Abstract
The induction of apoptosis in host cells is a prominent cytopathic effect of vesicular stomatitis virus (VSV) infection. The viral matrix (M) protein is responsible for several important cytopathic effects, including the inhibition of host gene expression and the induction of cell rounding in VSV-infected cells. This raises the question of whether M protein is also involved in the induction of apoptosis. HeLa or BHK cells were transfected with M mRNA to determine whether M protein induces apoptosis when expressed in the absence of other viral components. Expression of M protein induced apoptotic morphological changes and activated caspase-3 in both cell types, indicating that M protein induces apoptosis in the absence of other viral components. An M protein containing a point mutation that renders it defective in the inhibition of host gene expression (M51R mutation) activated little, if any, caspase-3, while a deletion mutant lacking amino acids 4 to 21 that is defective in the virus assembly function but fully functional in the inhibition of host gene expression was as effective as wild-type (wt) M protein in activating caspase-3. To determine whether M protein influences the induction of apoptosis in the context of a virus infection, the M51R M protein mutation was incorporated onto a wt background by using a recombinant infectious cDNA clone (rM51R-M virus). The timing of the induction of apoptosis by rM51R-M virus was compared to that by the corresponding recombinant wt (rwt) virus and to that by tsO82 virus, the mutant virus in which the M51R mutation was originally identified. In HeLa cells, rwt virus induced apoptosis faster than did rM51R-M virus, demonstrating a role for M protein in the induction of apoptosis. In contrast to the results obtained with HeLa cells, rwt virus induced apoptosis more slowly than did rM51R-M virus in BHK cells. This indicates that a viral component other than M protein contributes to induction of apoptosis in BHK cells and that wt M protein acts to delay induction of apoptosis by the other viral component. tsO82 virus induced apoptosis more rapidly than did rM51R-M virus in both HeLa and BHK cells. These two viruses contain the same point mutation in their M proteins, suggesting that sequence differences in genes other than that for M protein affect their rates of induction of apoptosis.
The induction of apoptosis in host cells is an important aspect of viral pathogenesis in many virus systems (33). One of the key questions to be addressed is which viral components induce apoptosis in virus-infected cells. Vesicular stomatitis virus (VSV), the prototype rhabdovirus, was an early example of a virus that was shown to induce the morphological changes and DNA fragmentation associated with apoptosis (20). Many of the cytopathic effects of VSV infection have been attributed to the activity of the viral matrix (M) protein. For example, M protein plays a major role in the inhibition of host gene expression (5, 6, 12, 30) and in the induction of cell rounding (7, 26) that are characteristic of VSV-infected cells. M protein is a structural component of the virion and performs several important functions in virus assembly. However, the functions of M protein in virus assembly are genetically separable from its role in cytopathogenesis (6, 26). The involvement of M protein in the cytopathic effects of VSV infection raises the question of whether M protein is also involved in the induction of apoptosis.
M protein affects several important cellular processes, whose inhibition could contribute to the induction of apoptosis, both in VSV-infected cells and when expressed in transfected cells in the absence of other viral components. For example, M protein inhibits transcription by all three host RNA polymerases (1). In the case of host RNA polymerase II, the target of the inhibition was identified as transcription factor TFIID (38, 39). M protein also blocks nucleocytoplasmic transport of RNAs and proteins that are dependent on Ran guanosine triphosphatase (17). Recently, M protein has been shown to interact with one or more nuclear pore components, including the nucleoporin Nup98 (31, 36). This interaction appears to account for M protein-induced inhibition of nucleocytoplasmic transport. M protein has also been shown to cause cell rounding in the absence of other viral components and is the only viral component that causes cell rounding at early times postinfection (7). Cell rounding induced by M protein involves disruption of all three types of cytoskeletal elements, including actin, vimentin, and tubulin (26). M protein interacts with tubulin in vitro and tubulin coimmunoprecipitates with M protein from VSV-infected cells, suggesting that an in vivo interaction between tubulin and M protein may contribute to cell rounding (29).
The goal of the experiments presented here was to determine whether M protein is involved in the induction of apoptosis by VSV. This was addressed by expressing M protein in the absence of other viral components and by using mutant M proteins that are either defective in the inhibition of host gene expression or in viral assembly functions (6). Our results demonstrate that M protein induces apoptosis when expressed in the absence of other viral components. The induction of apoptosis by M protein was genetically correlated with its ability to inhibit host gene expression and not with its virus assembly functions. The influence of M protein on the induction of apoptosis in the context of a virus infection was tested by using viruses containing a mutant M protein that was defective in the ability to inhibit host gene expression and in the ability to induce apoptosis. In HeLa cells, VSV M protein mutants induced apoptosis more slowly than did the wild-type (wt) strains from which they were derived. This indicates that M protein is an important inducer of apoptosis in VSV-infected HeLa cells. However, in BHK cells, the VSV M protein mutants induced apoptosis more rapidly than did wt VSV. This indicates that a viral component other than M protein contributes to the induction of apoptosis by VSV in BHK cells and that wt M protein acts to delay induction of apoptosis by the other viral component. Thus, both M protein and another viral component contribute to the induction of apoptosis in cells infected with VSV.
MATERIALS AND METHODS
Cells and viruses.
