Abstract
Single-cell analyses in humans and mice have revealed the identity of the erythropoietin producing cells of the kidney — opening up new avenues of research for anemia and related disorders.
More than 100 years ago, Carnot and Deflandre found that administration of serum from anemic rabbits led to increased red blood cell numbers in non-anemic rabbits, concluding that a humoral regulator of red blood cell production (erythropoiesis) must exist1. Decades later, Eugene Goldwasser recognized the crucial role of the kidney in the production of erythropoietin (EPO), the ‘master regulator’ of erythropoiesis. In 1977, Goldwasser and colleagues were the first to successfully isolate human EPO2. The gene was cloned just six years later, and human recombinant EPO treatment was approved in 1989 for the treatment of anemia. To this day, EPO continues to be used worldwide to treat millions of patients with anemia. However, recombinant EPO is not without limitations, including potential side effects and challenging administration.
Despite remarkable advances, the specific cells responsible for EPO production in the human kidney and the precise mechanisms regulating this process have remained elusive. In this issue of Nature Medicine, Kragesteen et al.3 use single-cell gene expression analysis (scRNA-seq), single-cell analysis of open chromatin (scATAC-seq) and mouse models to identify the EPO-expressing cells and to characterize the mechanism of EPO expression in mouse and human kidneys3. They discover that EPO-producing cells are a subtype of stromal cells that they named ‘Norn’ cells, inspired by Norse mythology — where Norn represents any of the three goddesses responsible for determining the destinies of gods and mortals.
In healthy people without hypoxia, EPO levels are very low, at around 10 mU ml−1. Tissue hypoxia is the most important regulator of EPO. During hypoxic stress, EPO levels can increase by 1,000-fold. The hormone binds to its receptor expressed on red blood cell precursors in the bone marrow, where it stimulates red blood cell production. Erythrocytes transport oxygen to the kidney and provide negative feedback in this regulatory loop. This beautiful example of evolutionarily conserved inter-organ communication is disrupted in millions of patients suffering from chronic kidney disease (CKD)4 and other disease states — resulting in anemia. To help maintain normal erythrocyte levels, EPO or various EPO-analogue erythropoiesis-stimulating agents (ESAs) are commonly used in patients with kidney disease. However, the initiation of ESA therapy with recombinant EPO must be carefully considered because of the risk of severe side effects (such as hypertension) and a higher risk of thrombotic events (such as stroke and myocardial infarction), as well as tumor progression or recurrence in patients with cancer5.
In their study, Kragesteen et al.3 showed that Norn cells express a highly specific gene signature (Fig. 1). The authors performed single-cell multi-omics analysis (combined scRNA-seq and scATAC-seq) in mouse kidneys to identify the motifs and transcription factors that regulate Epo expression in Norn cells. They discovered five open chromatin regions upstream and downstream of the Epo gene. The Norn Epo enhancer elements showed enrichment for TCF21 and C/EBP transcription factor binding sites. The team also identified 5,844 unique open chromatin regions, many of which regulate nearby genes expressed specifically in Norn cells — such as Epo, Prrx1, Il33 and Cxcl14. Finally, the authors identified more than 30 transcription factor motifs that were significantly enriched in Norn-specific open chromatin regions and determined that TCF21, C/EBP and GATA6 motifs were the most specific for Norn cells. More importantly, they discovered that the basic-helix-loop-helix (bHLH) family of transcription factor binding sites was overrepresented in Norn cells. The hypoxia-response element (HRE) motif was enriched in Norn-specific regions of the bHLH-e subfamily, albeit at a low frequency.
Fig. 1 |. The identification of erythropoietin-producing ‘Norn’ cells.
