kit (Bio-Rad, Hercules, Calif.). Five clones encoding the desired changes (K322A, L234A, L235E, G237A, and L234A, L235A) were identified by automated DNA sequencing. The mutated SacI-SalI fragments were then cloned back into in the pDR12 expression vector.
Expression and purification of antibodies.
Recombinant antibody was expressed in the vector pDR12 (provided by Raju Koduri and Dean Sauer). It contains a b12 light chain and heavy-chain expression cassette in which transcription is driven from a human cytomegalovirus promoter. The heavy-chain expression cassette contains the genomic human IgG1 gene. Selection and amplification of the plasmid was done on the basis of expression of the gene for glutamine synthetase (4).
IgG1 b12 mutant DNAs, prepared as described above, were cut with SalI and transfected into Chinese hamster ovary cells (CHO-K1 cells; American Type Culture Collection, Manassas, Va.) using lipofectin reagent per the manufacturer's recommendations (Life Technologies, Grand Island, N.Y.). Cells were distributed in six-well tissue culture plates, and clones were selected with l-methionine sulfoximine ranging in concentration from 40 to 100 μM (Sigma, St Louis, Mo.). Wells containing discrete colonies were assayed by enzyme-linked immunosorbent assay (ELISA) for antibody production. The highest producers were cloned by limiting dilution, expanded, and grown in 3-liter spinner flasks.
Recombinant IgG1 was expressed in CHO-K1 cells in glutamine-free Glasgow minimum essential medium (GMEM supplemented with 10% dialyzed fetal bovine serum [FBS]) (Tissue Culture Biologicals, Tulare, Calif.), MEM nonessential amino acids (Gibco-BRL, Grand Island, N.Y.), 1 mM MEM sodium pyruvate (Gibco-BRL), 500 μM l-glutamic acid, 500 μM l-asparagine, 30 μM adenosine, 30 μM guanosine, 30 μM cytidine, 30 μM uridine, 10 μM thymidine (Sigma), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 μM l-methionine sulfoximine (Sigma) in a 3-liter spinner flask. The supernatants were sterile filtered and purified over protein A-Sepharose Fast Flow (Pharmacia, Arlington Heights, Ill.). The antibody was eluted in 0.1 M citric acid, pH 3.0. The pH of the antibody solution was immediately brought to neutrality by the addition of 1 M Tris (pH 9.0), and the antibody was dialyzed against phosphate-buffered saline (PBS). Antibody concentrations were determined by the absorbance at 280 nm and confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antibody yields using this method ranged from 5 to 25 mg/liter.
Recombinant gp120 ELISA.
IgG1 b12 and the Fc mutants were tested for binding to gp120 in ELISA essentially as described previously (17). Briefly, recombinant monomeric gp120JR-FL (provided by Paul Maddon and Bill Olson, Progenics, Tarrytown, N.Y.) was coated to the wells of a microtiter plate by incubating overnight at 4°C. The plates were washed four times with PBS containing 0.05% (vol/vol) Tween 20 and blocked with 3% bovine serum albumin (BSA). The blocking solution was removed, and serial dilutions of the antibody set were added in duplicate (diluted in PBS–1% BSA–0.05% Tween 20) and incubated for 1 h at 37°C. The wells were washed and incubated for 1 h at 37°C with alkaline phosphatase-labeled goat anti-human IgG F(ab′)2 fragments (Pierce, Rockford, Ill.) (1:500 dilution in PBS–1% BSA–0.05% Tween 20). The plates were washed and developed with nitrophenol substrate (Sigma), and the absorbance was read at 405 nm.
HIV-1 neutralization.
Neutralization of HIV-1 primary isolates was assessed using a phytohemagglutinin (PHA)-activated peripheral blood mononuclear cell (PBMC)-based assay as described previously (60). PBMCs (from three CCR5 wild-type donors) were isolated and stimulated with PHA (5 μg/ml) (Sigma) for 48 h, followed by PHA and interleukin-2 (40 U/ml) (obtained from the ARRRP, contributed by Hoffman-La Roche) for 72 h in RPMI 1640 medium containing 10% heat-inactivated FBS, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine. The antibodies were diluted, and 50 μl per well was pipetted into round-bottom microtiter plates, after which an equal volume containing 100 50% tissue culture infective doses of HIV-1 stock was added. The antibody-virus mixture was incubated for 1 h at 37°C. Next, 100 μl of PHA-activated PBMCs (5 × 105/ml) was added to each well. After an overnight incubation, the cells were washed two times with tissue culture medium. On day 7, the cultures were collected and treated with 1% (vol/vol) Empigen (Calbiochem, La Jolla, Calif.). Triplicate samples were then tested for p24 content using an ELISA, as originally described by Moore et al. (37). In brief, sheep anti-p24 Ab D7320 (Aalo Bioreagents) was coated overnight on 96-well polystyrene enzyme immunoassay (EIA) plates (Costar) in 100 mM NaHCO3, pH 8.5. The plates were washed in PBS, and p24 was captured from serial dilutions of the HIV-1-containing samples in PBS–0.1% Empigen. After a 3-h incubation, unbound p24 was washed away and bound p24 was detected with alkaline phosphatase-labeled antibody BC1071 (International Enzymes) diluted 1:3,000 in PBS containing 20% sheep serum and 2% nonfat dry milk. After a 1-h incubation, the plates were washed and developed with an AMPAK kit (Dako Diagnostics) as recommended by the manufacturer. Production of p24 antigen in the antibody-containing cultures was compared to p24 production in cultures without antibody run in the same assay, and the antibody concentrations resulting in a 90% reduction in p24 content were determined.