BHK cells and HeLa cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). wt VSV (Indiana serotype, Orsay strain) and the tsO82 virus were grown in BHK cells as previously described, except that tsO82 virus was grown at 31°C (27). The infectious VSV cDNA clone previously described (37) was modified to contain three additional restriction sites to facilitate cloning of M gene mutations. The new sites were introduced into an XbaI-KpnI fragment containing the M gene by PCR mutagenesis as previously described (34). The new sites (underlined) and the surrounding sequences (positive sense) were SpeI (5′CTGTAGACTAGTACGTACTATGAAAAAAA3′), AflII (5′GAGTTCCTTAAGGAAGATTCTC3′), and AscI (5′TCTCCTAATTGGCGCGCCTCTCGAACAAC3′). Recombinant virus was isolated from the modified cDNA clone as previously described and was designated rwt virus (21, 37). The M51R mutation was introduced into the M gene of the modified cDNA clone as previously described (6), and the virus isolated from this cDNA was designated rM51R-M virus. Stocks were prepared from recombinant viruses that were plaque isolated twice, and the sequences of their M genes were confirmed by automated DNA sequencing of reverse transcription-PCR products prepared as previously described (6). For individual experiments, infections were performed with a multiplicity of infection (MOI) of 20 PFU/cell in DMEM containing 2% FBS.
Fluorescence microscopy.
Plasmids encoding M protein and enhanced green fluorescent protein (EGFP) were used for in vitro transcription of mRNA as previously described by using a commercial kit (Message Machine; Ambion, Inc.) (4). The resulting mRNAs contained 5′ caps and 3′ poly(A) sequences that enhance expression in transfected cells. BHK cells were grown in 35-mm-diameter dishes to about 50% confluency and cotransfected with 1 μg of M mRNA and 1 μg of EGPF mRNA or transfected with 1 μg of EGPF mRNA alone by using Lipofectin reagent as previously described (4). At 24 h posttransfection, the media were replaced with HEPES-buffered saline (HBS). The cells were analyzed by fluorescence microscopy using a 25× water immersion objective. The fluorescein channel was used to detect the presence of EGFP to indicate transfected cells.
Caspase-3 activity assay.
HeLa or BHK cells were grown in 24-well plates to about 50% confluency and were either transfected with M mRNAs or infected with wt and M mutant viruses. For Fig. 2, cells were transfected with 300 ng of yeast RNA (control), 300 ng of EGFP mRNA (control), 30 ng of wt M mRNA, 30 ng of MN1 M mRNA, or 300 ng of M51R-M mRNA for 24 h. The total amount of RNA transfected was held constant at 300 ng in all samples with the addition of yeast RNA. For Fig. 3, HeLa or BHK cells were transfected with the amount of M mRNA indicated and for the times indicated with the total amount of RNA transfected held constant at 300 ng in all samples with the addition of yeast RNA. The cells were lysed, and caspase-3 activity was determined by using a fluorogenic substrate (DVED-AFC; R&D Systems, Inc.) in accordance with the protocol supplied by the manufacturer. Each sample was incubated for 2 h with the peptide substrate for caspase-3, and the reaction was stopped by the addition of 900 μl of 10 mM Tris–10 mM NaCl, pH 8.1. Fluorescence intensities were measured at excitation and emission wavelengths of 400 and 490 nm, respectively, and compared to standards of 7-amino-4-trifluoromethyl-coumarin at known concentrations.
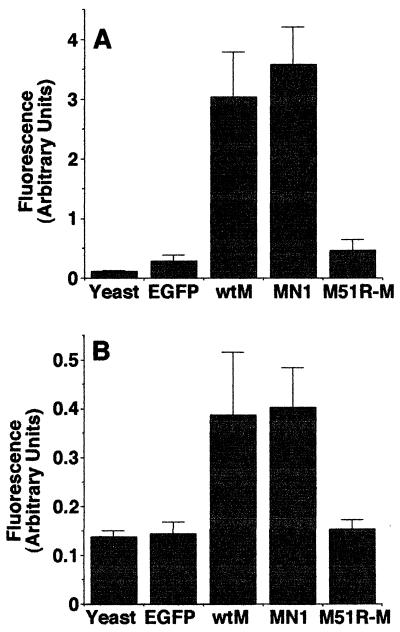
FIG. 2.
Caspase-3 activity in cells expressing wt and mutant M proteins. HeLa (A) or BHK (B) cells were transfected with 300 ng of yeast RNA (control), 300 ng of EGFP mRNA (control), 30 ng of wt M mRNA, 30 ng of MN1 M mRNA, or 300 ng of M51R-M mRNA. Transfection with these amounts of mRNA has been shown to result in comparable levels of expression of the wt and mutant M proteins (26). At 24 h posttransfection, the cells were lysed and caspase-3 activity was measured with a fluorogenic substrate. The amount of caspase-3 activated is expressed in arbitrary fluorescence units. The data represent the average ± the standard deviation of four experiments.
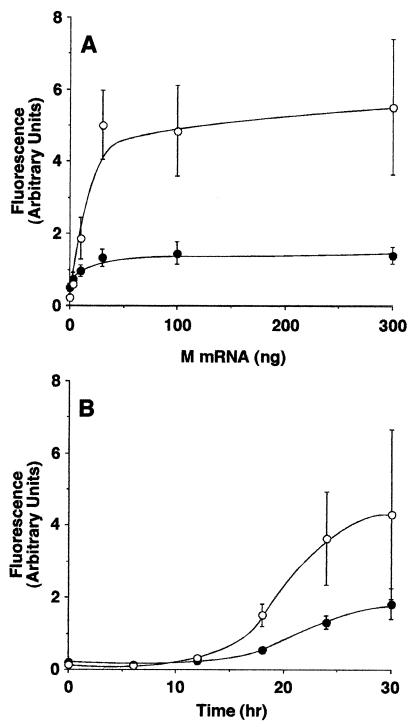
FIG. 3.
M protein expression induces activation of caspase-3. (A) HeLa cells (open circles) or BHK cells (closed circles) were transfected with the indicated amounts of M mRNA for 24 h. The cells were analyzed for caspase-3 activity as described in the legend to Fig. 2. The amount of caspase-3 activated is expressed in arbitrary fluorescence units. (B) BHK cells (closed circles) or HeLa cells (open circles) were transfected with 30 ng of M mRNA for the indicated times. Cells were lysed and assayed for caspase-3 activity. The data represent the average ± the standard deviation of three experiments.
