Abstract
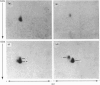
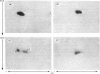
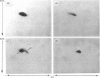
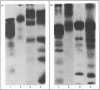
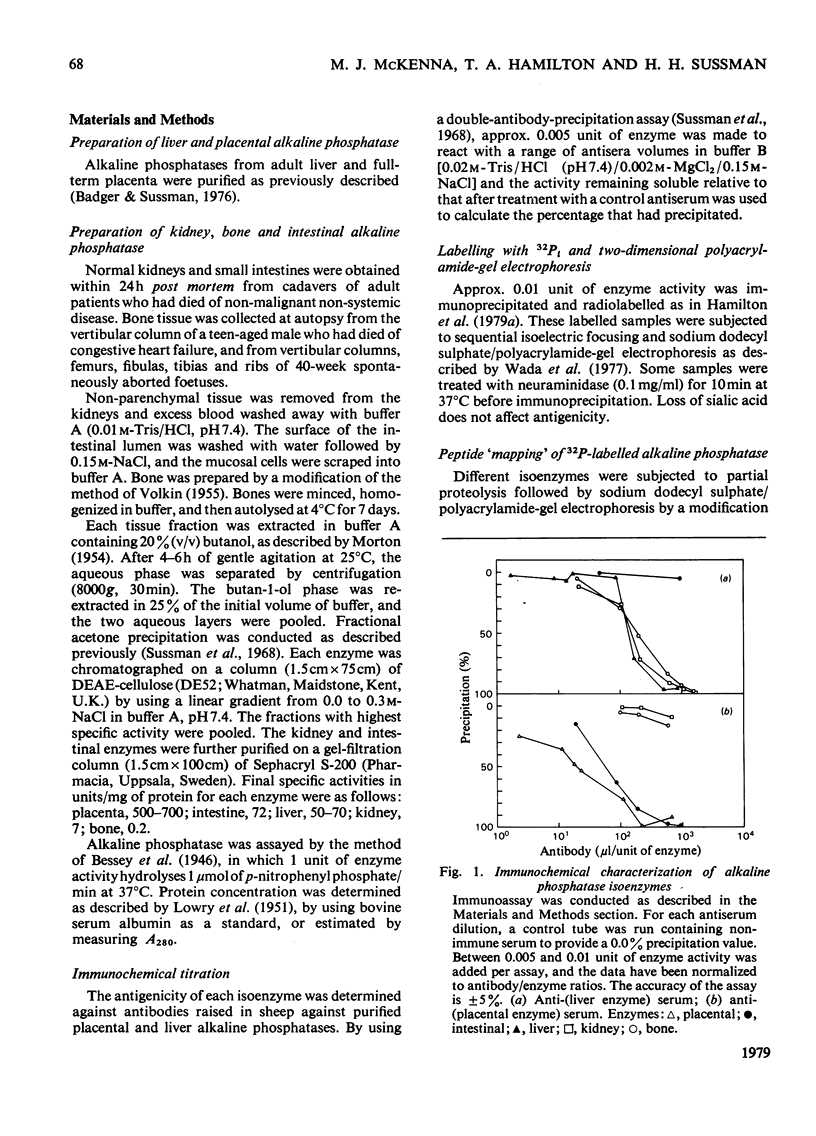
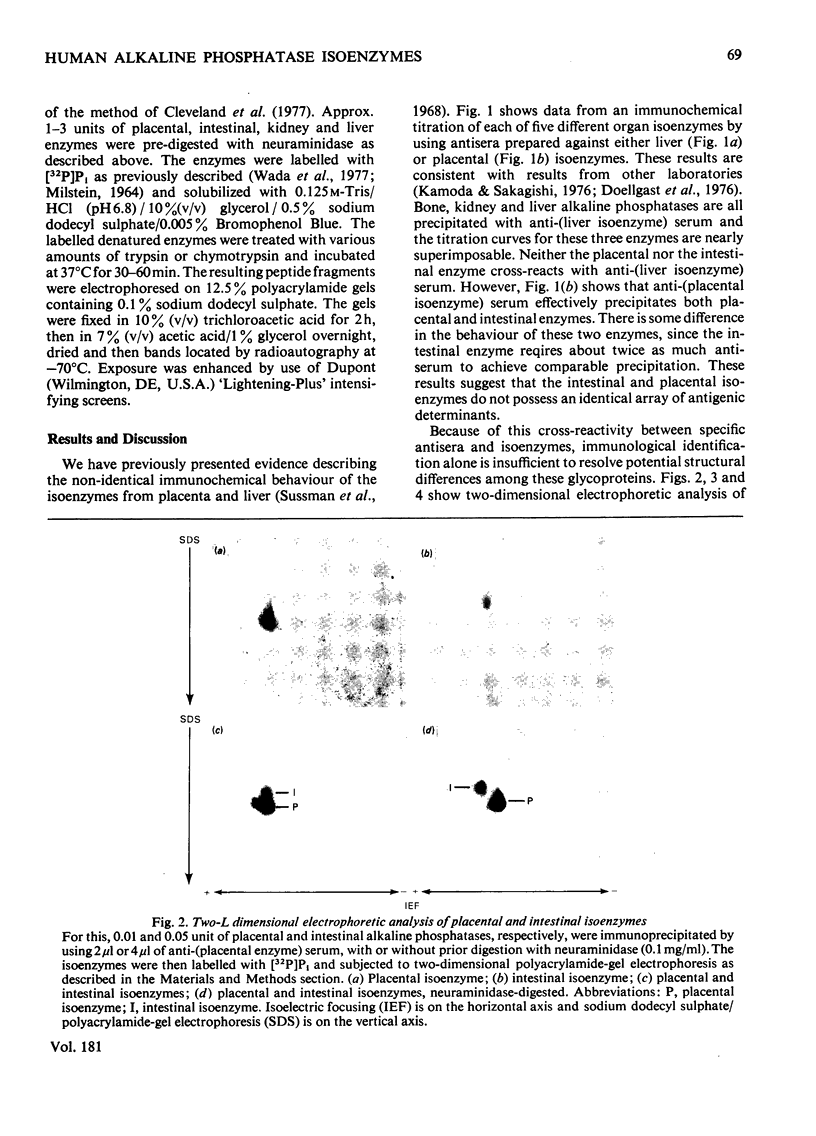
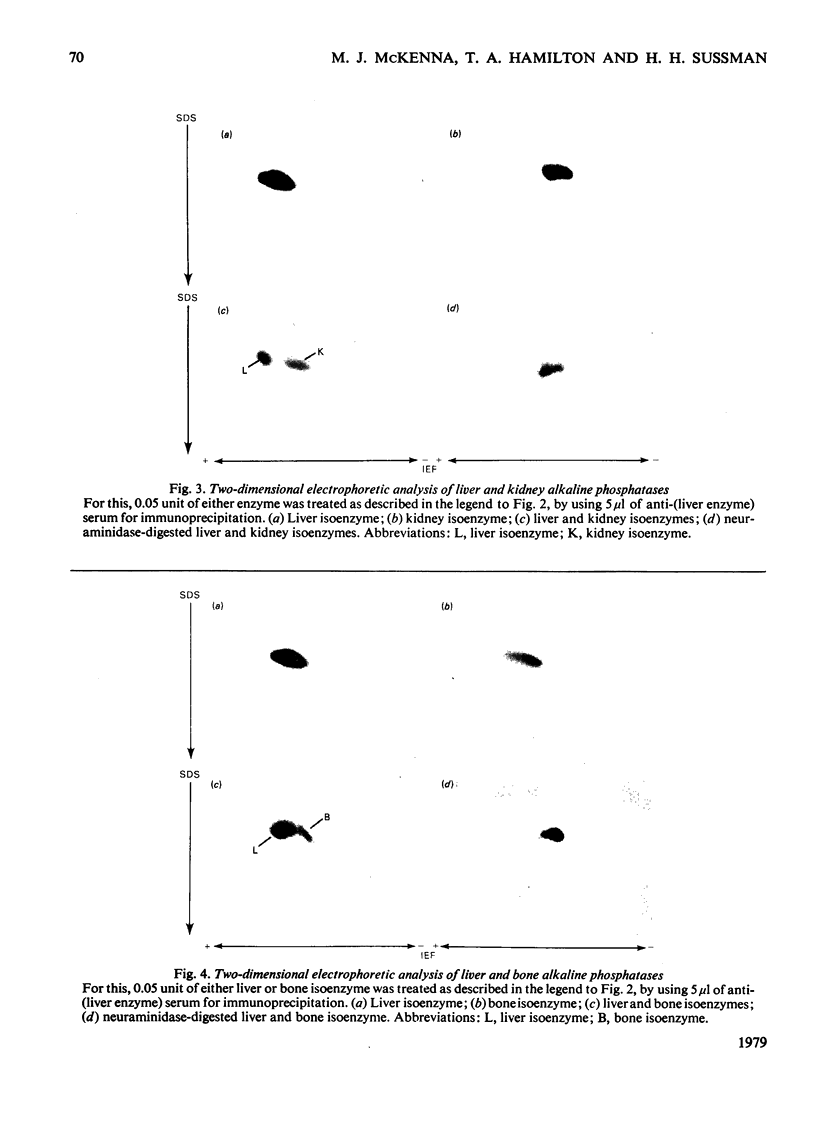
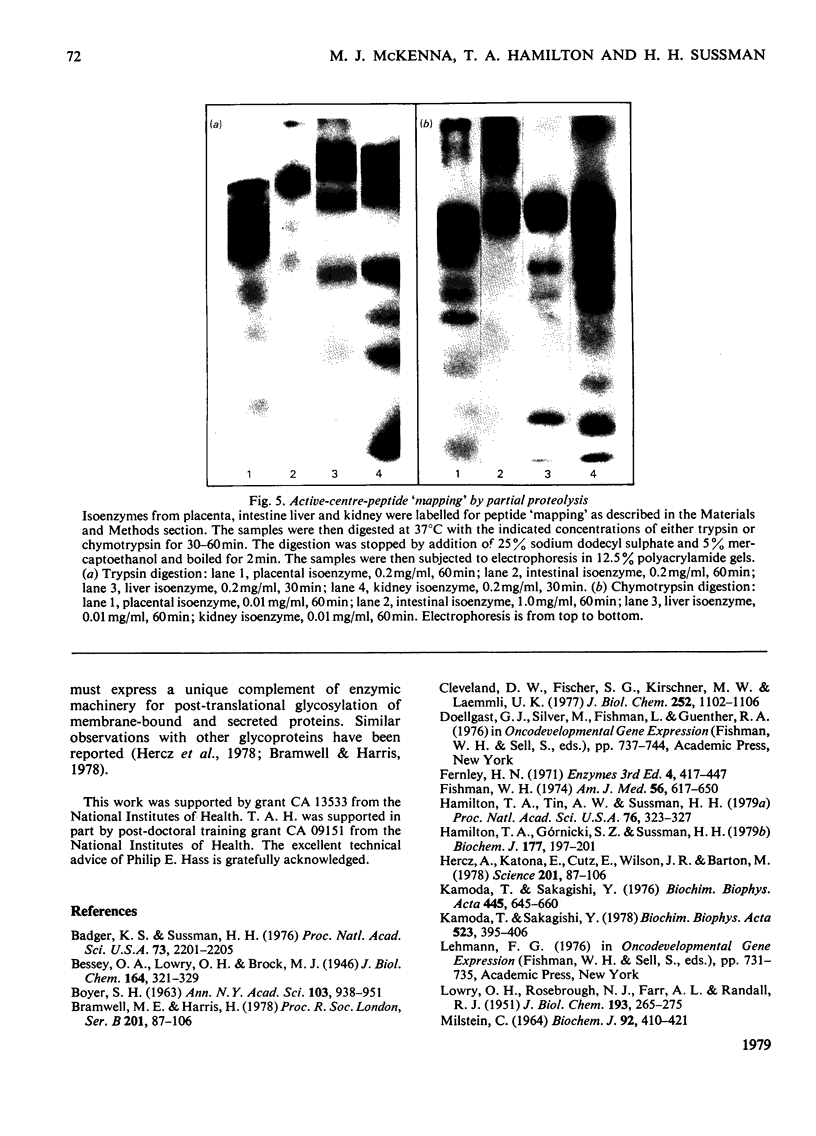
The structural relationships among human alkaline phosphatase isoenzymes from placenta, bone, kidney, liver and intestine were investigated by using three criteria. 1. Immunochemical characterization by using monospecific antisera prepared against either the placental isoenzyme or the liver isoenzyme distinguishes two antigenic groups: bone, kidney and liver isoenzymes cross-react with anti-(liver isoenzyme) serum, and the intestinal and placental isoenzymes cross-react with the anti-(placental isoenzyme) antiserum. 2. High-resolution two-dimensional electrophoresis of the 32P-labelled denatured subunits of each enzyme distinguishes three groups of alkaline phosphatase: (a) the liver, bone and kidney isoenzymes, each with a unique isoelectric point in the native form, can be converted into a single form by treatment with neuraminidase; (b) the placental