FcγR binding assays.
FcγR binding assays were performed essentially as described by Parren et al. (45). Binding of antibody to FcγR was assessed using antibody monomers or dimers as indicated. Antibody dimers were prepared by incubating overnight with F(ab′)2 fragments of mouse anti-human κ-light chain MAb K35 in a molar ratio of 1:1, which resulted in stable tetrameric complexes as detailed by Huizinga et al. (26). FcγR-transfected cells (3 × 105 in 100 μl of PBS–1% BSA) were incubated with 25 μl of serial dilutions of antibody monomers or dimers for 45 min at 4°C, washed, and then incubated with fluorescein isothiocyanate-labeled F(ab′)2 fragments of goat anti-human IgG. After washing, cell-bound antibody was detected using flow cytometry. The assays were performed using IIA1.6 cells transfected with FcγRIA and γ-chain, or FcγRIIa (H131), and Jurkat cells transfected with FcγRIIIa (24, 45, 54, 55).
C1q ELISA.
Antibody was diluted to 1.25 μg/ml in PBS and coated overnight at room temperature onto EIA ELISA plates (Costar, Corning, N.Y.). Plates were washed three times with PBS–0.05% Tween 20 and a titration of human C1q (Calbiochem) prepared in PTG (PBS–0.02% Tween–0.1% gelatin) was added. After a 4-h incubation at room temperature, the plates were washed four times with PBS–0.05% Tween 20. A mixture of goat anti-human C1q (Calbiochem) and rabbit anti-goat IgG alkaline phosphatase conjugate (Sigma), both diluted at 1/1,000 in PTG, was added, and the plates were incubated for 1 h at room temperature. The plates were washed four times and developed using nitrophenol substrate (Sigma). Absorbance was measured at 405 nm. All data are expressed as means of triplicates.
ADCC with TCLA HIV-1.
ADCC was assessed in standard chromium release assays (56). Effector cells were either PBMCs or adult human elutriated monocytes, as noted in the text. The PBMCs were isolated by centrifugation over Histopaque-1077 (Sigma). Cells were washed in PBS and resuspended in RPMI 1640 containing 10% FCS, 2 mM l-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml) at a density of approximately 4 × 106 cells/ml and incubated overnight at 37°C prior to use as effector cells in ADCC. The following day, 106 target cells were labeled with Na251CrO4 (Amersham, Arlington Heights, Ill.) for approximately 2 h in 50 μl of fetal calf serum (FCS) containing 10 mM HEPES. These target cells were either uninfected CEM-NKr cells or CEM-NKr cells chronically infected with HIV-1MN. After labeling, target cells were washed four times with RPMI 1640–10 mM HEPES. In each well of a microtiter plate, 106 washed 51Cr-labeled target cells were incubated with antibody for 30 min at 37°C in a total volume of 150 μl. Then, 106 PBMCs or 6 × 105 human elutriated monocytes (monocytes were thawed rapidly at 37°C and washed once with RPMI 1640 before addition to target cells) were added as a source of effector cells in 50 μl of assay medium, bringing the total volume to 200 μl. The plates were spun at 1,000 rpm for 5 min in a Beckman GS-6R centrifuge to pellet the cells prior to a 4-h incubation at 37°C. At the end of this incubation period, plates were spun another time as described above, 100 μl of supernatant was collected, and 51Cr release was measured in a gamma counter (Packard, Meriden, Conn.). The percent specific lysis was calculated as follows: (experimental release − spontaneous release)/(total release − spontaneous release) × 100%. Total 51Cr release was determined by substituting 50 μl of antibody for Empigen detergent (Calbiochem). All data are expressed as the means of triplicate determinations.
CDC assay.