Time lapse microscopy.
BHK and HeLa cells were grown to about 50% confluency in 25-cm flasks and infected at an MOI of 20 PFU/cell. Each flask was placed on a rocker for 30 min at room temperature and then placed on the stage of a Zeiss inverted Axiovert phase-contrast time lapse microscopy system equipped with an incubator containing an atmosphere of 5% CO2 and a temperature of 37°C as previously described (8). The progression of the cells was followed with phase-contrast time lapse microscopy using a Dage MTI-100 video camera affixed to the microscope at a time lapse ratio of 600:1. The time when each of 50 to 130 cells entered apoptosis was determined from a time-date generator record on the videotape. Images for Fig. 3 were digitized and captured from the videotape record.
Fluorescence time lapse microscopy was performed to determine the fate of cells expressing M protein and EGFP. BHK or HeLa cells were transfected with M and EGPF mRNAs or EGPF mRNA alone as described above. Five hours posttransfection, when sufficient EGFP was being expressed to be viewed by fluorescence microscopy, the dish was placed in the microscope incubator. A fluorescence image was recorded to determine which cells were transfected. The progression of the cells was then followed with phase-contrast time lapse microscopy. Additional fluorescence images were taken at 24 h posttransfection.
Cell membrane permeability assay.
BHK or HeLa cells grown in six-well dishes to about 60% confluency were infected at an MOI of 20 PFU/cell. At the time points indicated in Fig. 5, the cells were washed with HBS and 4 μmol of ethidium homodimer-1 (Molecular Probes, Inc.) was added to each sample for 15 min to label cells with permeable cell membranes. The cells were washed three times with HBS and harvested by lifting with trypsin and then adding DMEM containing 10% FBS. Each sample was filtered and analyzed by flow cytometry using a Becton-Dickinson FACStar Plus flow cytometer. The percentage of intact cells that had permeable cell membranes was determined by using CellQuest software (Becton-Dickinson, Inc.). It was noted that VSV-infected cells underwent a slight increase in permeability to ethidium homodimer-1 at early times postinfection, when the cells were still alive. Dead cells were clearly resolved as a separate population with much higher fluorescence intensity. The flow cytometry analysis was gated by forward and side scatter so that only intact cells were analyzed. In order to account for the cells that disintegrated and could not be analyzed by flow cytometry, intact cells were counted in a Coulter counter. For each time point, uninfected cells were lifted and fixed with 4% formaldehyde at the same time that another cell sample was infected, so that the original number of infected cells could be determined. Infected cells corresponding to each time point were fixed at the end of the experiment. All of the cells were counted, and the number of cells that disintegrated was determined by subtracting the number of intact infected cells from the number of uninfected cells at each time point. The total percentage of dead cells was calculated by adding the percentage of intact cells labeled with ethidium homodimer-1 using flow cytometry to the percentage of cells that disintegrated as measured by the Coulter counter.
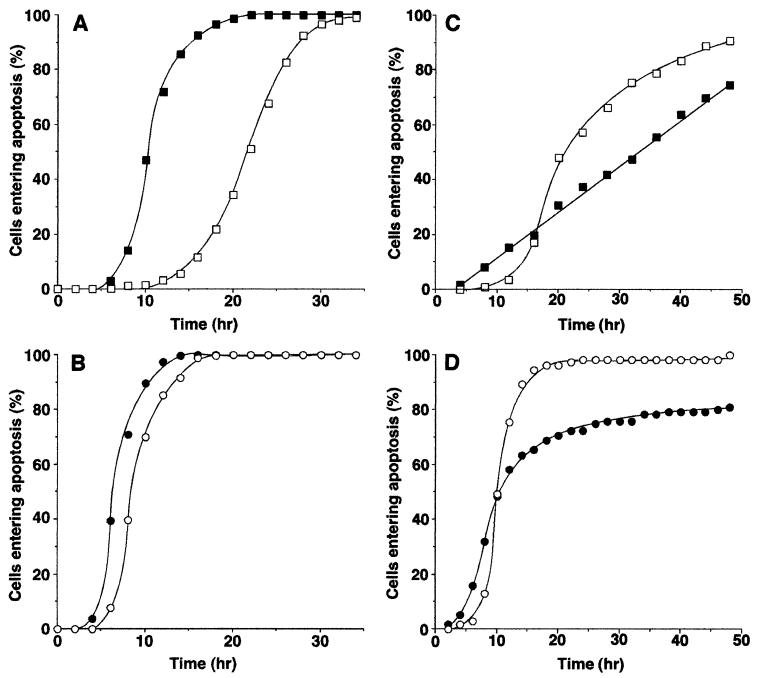
FIG. 5.
Induction of apoptosis in cells infected with wt VSV and VSV M gene mutants. HeLa cells (A and B) or BHK cells (C and D) were infected with rwt virus (closed squares), rM51R-M virus (open squares), wtO virus (closed circles), or tsO82 virus (open circles) and analyzed by time lapse microscopy as described in the legend to Fig. 4. The time at which each of 50 to 130 cells entered apoptosis was determined from the time-date record on the videotape. Data shown are the cumulative percentage of cells entering apoptosis as a function of time postinfection. The data represent an average of two experiments.
Viral growth rate.
HeLa and BHK cells were grown in 60-mm-diameter dishes to about 90% confluency and infected at an MOI of 20 PFU/cell. One hour postinfection, the medium was aspirated and the cells were washed three times with phosphate-buffered saline and refed with 5 ml of DMEM with 2% FBS. At each time point, 100-μl aliquots were removed from each plate and stored at −70°C. Titers of infectious virus released from the cells were determined by plaque assay with BHK cells.