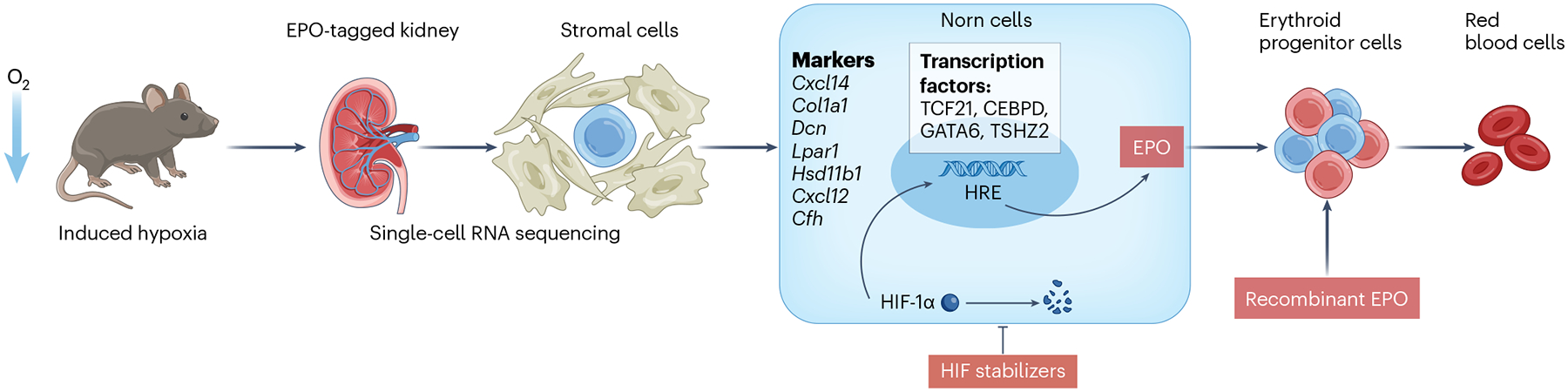
Hypoxia is sensed by hypoxia-inducible factor (HIF), which is the primary regulator of erythropoietin (EPO) production. EPO acts on erythroid progenitors to stimulate red blood cell production. Using an Epo-tagged kidney mouse model (in which EPO expression is linked to fluorescent protein) under hypoxic conditions and single-cell RNA sequencing, the research team identified a subpopulation of stromal cells, which they termed ‘Norn cells’, as the primary source of EPO in the kidney. Norn cells express Cxcl14, Col1a1, Dcn, Lpar1, Hsd11b1, Cxcl12 and Cfh and are enriched for a variety of transcription factors including TCF21, CEBPD, GATA6 and TSHZ2. Understanding these cells may reveal new therapeutic targets for combating anemia.
After they had created a gene signature from mouse Norn cells, Kragesteen et al.3 identified these cells in published scRNA-seq datasets of developing mouse and human kidneys. Using mRNA in situ hybridization staining on human kidney sections from carbon monoxide poisoning victims (which would be hypoxic), they confirmed that EPO expression in the hypoxic kidney was highly associated with Norn cell markers. Therefore, they concluded that Norn cells are the primary EPO-producing cells in the kidney and are conserved from mice to humans. We also searched for Norn cells and their gene signature in a recently available human kidney single-cell gene expression dataset and were able to identify Norn cells and their characteristic gene expression signature6.
Besides the role of hypoxia, previously little has been known about Epo regulation. The work of Kragesteen et al.3 is fundamental as it identified the full regulatory region of Epo and the entire transcriptional machinery of Norn cells. Furthermore, although the mouse model used in this study has previously been used to identify EPO-producing cells7, these cells had not been identified and fully characterized in the human kidney in their native state, which has now been enabled by the application of single-cell technology to investigate the full molecular and genomic contexts.
The identification of Norn cells could have substantial clinical implications. Recently, hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI), also known as HIF stabilizers, have been approved as a new means to treat anemia, beyond recombinant EPO. These inhibitors prevent the degradation of HIF, an essential transcription factor that facilitates EPO expression (Fig. 1). One advantage of HIF stabilizers is that they can be administered orally, unlike conventional ESAs that require injection8. The discovery of Norn cells, a specific subset of renal interstitial cells responsible for EPO production, bridges the knowledge gap about physiological EPO regulation. These findings could lead to novel therapeutic targets beyond HIF and improved therapeutic approaches to maintaining physiological EPO levels — not only in individuals with kidney disease but also in other forms of anemia.
Now, further research is required to identify the mechanisms by which specific interstitial Norn cells become disrupted in kidney disease and to identify strategies for preserving their function. The current study focused on analyzing hypoxic mice and therefore may not have captured the full range of mechanisms regulating EPO production in vivo. Furthermore, the study focused on the transcriptional and epigenetic landscape of Norn cells and EPO production; but protein expression, stability and post-translational modifications, which have key roles in hypoxia sensing, should be tested in future studies.
In summary, the study from Kragesteen et al.3 represents a major advance in our understanding of how the body senses tissue hypoxia and regulates red blood cell production. Ultimately, these advancements could lead to promising new interventions to combat renal and other forms of anemia in patients.
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- 1.Jelkmann W Respir. Physiol 63, 257–266 (1986). [DOI] [PubMed] [Google Scholar]
- 2.Wojchowski D Nature 470, 40–40 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Kragesteen AG et al. Nat. Med 10.1038/s41591-023-02314-7 (2023). [DOI] [Google Scholar]
- 4.Semenza GL Annu. Rev. Med 74, 307–319 (2023). [DOI] [PubMed] [Google Scholar]
- 5.KDIGO. Kidney Int Suppl. 2, 283–287 (2012). [Google Scholar]
- 6.Abedini A et al. Preprint at bioRxiv 10.1101/2022.10.24.513598 (2022). [DOI] [Google Scholar]
- 7.Imeri F et al. Kidney Int. 95, 375–387 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Singh AK et al. N. Engl. J. Med 385, 2325–2335 (2021). [DOI] [PubMed] [Google Scholar]