Target cells (CEM-NKr cells; uninfected or infected with HIV-1MN) were labeled by Na251CrO4 as described above. After three washes, the labeled cells were sensitized by adding wild-type or mutant IgG1 b12 to the cells at a final concentration of 10 μg/ml and were incubated at 4°C for 1 h. After three washes, 2 × 105 sensitized target cells were dispensed into 96-well U-bottom microtiter plates. Rabbit serum (Calbiochem) was used as a source of complement and was serially diluted with RPMI 1640. The latter was added to sensitized target cells at 100 μl/well, and the plates were incubated at 37°C for 1 h. At the end of this time, plates were spun and 100 μl of supernatant was used to measure the 51Cr release as described above.
RESULTS
Antigen recognition and neutralization.
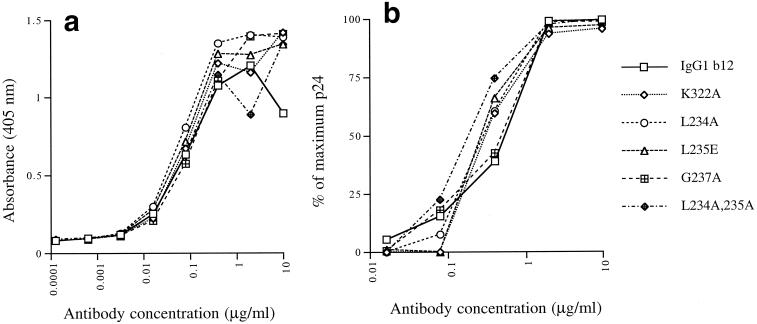
Five IgG1 b12 mutants were constructed by introducing point mutations in the lower hinge (L234A, L235E, G237A, and double mutant L234A, L235A) and the N-terminal end of the CH2 domain (K322A). Although it was unlikely that these mutations would influence the affinity of the antibody for gp120, all five antibodies were tested for binding to recombinant HIV-1JRFL gp120 in ELISA. All mutants bound similarly to gp120 as expected (Fig. 1a). In addition, we tested all mutants in a PHA-activated PBMC-based neutralization assays with HIV-1JR-FL (Fig. 1b), HIV-1JR-CSF, and HIV-189.6 (Table 1). All mutants neutralized these primary isolates similarly (Fig. 1b; Table 1).
FIG. 1.
Binding of wild-type and mutant IgG1 b12 to recombinant HIV-1JR-FL gp120 in ELISA (a) and neutralization of HIV-1JR-FL (b).
TABLE 1.
Neutralization of HIV-1 primary isolates by wild-type and mutant IgG1 b12
| Antibody | 90% Inhibitory concn (μg/ml)
|
||
|---|---|---|---|
| HIV-1JR-FL | HIV-1JR-CSF | HIV-189.6 | |
| IgG1 b12 | 2 | 50 | 6.3 |
| K322A | 2 | 50 | 6.3 |
| L234A | 2 | 50 | 6.3 |
| G237A | 2 | 25 | 12.5 |
| L235E | 2 | 50 | 3.1 |
| L234A, L235A | 2 | 50 | 6.3 |
Binding of wild-type and mutant IgG1 b12 to FcγRI, FcγRIIa, and FcγRIIIa.
The ability of antibodies to mediate ADCC is dependent on the relative affinity of the antibody for FcγRI, -II, and -III. We first measured the ability of IgG1 b12 and the mutant antibodies described above to bind to FcγRI by flow cytometry using both monomeric and dimeric IgG1 (cross-linked with an anti-light chain MAb) (26). Binding of IgG by the high-affinity receptor FcγRI is usually studied using monomeric IgG. We, however, also included dimeric IgG which allowed us to also examine possible lower-affinity interactions. In these binding studies, we used FcγRIa- and γ-chain-transfected IIA1.6 cells (54). Binding of both dimeric and monomeric wild-type and mutant IgG1 b12 to IIA1.6 cells revealed that the mutation at position 234 reduced the affinity for FcγRI about fivefold, whereas the other two mutations in the lower hinge (L235E and G237A) reduced IgG1 b12 affinity for FcγRI about 40-fold. Combining the 234 and 235 mutations completely abolished FcγRI binding, and binding was reduced to undetectable levels even in the IgG dimer-binding assay. The FcγRI binding affinity of K322A was not affected. The rank order of FcγRI-binding by the IgG1 b12 variants therefore is as follows: b12 = K322A > L234A ≫ G237A, L235E, double-mutant L234A, L235A (Table 2).
TABLE 2.
Binding of IgG1 b12 and IgG1 b12 Fc mutants to FcγR
| Antibodies | K50 (μg/ml)a
|
|||
|---|---|---|---|---|
| FcγRI (monomer binding) | FcγRI (dimer binding) | FcγRIIa | FcγRIIIa | |
| b12 | 0.16 | 0.3 | 4.8 | 7 |
| K322A | 0.17 | 0.4 | 8.3 | 13 |
| L234A | 1.1 | 1.68 | >50 | >50 |
| G237A | 6 | 7.6 | >50 | >50 |
| L235E | 7 | 12 | >50 | >50 |
| L234A, L235A | >50 | >50 | >50 | >50 |
| IgG1-CLB | 0.12 | 0.2 | 1.8 | 10 |
Data are expressed as the antibody concentration at which half maximal binding was achieved, K50 of >50 μg/ml indicate that this was not achieved.