Rate of viral protein synthesis.
BHK and HeLa cells were grown in six-well dishes to about 90% confluency and infected at an MOI of 20 PFU/cell. At 4, 8, and 12 h postinfection, cells were labeled with [35S]methionine as previously described (6). Cells were solubilized with 0.3 ml of 2% sodium dodecyl sulfate (SDS) disruption buffer. The DNA in the samples was sheared in a syringe with a 26-gauge needle, and the proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% polyacrylamide gels. The gels were fixed, dried, and analyzed by phosphorescence imaging. The radioactivity of the M protein bands was quantified with ImageQuant software (Molecular Dynamics, Inc.).
RESULTS
M protein induces apoptosis in the absence of other viral components.
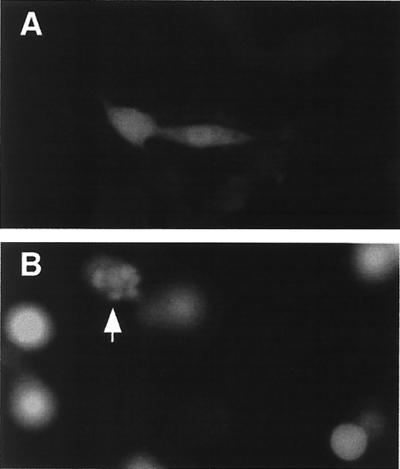
BHK or HeLa cells were transfected with in vitro-transcribed M mRNA to determine whether they undergo the characteristic morphological and biochemical changes associated with apoptosis. Cells were transfected with M mRNA rather than plasmid DNA containing the M gene because M protein inhibits its own expression from DNA vectors that depend on host transcriptional activity (5). Cells were cotransfected with mRNA encoding EGFP so transfected cells could be distinguished from nontransfected cells by fluorescence microscopy. Fluorescence images of transfected BHK cells obtained at 24 h posttransfection are shown in Fig. 1. Control BHK cells transfected with EGFP mRNA retained their normal elongated morphology (Fig. 1A). BHK cells cotransfected with M mRNA and EGFP mRNA showed the characteristic round morphology of cells expressing M protein (Fig. 1B). The cell in panel B denoted by the arrow appears to be undergoing membrane blebbing, a key morphological change associated with the induction of apoptosis. In cells undergoing apoptosis, membrane blebbing is followed by a period of cessation of membrane activity. Thus, it is possible that all of the transfected cells in Fig. 1B were apoptotic, but most of the cells had undergone membrane blebbing earlier and had already ceased membrane activity. Indeed, time lapse microscopy demonstrated that all of the BHK cells transfected with M mRNA underwent membrane blebbing between 10 and 26 h posttransfection (data not shown). Membrane blebbing was not observed in control cells transfected with EGFP mRNA alone. Similar results were obtained with HeLa cells cotransfected with M and EGFP mRNAs (data not shown). These results suggest that M protein induces cells to undergo apoptosis when it is expressed in the absence of other viral components.
FIG. 1.
M protein expression induces morphological changes associated with apoptosis. BHK cells were transfected with EGPF mRNA (A) or M mRNA and EGPF mRNA (B). At 24 h posttransfection, cells were analyzed by fluorescence microscopy using a 25× water immersion objective. The fluorescein channel was used to detect the presence of EGFP to indicate transfected cells. Digital fluorescence images of transfected cells expressing EGFP are shown in both panels. the arrow in panel B indicates a cell undergoing membrane blebbing.
The induction of apoptosis by M protein was confirmed by assaying the activity of caspase-3 in cells transfected with M mRNA. Caspase-3 activity was chosen as an indicator of apoptosis because most apoptotic pathways involve the activation of caspase-3, while necrotic death is typically not associated with the activation of caspases. HeLa cells (Fig. 2A) or BHK cells (Fig. 2B) were transfected with mRNA encoding wt M protein. Cells were also transfected with mRNAs encoding mutant M proteins to determine whether the induction of apoptosis was genetically correlated with the virus assembly functions of M protein or with its ability to inhibit host gene expression. A mutant M protein containing a substitution of arginine for methionine at position 51 of the 229-amino-acid M protein (M51R mutation) is defective in the ability to inhibit host gene expression but is fully functional in virus assembly (6, 9, 12, 19, 26). In contrast, an M protein deletion mutant, MN1, missing amino acids 4 to 21 is defective in the virus assembly functions but inhibits host gene expression and causes cell rounding as effectively as does wt M protein (6, 26). Cells were also transfected with yeast RNA or EGFP mRNA as negative controls. Twenty-four hours posttransfection, the cells were lysed and caspase-3 activity was determined with a fluorogenic substrate. In these experiments, we used an amount of wt or MN1 M mRNA (30 ng per culture) that was 10-fold less than that of M51R-M or EGFP mRNA (300 ng per culture) in order to achieve comparable levels of protein expression. This is because the cytopathic activity of wt and MN1 M proteins enhances translation of transfected mRNAs (4). The enhanced expression from transfected mRNA of wt M protein compared to that of the M51R-M protein described previously (26) was reconfirmed during the course of these experiments (data not shown). The level of M protein expression in these experiments was approximately 1,000-fold lower than that in VSV-infected cells (27).
Expression of wt M protein induced activation of caspase-3 in both HeLa cells (Fig. 2A) and BHK cells (Fig. 2B), indicating that M protein can induce apoptosis in the absence of other viral components. Expression of MN1 mutant M protein, which is defective in viral assembly functions such as membrane budding, induced levels of caspase-3 comparable to those obtained with wt M protein in both cell types. In contrast, expression of the M51R mutant M protein activated little, if any, caspase-3 in either cell type. These data indicate that the M51R mutant M protein, which is defective in the ability to inhibit host gene expression, is also defective in the ability to induce apoptosis. Thus, the induction of apoptosis by M protein is genetically correlated with the inhibition of host gene expression and not with its virus assembly functions.