isoenzyme, whose position also shifts after removal of sialic acid; and (c) the intestinal isoenzyme, which is distinct from all other phosphatases and is unaffected by neuraminidase digestion. 3. Finally, we compare the primary structure of each enzyme by partial proteolytic-peptide 'mapping' in dodecyl sulphate/polyacrylamide gels. These results confirm the primary structural identity of liver and kidney isoenzymes and the non-identity of the placental and intestinal forms. These data provide direct experimental support for the existence of at least three alkaline phosphatase genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER S. H. Human organ alkaline phosphatases: discrimination by several means including starch gel electrophoresis of antienzyme-enzyme supernatant fluids. Ann N Y Acad Sci. 1963 May 8;103:938–951. doi: 10.1111/j.1749-6632.1963.tb53746.x. [DOI] [PubMed] [Google Scholar]
- Badger K. S., Sussman H. H. Structural evidence that human liver and placental alkaline phosphatase isoenzymes are coded by different genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2201–2205. doi: 10.1073/pnas.73.7.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. An abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Fishman W. H. Perspectives on alkaline phosphatase isoenzymes. Am J Med. 1974 May;56(5):617–650. doi: 10.1016/0002-9343(74)90631-7. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Górnicki S. Z., Sussman H. H. Alkaline phosphates from human milk. Comparison with isoenzymes from placenta and liver. Biochem J. 1979 Jan 1;177(1):197–201. doi: 10.1042/bj1770197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Tin A. W., Sussman H. H. Regulation of alkaline phosphatase expression in human choriocarcinoma cell lines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):323–327. doi: 10.1073/pnas.76.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoda T., Sakagishi Y. Partial purification of human intestinal alkaline phosphatase with affinity chromotography. Some properties and interaction of concanavalin A with alkaline phosphatase. Biochim Biophys Acta. 1976 Oct 11;445(3):645–660. doi: 10.1016/0005-2744(76)90117-0. [DOI] [PubMed] [Google Scholar]
- Komoda T., Sakagishi Y. The function of carbohydrate moiety and alteration of carbohydrate composition in human alkaline phosphatase isoenzymes. Biochim Biophys Acta. 1978 Apr 12;523(2):395–406. doi: 10.1016/0005-2744(78)90042-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTON R. K. The purification of aklaline phosphatases of animal tissues. Biochem J. 1954 Aug;57(4):595–603. doi: 10.1042/bj0570595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. W., Shakespeare M. J., Thomas D. M. Observations on the heat-stability of alkaline phosphatase isoenzymes in serum. Clin Chim Acta. 1972 Aug;40(1):35–41. doi: 10.1016/0009-8981(72)90248-3. [DOI] [PubMed] [Google Scholar]
- Mulivor R. A., Hannig V. L., Harris H. Developmental change in human intestinal alkaline phosphatase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3909–3912. doi: 10.1073/pnas.75.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Mennuti M., Zackai E. H., Harris H. Prenatal diagnosis of hypophosphatasia; genetic, biochemical, and clinical studies. Am J Hum Genet. 1978 May;30(3):271–282. [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- Wada H. G., Górnicki Z., Sussman H. H. The sialoglycoprotein subunits of human placental brush border membranes characterized by two-two-dimensional electrophoresis. J Supramol Struct. 1977;6(4):473–484. doi: 10.1002/jss.400060402. [DOI] [PubMed] [Google Scholar]