Next, we tested the ability of our antibody panel to bind to the low-affinity receptors FcγRIIa and FcγRIIIa. The mutation in the CH2 domain (K322A) only slightly reduced the binding affinity to both FcγRIIa and FcγRIIIa compared to wild-type IgG1 b12 (Table 2). All mutations in the lower hinge region in contrast abolished binding to both FcγRIIa and FcγRIIIa (Table 2).
Therefore, the importance of L235 for FcγRI binding was confirmed, but binding could only be completely abrogated by introducing a double mutation at L234 and L235. In contrast to previous studies on hIgG3 (34), mutagenesis of L234 as well as L235 in hIgG1 abolished both FcγRII and FcγRIII binding.
ADCC.
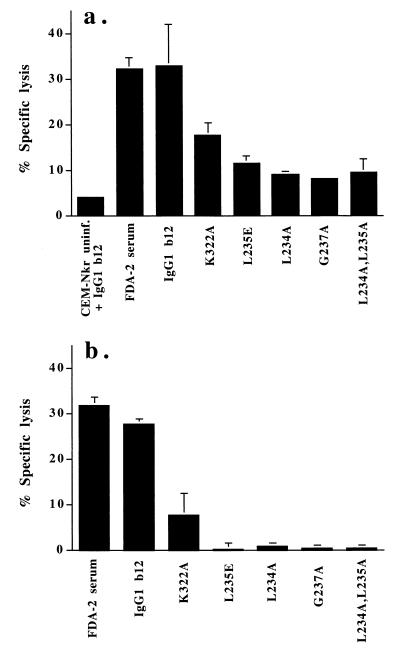
Antibody (IgG1 b12) bound to envelope expressed on the surface of HIV-1-infected cells may recruit effector cells, such as NK cells or monocytes, by interacting with specific Fc receptors and induce ADCC leading to lysis of the infected cells. In order to measure the ability of IgG1 b12 and the mutants described above to mediate ADCC of HIV-1-infected cells, we incubated serial dilutions of these antibodies with 51Cr-labeled HIVMN-infected CEM-Nkr cells in the presence of PBMCs and purified monocytes as effector cells. As shown in Fig. 2a and b, IgG1 b12 mediated specific cell lysis of HIVMN-infected CEM-NKr cells in the presence of both human PBMCs and purified monocytes, whereas ADCC by mutant K322A was reduced. The ability of IgG1 b12 mutants L235E, L234A, G237A, and double mutant L234A, L235A to mediate ADCC of HIV-1MN-infected CEM-NKr cells was strongly reduced with PBMCs and abolished with monocytes as effector cells (Fig. 2a and b). The positive control serum, FDA-2, is a potently neutralizing serum with a 90% HIV-1MN neutralization titer of 1:4,000 (44); ADCC of IgG1 b12 on noninfected CEM-Nkr cells was used as a negative control.
FIG. 2.
ADCC of CEM-Nkr cells infected with HIV-1MN by PBMC and purified human monocytes. Uninfected and HIV-1MN-infected CEM-NKr cells were labeled with 51Cr for 2 h at 37°C. The labeled target cells were incubated with wild-type or mutant IgG1 b12 before addition of cultured PBMCs (a) or purified human monocytes (b) as effector cells. Wild-type and mutant IgG1 b12 were used at 12.5 μg/ml. Serum from an HIV-1-seropositive patient (FDA-2 [44]) was used at a 1/4,000 dilution as a positive control. Uninfected CEM-NKr cells incubated with wild-type IgG1 b12 were included as a negative control. The assays were performed twice with similar results.
To verify the studies with the IgG1 mutant antibodies, we examined the IgG1 b12-mediated ADCC of HIVMN-infected CEM-NKr with PBMCs and monocytes in the presence and absence of anti-FcγR antibodies. F(ab′)2 fragments of an anti-FcγRIII antibody, 3G8, efficiently inhibited the PBMC-mediated ADCC of HIVMN-infected CEM-NKr (Fig. 3a). No inhibition of ADCC was observed with Fab fragments of anti-FcγRI 10.1 or anti-FcγRII IV.3. Thus, ADCC of HIV-infected cells by PBMCs is mediated through FcγRIIIa. This is in agreement with the strong reduction of ADCC in mutants in which binding to FcγRIIIa was abolished (Fig. 2a). In addition, the ability of mutant K322E to mediate ADCC was reduced about twofold compared to IgG1 b12, which corresponds with its reduction of binding to FcγRIIIa (Fig. 2a; Table 2).