M protein expression resulted in higher levels of caspase-3 activity in HeLa cells than in BHK cells. This is apparent from the difference in the scales of the y axes of Fig. 2A and B. wt M protein activated 10.4-fold higher levels of caspase-3 than did the EGFP control in HeLa cells but only 2.7-fold higher levels in BHK cells. This indicates that HeLa cells are more sensitive to caspase-3 activation by M protein than are BHK cells.
To determine whether the difference in the levels of caspase-3 activation in HeLa and BHK cells was dependent on the amount of M mRNA transfected or the time of transfection, the concentration dependence and time course of M protein-induced apoptosis were determined in both cell types. Figure 3A shows the results of transfection of HeLa cells (open circles) and BHK cells (closed circles) with different amounts of M mRNA ranging from 0 to 300 ng. Twenty-four hours posttransfection, the cells were lysed and caspase-3 activity was determined with a fluorogenic substrate. Expression of M protein induced caspase-3 activation over similar titration ranges in both cell types. Figure 3B shows a time course of caspase-3 activation after transfection of HeLa cells (open circles) and BHK cells (closed circles) with 30 ng of M mRNA. Significant activation of caspase-3 began at about 18 h posttransfection in both cell types and continued throughout the duration of the time course. The levels of caspase-3 activity in BHK cells were approximately threefold less than in HeLa cells in these experiments. Thus, the difference in the levels of caspase-3 activity induced by M protein in HeLa cells versus BHK cells was not dependent on the amount of M mRNA transfected or the time posttransfection at which the cells were analyzed.
M protein and another viral component contribute to induction of apoptosis in cells infected with VSV.
The conclusion that M protein can induce apoptosis in the absence of other viral components raises the question of whether M protein is also involved in induction of apoptosis in the context of a virus infection. This question was addressed by using viruses containing the M51R mutation. The M51R mutation was originally identified in the M protein of tsO82 virus, but it was not known at the time whether the tsO82 virus contained additional mutations in genes other than M. To address this possibility, the M51R mutation was introduced onto the wt background of an infectious VSV cDNA clone. The recombinant virus containing the mutation was designated rM51R-M virus, and its properties were compared to those of the corresponding rwt virus. Likewise, the tsO82 virus was compared to the wt Orsay (wtO) strain from which it was derived.
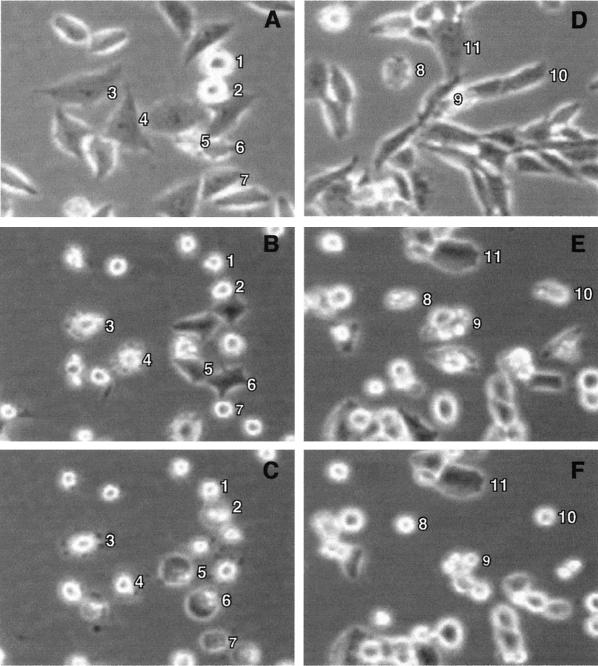
Time lapse microscopy was used to determine the timing of morphological changes associated with the induction of apoptosis by wt and M protein mutant viruses. Cells undergoing apoptosis proceed through a characteristic sequence of morphological changes beginning with cell rounding followed by membrane blebbing. This is followed by a period of cessation of membrane activity. The final stages of apoptosis involve the protrusion of thin surface microspikes, cell surface blistering, and membrane rupture. Examples of these morphological changes can be seen in Fig. 4, which shows images obtained by phase-contrast time lapse microscopy of HeLa or BHK cells infected with wtO virus. Figure 4A to C show the same field containing HeLa cells infected with wtO virus at various times postinfection. At early times postinfection (e.g., 4 h, panel A), the only cells that were round were those undergoing mitosis (cells 1 and 2). Cells entering mitosis during the first few hours postinfection, such as cells 1 and 2, regained their flattened morphology upon completion of mitosis (data not shown). When VSV-infected HeLa cells entered apoptosis, cell rounding was closely followed by the onset of membrane blebbing. This is evident in panel B (9.5 h postinfection), in which cells 3 and 4 were undergoing membrane blebbing. The other round cells in the field had undergone membrane blebbing previously and had ceased membrane activity by this point. By 15.5 h postinfection (panel C), all of the cells were round and cells 3 and 4 had ceased membrane blebbing. There were noticeable apoptotic bodies surrounding cell 3, and cells 5 to 7 were undergoing membrane blistering, which is a late stage of apoptosis that precedes membrane rupture.
FIG. 4.
Time lapse microscopy analysis of morphological changes in VSV-infected cells. HeLa cells (A to C) or BHK cells (D to F) were infected with wtO virus and then analyzed by phase-contrast time lapse microscopy. Digital images of the same fields captured at different times postinfection are shown. The images of infection of HeLa cells were taken at 4 h (A), 9.5 h (B), and 15.5 h (C). The images of infection of BHK cells were taken at 0.5 h (A), 11 h (B), and 18 h (C). Numbered cells were chosen to illustrate cell rounding due to mitosis (cells 1 and 2), membrane blebbing (cells 3, 4, 8, 9, and 10), and cell blistering due to apoptosis (cells 5, 6, and 7), and a BHK cell that remained elongated at a late time postinfection (cell 11).