FIG. 3.
Inhibition of ADCC by anti-FcγR antibodies. ADCC of HIV-1MN-infected CEM-NKr cells by PBMC (a) or purified human monocytes (b) in the presence and absence of F(ab′)2 fragments of anti-FcγRIII MAb 3G8 (20 μg/ml), Fab fragments of anti-FcγRII MAb IV.3 (5 μg/ml), and Fab fragments of anti-FcγRI MAb 10.1 (20 μg/ml). IgG1 b12 was used at a concentration of 12.5 μg/ml. Serum from an HIV-1-seropositive patient (FDA-2 [44]) was used at a 1/4,000 dilution as a positive control. The assays were performed twice with similar results.
As shown in Fig. 3b, anti-FcγRI (Fab 10.1) but not anti-FcγRII (Fab IV.3) or anti-FcγRIII [F(ab′)23G8], inhibited monocyte-mediated ADCC of HIV-1MN-infected CEM-Nkr-MN cells. These results suggest that ADCC of HIV-infected cells by monocytes is mediated through FcγRI. A relatively small (fivefold) reduction in FcγRI binding affinity as observed for the mutant L234A is therefore sufficient to abrogate the monocyte-mediated ADCC of HIV-1-infected cells (Fig. 2b).
C1q binding and complement activation.
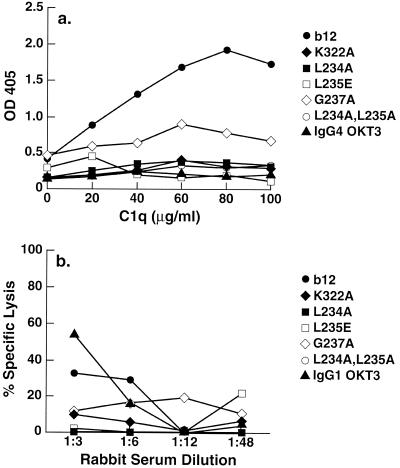
The ability of b12 and the mutant antibodies to bind C1q and CDC of HIV-1-infected cells was investigated. As shown in Fig. 4a and b, wild-type IgG1b12 bound well to C1q and mediated a potent CDC of HIV-1MN-infected CEM-Nkr cells in the presence of rabbit serum as a source for complement. The ability of the K322A mutant to bind C1q and mediate CDC was strongly reduced. All b12 variants with mutations in the lower hinge region (L235E, L234A, G237A and L234A, L235A) furthermore were reduced in their ability to mediate CDC of HIV-1-infected cells as well (Fig. 4a and b).
FIG. 4.
Binding of wild-type and mutant IgG1 b12 to C1q (a) and complement-mediated lysis of HIV-1MN-infected CEM-Nkr-MN cells by IgG1 b12 and IgG1 b12 mutants (b). Isotype variants of anti-CD3 MAb OKT3 (IgG1 and IgG4) were used as controls.
These results strongly suggest that for hIgG1, the residue at position 322 at the N-terminal end of the CH2 domain as well as residues in the lower hinge region are involved in C1q binding and consequent complement activation.
DISCUSSION
The role of effector function in the anti-HIV-1 activity of antibody is poorly understood. It has been shown in a number of passive antibody transfer studies that MAbs or polyclonal antisera capable of neutralizing the challenge virus can protect against HIV-1 infection (2, 3, 20–22, 35, 36, 40, 41, 46). Significantly, passive immunization studies using broadly neutralizing antibodies, including b12, have recently been used to protect rhesus macaques from intravenous and mucosal challenge with pathogenic primary HIV-1 isolate-derived SHIVs (35, 36, 43).
Neutralizing antibody may limit the dissemination of an enveloped virus, such as HIV-1, by at least two separate mechanisms. First, the antibody may interact with free virus and neutralize it by interfering with the attachment or fusion of its target cells, thereby protecting the cells from infection. Second, the neutralizing antibody may bind to envelope expressed on the surface of infected cells and induce cell lysis by the recruitment of effector functions, including ADCC and CDC (reviewed by Parren and Burton [40]). The relative roles of neutralization and Fc-mediated effects in HIV-1 infection have not yet been directly determined. In a recent study, Binley and colleagues (7) suggested that a transient effect on viral load by the infusion of anti-SIV antibodies was likely due to killing of SIV-infected cells, although this conclusion could only be drawn by inference.
We set out to directly study the role of Fc-mediated effector function in HIV-1 infection by the preparation of a panel of Fc mutants of the broadly HIV-1-neutralizing MAb b12. The FcγR binding assays demonstrated that single mutations at residues 234 and 235 strongly reduced the binding to FcγRI and completely abolished binding to FcγRIIa and FcγRIIIa. Our results are in agreement with previous studies showing the importance of the lower hinge region of IgG1 in FcγRI, FcγRII, and FcγRIII binding (38, 48, 57, 59). We also showed that double mutation of amino acids 234 and 235 completely abolished binding to all FcγRs.