A similar pattern of morphological changes was observed in BHK cells infected with VSV (Fig. 4D to F), with the exception that in BHK cells, membrane blebbing did not always immediately follow cell rounding. Cells 8 to 10 are examples of BHK cells that were elongated at early times postinfection (Fig. 4D), underwent membrane blebbing at 11 h postinfection (Fig. 4E), and ceased membrane activity by 18 h postinfection (Fig. 4F). In contrast to HeLa cells, a few BHK cells remained elongated at late times postinfection (e.g., cell 11).
In order to compare apoptosis induced by wt VSV and M protein mutant viruses, time lapse microscopy was used to quantify the timing of the induction of apoptosis in cells infected with each of the different viruses. The time at which each of 50 to 130 cells in the field entered apoptosis was determined from the time-date record on the videotape. The data were expressed as the cumulative percentage of cells that had entered apoptosis as a function of time postinfection. The onset of membrane blebbing, rather than cell rounding, was chosen as the criterion for the time of entry into apoptosis because of the possibility that M protein has cell rounding activity that is independent of the induction of apoptosis.
Figure 5 shows the time lapse microscopy results obtained with HeLa cells (panels A and B) and BHK cells (panels C and D) infected with wt VSV and M protein mutant viruses. Panels A and C show results obtained with the recombinant viruses derived from cDNA clones, and panels B and D show results obtained with viruses derived from the Orsay strain. HeLa cells infected with rwt virus entered apoptosis rapidly so that nearly all of the cells had entered apoptosis by 16 h postinfection (Fig. 5A, closed squares). The rM51R-M virus (open squares) induced apoptosis more slowly in HeLa cells, so that about 10% of the cells had entered apoptosis by 16 h postinfection. These data indicate that the effect of wt M protein is to accelerate cell death in HeLa cells. Results of similar experiments performed with BHK cells contrast markedly with those obtained with HeLa cells. In the first 12 h postinfection, about 15% of the BHK cells infected with rwt virus entered apoptosis (Fig. 5C, closed squares) while few cells infected with rM51R-M virus showed apoptotic changes by this time (open squares). However, the majority of BHK cells infected with rwt virus entered apoptosis more slowly than did cells infected with rM51R-M virus. These results indicate that another viral component is the main inducer of apoptosis in BHK cells and that the effect of wt M protein is to delay apoptosis induced by this other viral component in most BHK cells. The prolonged period of induction of apoptosis by rwt virus in BHK cells resulted in a graph that was less sigmoid shaped than the others in Fig. 5. Similar results have been obtained with other cell types undergoing VSV-induced apoptosis in which M protein delays apoptosis (data not shown).
There was little difference in the timing of the induction of apoptosis in HeLa cells between the wtO and tsO82 viruses (Fig 5B). Particularly noteworthy is the fact that cells infected with tsO82 virus (Fig. 5B, open circles) entered apoptosis more rapidly than did cells infected with rM51R-M virus (Fig. 5A, open squares). In the case of BHK cells, most of the cells entered apoptosis more slowly when infected with wtO virus (Fig. 5D, closed circles) than when infected with tsO82 virus (open circles), similar to the results obtained with the recombinant viruses (Fig. 5C), supporting the hypothesis that another viral component induces apoptosis in BHK cells and the effect of wt M protein is to delay apoptosis in most VSV-infected BHK cells.
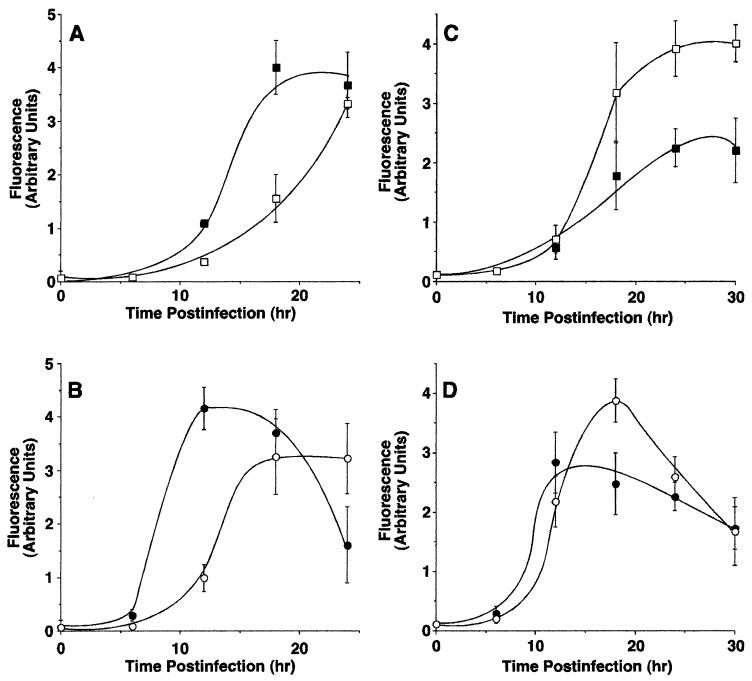
The differences in the induction of apoptosis between viruses with wt versus mutant M proteins were confirmed by assaying the activity of caspase-3. Figure 6 shows the results obtained with HeLa cells (panels A and B) and BHK cells (panels C and D) infected with the wt and M protein mutant viruses. Panels A and C show results obtained with the recombinant viruses derived from cDNA clones, and panels B and D show results obtained with viruses derived from the Orsay strain. rwt virus (Fig. 6A, closed squares) induced caspase-3 activity in HeLa cells more rapidly than did the rM51R-M virus (open squares). For example, 50% of maximum activity was induced by rwt virus by approximately 14 h postinfection, compared to 20 h postinfection with rM51R-M virus. These results support the conclusion that the effect of wt M protein is to accelerate cell death in HeLa cells, similar to the results obtained with time lapse microscopy (Fig. 5).
FIG. 6.
Caspase-3 activity in cells infected with wt VSV and VSV M gene mutants. HeLa cells (A and B) or BHK cells (C and D) were infected with rwt virus (closed squares), rM51R-M virus (open squares), wtO virus (closed circles), or tsO82 virus (open circles) for the times indicated. Duplicate samples were analyzed for caspase-3 activation as described in the legends to Fig. 2 and 3. The amount of caspase-3 activated is expressed as arbitrary fluorescence units. The data represent the average ± the standard deviation of three experiments.
Results of experiments performed with BHK cells contrast markedly with those obtained with HeLa cells. Infection with rM51R-M virus (Fig. 6C, open squares) activated much higher levels of caspase-3 than did infection with rwt virus (Fig. 6C, closed squares). These results indicate that another viral component is the main inducer of apoptosis in BHK cells and that the effect of wt M protein is to inhibit activation of caspase-3 by this other viral component. These results are also consistent with the results obtained with time lapse microscopy (Fig. 5).
wtO virus (Fig. 6B, closed circles) induced caspase-3 activity in HeLa cells more rapidly than did tsO82 virus (open circles), supporting the conclusion that wt M protein accelerates the induction of apoptosis in HeLa cells. The difference in the timing of the induction of apoptosis between wtO virus and tsO82 virus was more pronounced when measured by activation of caspase-3 (Fig. 6B) compared to the onset of membrane blebbing (Fig. 5B). In BHK cells, tsO82 virus (Fig. 6D, open circles) induced more caspase-3 activity than did wtO virus (closed circles), similar to the results obtained with time lapse microscopy (Fig. 5). These results further support the conclusion that another viral component induces apoptosis in BHK cells and the effect of wt M protein is to delay apoptosis in VSV-infected BHK cells. In HeLa cells infected with wtO virus and BHK cells infected with tsO82 virus, the level of caspase-3 declined at late times postinfection. We attribute this to a loss of caspase-3 following the loss of membrane integrity during cell death.
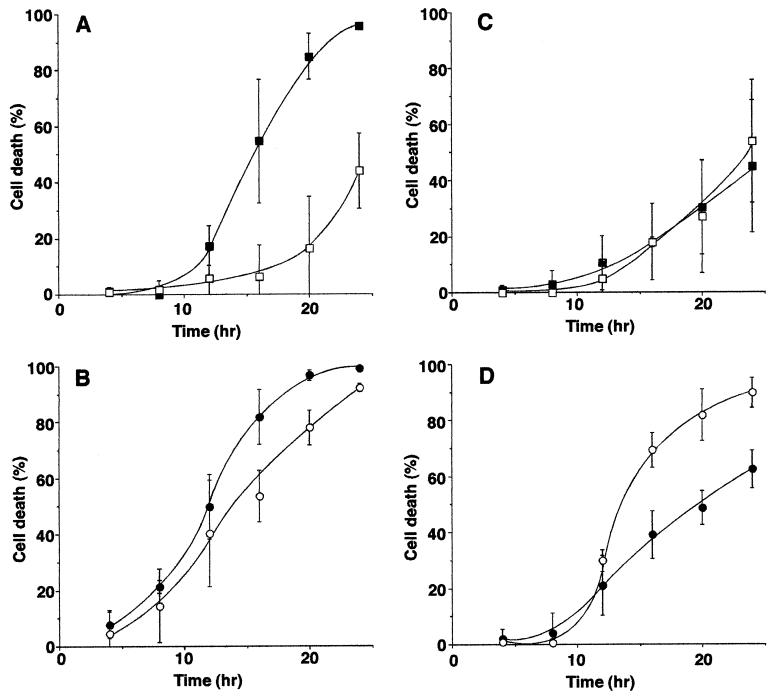
The time lapse microscopy and caspase-3 results were further confirmed by flow cytometry experiments that quantified cell death induced by M gene mutant viruses and their wt controls. Infected cells were labeled with ethidium homodimer-1, a fluorescent dye that penetrates cells with permeable cell membranes, thus making it a marker for dead cells. The percentage of labeled cells was determined by flow cytometry, which was gated so that only intact cells were analyzed. In order to account for the cells that had died and disintegrated and therefore were not analyzed by flow cytometry, intact infected cells were counted in a Coulter counter. For each time point, uninfected cells were lifted and fixed at the same time that another cell sample was infected, so that the original number of infected cells could be determined. The number of cells that disintegrated was determined by subtracting the number of intact infected cells from the number of uninfected cells at each time point. The total percentage of dead cells was calculated by adding the percentage of cells labeled with ethidium homodimer-1 to the percentage of cells that disintegrated as measured by the Coulter counter. Figure 7 shows the time course of cell death induced by wt VSV and M gene mutant viruses in HeLa cells (panels A and B) and BHK cells (panels C and D). rwt virus effectively killed HeLa cells so that nearly all of the cells were dead by 24 h postinfection (Fig. 7A, closed squares). The rM51R-M virus (open squares) was much less effective than rwt virus in killing HeLa cells so that fewer than half of the cells were dead at 24 h postinfection. These results support the conclusion that the effect of wt M protein is to accelerate cell death in HeLa cells, similar to the conclusions from time lapse microscopy (Fig. 5) and caspase-3 activation (Fig. 6). There was little difference in cell killing between wtO virus (Fig. 7B, closed circles) and tsO82 virus (open circles). tsO82 virus induced cell death at a greater rate than did rM51R-M virus, even though both viruses contain the M51R mutation in their M proteins, supporting the hypothesis that there is more than one component that contributes to the death of VSV-infected cells. This result is also similar to the results obtained with time lapse microscopy.