We examined the nature of the FcγRs involved in the ADCC of HIV-infected cells by using anti-FcγR antibodies. Our data indicate that ADCC by PBMCs is inhibited only in the presence of anti-FcγRIII antibody, while ADCC by monocytes is abolished in the presence of anti-FcγRI antibody. Alsmadi and Tilley (1) proposed previously that ADCC of HIV-infected cells is exclusively mediated through FcγRIII expressed on the surface of CD56+ NK cells. In their study, they measured ADCC activity using cultured PBMCs as effector cells which are likely reduced in monocyte content due to the adherence of these cells to plastic. Similarly, we found that PBMC-dependent ADCC was primarily mediated through FcγRIII, even though monocytes represented up to 8% of total cells in our PBMC preparations, as measured by fluorescence-activated cell sorting (FACS) using a CD14 marker (data not shown). The effective ADCC by purified monocytes in vitro in our study, however, indicates that it is likely that monocytes or macrophages expressing FcγRI contribute to the ADCC of HIV-1-infected cells in vivo. Therefore, we suggest that ADCC of HIV-infected cells in vivo may be mediated by both FcγRI and FcγRIIIa.
Human IgG1 has the ability to bind C1q and lyse cells by activating complement through the classical pathway. The binding site for C1q on murine IgG2b was mapped to residues 318, 320, and 322 (18). We measured the ability of b12 and the mutants described to bind to C1q and to activate complement-mediated cell lysis. We used heterologous rather than homologous complement, as this resulted in more efficient lysis of HIV-1-infected (human) cells (data not shown). Changing lysine 322 to alanine completely abolished binding to C1q and complement activation, which confirms a previous report (27). Less expected was the reduction of C1q binding and complement activation by mutations in the lower hinge. In an earlier study, Morgan and colleagues had reported on the reduction of C1q binding and complement activation by changes in residues 235 and 237 for a chimeric mouse/human antibody (38). We show that in a fully human IgG1, L235, G237, and, in addition, L234 in the lower hinge region appear to play a significant direct or indirect role in C1q binding.
A concern may be that the null phenotype of the L234A, L235A double mutation for FcγR as well as C1q binding is a result of more-drastic rearrangements of the IgG Fc structure of this mutant. However, in a recent study, we have shown that the binding of the L234A, L235A double mutant to FcRn was only slightly reduced (<25%) compared to wild-type b12 (57). Protein A and G binding furthermore were unaffected (not shown). Proteins A, G, and FcRn all bind to the CH2-CH3 interface. The retention of FcRn binding, in particular, is significant as this receptor has two important functions, namely the cross-placental transport of maternal IgG to the fetus and the protection of IgG from normal serum protein catabolism (23, 29, 49). The serum half-life of the L234A, L235A mutant should therefore not be significantly affected.
In summary, we demonstrated that manipulation of residues 234, 235, and 237 in the lower hinge region of hIgG1 modulates binding to FcγRs. Our data also indicated that, in hIgG1, both the lower hinge and N-terminal end of the CH2 domain are involved in C1q binding and complement lysis. Furthermore, using both PBMCs and purified monocytes as effector cells, we found that ADCC of HIV-infected cells is mediated through both FcγRI and FcγRIIIa. As shown in our results, mutant K322A was only slightly, up to twofold, reduced in its ability to mediate ADCC and did not mediate CDC. On the other hand, the double mutant L234A, L235A mediated neither CDC nor ADCC. Both mutants were unchanged in their ability to neutralize primary HIV-1 isolates. Therefore, these mutants display specific valuable features that can be used in future in vivo studies. Our goal will be to use these mutants in in vivo passive antibody transfer-SHIV challenge studies in rhesus macaques to elucidate the relative roles of neutralization and of ADCC and complement activation in protection against HIV-1 infection and pathogenesis.
ACKNOWLEDGMENTS
We are grateful to Dennis Burton for his support and many valuable discussions. We thank Rowena Aguilar-Sino, Dawn Slifka, and Nomdo Westerdaal for technical assistance. We acknowledge the assistance of the General Clinical Research Center of TSRI (M01 RR00833).
This work was supported by NIH grant number AI40377.
REFERENCES
- 1.Alsmadi O, Tilley S A. Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope and chimpanzee monoclonal antibodies of different epitope specificities. J Virol. 1998;72:286–293. doi: 10.1128/jvi.72.1.286-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrus L, Prince A M, Bernal I, McCormack P, Lee D H, Gorny M K, Zolla-Pazner S. Passive immunization with human immunodeficiency virus type 1-neutralizing monoclonal antibody in hu-PBL-SCID mice: isolation of a neutralization escape variant. J Infect Dis. 1998;177:889–897. doi: 10.1086/515251. [DOI] [PubMed] [Google Scholar]
- 3.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 4.Bebbington C R, Renner G, Thomson S, King D, Abrams D, Yarranton G T. High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Bio/Technology. 1992;10:169–175. doi: 10.1038/nbt0292-169. [DOI] [PubMed] [Google Scholar]
- 5.Bindon C I, Hale G, Waldmann H. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988;18:1507–1514. doi: 10.1002/eji.1830181006. [DOI] [PubMed] [Google Scholar]
- 6.Bindon C I, Hale G, Waldmann H. Complement activation by immunoglobulin does not depend solely on C1q binding. Eur J Immunol. 1990;20:277–281. doi: 10.1002/eji.1830200208. [DOI] [PubMed] [Google Scholar]
- 7.Binley J M, Clas B, Gettie A, Vesanen M, Montefiori D C, Sawyer L, Booth J, Lewis M, Marx P A, Bonhoeffer S, Moore J P. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology. 2000;270:237–249. doi: 10.1006/viro.2000.0254. [DOI] [PubMed] [Google Scholar]
- 8.Brekke O H, Michaelsen T E, Aase A, Sandin R H, Sandlie I. Human IgG isotype-specific amino acid residues affecting complement-mediated cell lysis and phagocytosis. Eur J Immunol. 1994;24:2542–2547. doi: 10.1002/eji.1830241042. [DOI] [PubMed] [Google Scholar]
- 9.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauder A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 10.Burton D R. Immunoglobulin G: functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 11.Burton D R, Barbas C F, I. I I, Persson M A A, Koenig S, Chanock R M, Lerner R A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton D R, Boyd J, Brampton A D, Easterbrook-Smith S B, Emanuel E J, Novotny J, Rademacher T W, van Schravendijk M R, Sternberg M J E, Dwek R A. The C1q receptor site on immunoglobulin G. Nature. 1980;288:338–344. doi: 10.1038/288338a0. [DOI] [PubMed] [Google Scholar]
- 13.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 14.Burton D R, Woof J M. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 15.Canfield S M, Morrison S L. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991;173:1483–1493. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodefiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ditzel H J, Parren P W H I, Binley J M, Sodroski J, Moore J P, Barbas C F, Burton D R. Mapping the protein surface of human immunodeficiency virus type 1 gp120 using human monoclonal antibodies from phage display libraries. J Mol Biol. 1997;267:684–695. doi: 10.1006/jmbi.1997.0912. [DOI] [PubMed] [Google Scholar]
- 18.Duncan A R, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 19.Duncan A R, Woof J M, Partridge L J, Burton D R, Winter G. Localisation of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988;332:563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- 20.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 21.Gauduin M C, Parren P W H I, Weir R, Barbas III C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 22.Gauduin M C, Safrit J T, Weir R, Fung M S, Koup R A. Pre- and post-exposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J Infect Dis. 1995;171:1203–1209. doi: 10.1093/infdis/171.5.1203. [DOI] [PubMed] [Google Scholar]
- 23.Ghetie V, Hubbard J G, Kim J K, Tsen M F, Lee Y, Ward E S. Abnormally short serum half-lives of IgG in β2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 24.Heijnen I A, van de Winkel J G J. Human IgG Fc receptors. Int Rev Immunol. 1997;16:29–55. doi: 10.3109/08830189709045702. [DOI] [PubMed] [Google Scholar]
- 25.Hughes-Jones N C, Gardner B. Reaction between the isolated globular sub-units of the complement component C1q and IgG-complexes. Mol Immunol. 1979;16:697–701. doi: 10.1016/0161-5890(79)90010-5. [DOI] [PubMed] [Google Scholar]
- 26.Huizinga T W J, Kerst M, Nuijens J H, Vlug A A E, von dem Borne K, Roos D, Tetteroo P A T. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J Immunol. 1989;142:2359–2364. [PubMed] [Google Scholar]
- 27.Idusogie E E, Presta L G, Gazzano-Santoro H, Totpal K, Wong P Y, Ultsch M, Meng Y G, Mulkerrin M G. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 28.Jefferis R, Lund J, Pound J. Molecular definition of interaction sites on human IgG for Fc receptors (huFcγR) Mol Immunol. 1990;27:1237–1240. doi: 10.1016/0161-5890(90)90027-w. [DOI] [PubMed] [Google Scholar]
- 29.Junghans R P, Anderson C L. The protection receptor for IgG catabolism is the beta 2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler J A, McKenna P M, Emini E A, Chan C P, Patel M D, Gupta S K, Mark III G E, Barbas C F, Burton D R, Conley A J. Recombinant human monoclonal antibody IgG1 b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retrovir. 1997;13:575–581. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 31.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel T A, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 33.Lund J, Pound D, Jones P T, Duncan A R, Bentley T, Goodall M, Levine B A, Jefferis R, Winter G. Multiple binding sites on the CH2 domain of IgG for mouse FcγRII. Mol Immunol. 1992;29:53–59. doi: 10.1016/0161-5890(92)90156-r. [DOI] [PubMed] [Google Scholar]
- 34.Lund J, Winter G, Jones P T, Pound J D, Tanaka T, Walker M R, Artymiuk P J, Arata Y, Burton D R, Jefferis R, Woof J M. Human FcγRI and FcγRII interact with distinct but overlapping sites on human IgG. J Immunol. 1991;147:2657–2662. [PubMed] [Google Scholar]
- 35.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic SHIV-89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 37.Moore J P, McKeating J A, Weiss R A, Sattentau Q J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 38.Morgan A, Jones N D, Nesbitt A M, Chaplin L, Bodmer N W, Emtage J S. The N-terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, FcγRI and FcγRIII binding. Immunology. 1995;86:319–324. [PMC free article] [PubMed] [Google Scholar]
- 39.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parren P W H I, Burton D R. The anti-viral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parren P W H I, Ditzel H J, Gulizia R J, Binley J M, Barbas C F, III, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Parren P W H I, Gauduin M C, Koup R A, Poignard P, Sattentau Q J, Fisicaro P, Burton D R. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;58:125–132. doi: 10.1016/s0165-2478(97)00109-0. [DOI] [PubMed] [Google Scholar]
- 43.Parren P W H I, Marx P A, Hessell A J, Luckay A, Harouse J, Cheng-Mayer C, Moore J P, Burton D R. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parren P W H I, Wang M, Trkola A, Binley J M, Purtscher M, Katinger H, Moore J P, Burton D R. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type-1. J Virol. 1998;72:10270–10274. doi: 10.1128/jvi.72.12.10270-10274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parren P W H I, Warmerdam P A, Boeije L C, Arts J, Westerdaal N A, Vlug A, Capel P J, Aarden L A, van de Winkel J G. On the interaction of IgG subclasses with the low affinity FcγRIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Investig. 1992;90:1537–1546. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince A M, Reesink H, Pascual D, Horowitz B, Hewlett I, Murthy K K, Cobb K E, Eichberg J W. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res Hum Retrovir. 1991;7:971–973. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 47.Sarmay G, Lund J, Rozsnayay Z, Gergeley J, Jefferis R. Mapping and comparison of the interaction sites on the Fc region of IgG responsible for triggering antibody dependent cellular cytotoxicity (ADCC) through different types of human Fcγ receptor. Mol Immunol. 1992;29:633–639. doi: 10.1016/0161-5890(92)90200-h. [DOI] [PubMed] [Google Scholar]
- 48.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-Å crystal structure of the human IgG1 Fc fragment-FcγRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 49.Story C M, Mikulska J E, Simister N E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan L K, Shopes R J, Oi V T, Morrison S L. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci USA. 1990;87:162–166. doi: 10.1073/pnas.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao M-H, Smith R I F, Morrison S L. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178:661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trkola A, Pomales A P, Yuan H, Korber B, Maddon P J, Allaway G, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type I. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Vugt M J, Heijnen A F, Capel P J, Park S Y, Ra C, Saito T, Verbeek J S, van de Winkel J G J. FcR gamma-chain is essential for both surface expression and function of human FcγRI (CD64) in vivo. Blood. 1996;87:3593–3599. [PubMed] [Google Scholar]
- 55.Warmerdam P A M, van de Winkel J G J, Vlug A, Westerdaal N A, Capel P J. A single amino acid change in the second Ig-like domain of the human Fcγ receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–1443. [PubMed] [Google Scholar]
- 56.Weinhold K J, Tyler D S, Lyerly H K. Measurement of direct and indirect forms of anti-HIV ADCC: implications for other retroviral disease. Dev Biol Stand. 1990;72:343–348. [PubMed] [Google Scholar]
- 57.Wines B D, Powell M S, Parren P W H I, Barnes N, Hogarth P M. The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors FcγRI and FcγRIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol. 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 58.Woof J M, Partridge L J, Jefferis R, Burton D R. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986;23:319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- 59.Xu D, Alergre M-L, Varga S S, Rothermel A L, Collins A M, Pulito V L, Hanna L S, Dolan K P, Parren P W H I, Bluestone J A, Joliffe L K, Zivin R A. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 60.Zwick M B, Labrijn A F, Wang M, Spenlehauer C, Ollmann Saphire E, Binley J M, Moore J P, Stiegler G, Katinger H, Burton D R, Parren P W H I. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]