FIG. 7.
Induction of cell death by wt VSV and VSV M gene mutants as measured by membrane permeability. HeLa cells (A and B) or BHK cells (C and D) were infected with rwt virus (closed squares), rM51R-M virus (open squares), wtO virus (closed circles), or tsO82 virus (open circles). Duplicate samples were labeled with ethidium homodimer-1 and analyzed by flow cytometry. The percentage of intact cells that had permeable cell membranes was determined by using CellQuest software. In order to account for the cells that disintegrated and could not be analyzed by flow cytometry, intact cells were counted in a Coulter counter. For each time point, uninfected cells were lifted and fixed with 4% formaldehyde at the same time that another cell sample was infected. Infected samples corresponding to each time point were fixed at the end of the experiment. All of the cells were counted, and the number of cells that disintegrated was determined by subtracting the number of intact infected cells from the number of uninfected cells at each time point. The total percentage of dead cells was calculated by adding the percentage of intact cells labeled with ethidium homodimer-1 as measured by flow cytometry to the percentage of cells that disintegrated as measured by the Coulter counter. The data represent the average ± standard deviation of three experiments.
In BHK cells, there was little difference in the cell death caused by rwt virus and that caused by rM51R-M virus over the 24-h time course (Fig. 7C), and tsO82 virus killed BHK cells faster than did wtO virus (Fig. 7D). These results are similar to those obtained by time lapse microscopy and caspase-3 activation, although the onset of membrane blebbing and the activation of caspase-3 are earlier events than the membrane rupture measured by these experiments. Thus, we can again conclude that another viral component is the main inducer of apoptosis in BHK cells. The effect of wt M protein is to delay the induction of apoptosis by this other viral component in BHK cells infected with VSV. This delay was apparent when the tsO82 and wtO viruses were compared (Fig. 7D). In the case of the rM51R-M and rwt viruses, the delay was not apparent in Fig. 7C because fewer infected cells died within the 24-h time course.
Viral growth and M protein expression by wt and M protein mutant viruses.
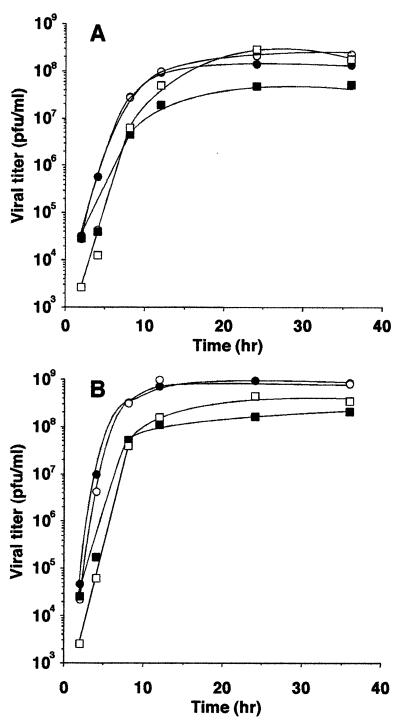
Rates of wt and M protein mutant virus growth and protein expression in HeLa or BHK cells were determined in order to determine whether the induction of apoptosis by these viruses is related to the level of virus replication or M protein expression. Cells were infected with each of the two different M protein mutant viruses (rM51R-M or tsO82) or the corresponding wt virus (rwt or wtO), and the virus yield was determined by plaque assay as a function of time postinfection. The results of two viral growth experiments performed with HeLa cells were averaged and are shown in Fig. 8A. RM51R-M virus (open squares) produced levels of progeny similar to those produced by rwt virus (closed squares) at early times postinfection but accumulated to approximately 10-fold higher titers than did rwt virus by 24 h postinfection. In repeated experiments at 24 h postinfection, this trend in virus yield, although reproducible, was not statistically significant, 4.0 × 107 ± 2.0 × 107 for rwt virus and 1.8 × 108 ± 1.3 × 108 for rM51R-M virus (n = 4, P = 0.1). These data indicate that the M51R M protein mutation does not have a deleterious effect on virus growth and that in HeLa cells, it slightly enhanced the yield of the recombinant virus. There was little difference between the growth of tsO82 virus (open circles) and that of the wtO virus from which it was derived (closed circles). tsO82 virus was originally identified as a ts mutant in chicken embryo cells (13) but is not temperature sensitive for virus growth in most cell types. These results obtained at 37°C are consistent with the lack of temperature sensitivity of this virus for growth in HeLa cells. rwt virus grew to lower titers than either of the viruses derived from the Orsay strain. This appears to reflect strain differences in virus growth.
FIG. 8.
Effects of M gene mutations on virus growth. HeLa cells (A) or BHK cells (B) were infected with wtO virus (closed circles), tsO82 virus (open circles), rwt virus (closed squares), or rM51R-M virus (open squares). One hour postinfection, the cells were washed with phosphate-buffered saline and 5 ml of DMEM with 2% FBS was added to each dish. At the indicated time point, 100 μl of supernatant was removed from each plate and stored at −70°C. Plaque assays were performed in duplicate with BHK cells. The data reported are averages of two experiments.
The results of similar experiments performed with BHK cells are shown in Fig. 8B. RM51R-M virus (open squares) grew to higher titers than did rwt virus (closed squares), although there was less difference between the viral growth rates in BHK cells than in HeLa cells. The viruses derived from the Orsay strain (circles) grew to higher titers than did the viruses derived from the infectious cDNA clone (squares). As in HeLa cells, it is also apparent that the tsO82 virus is not temperature sensitive in BHK cells. The important conclusion to be drawn from these data is that the M51R M protein mutation does not have a deleterious effect on virus growth.
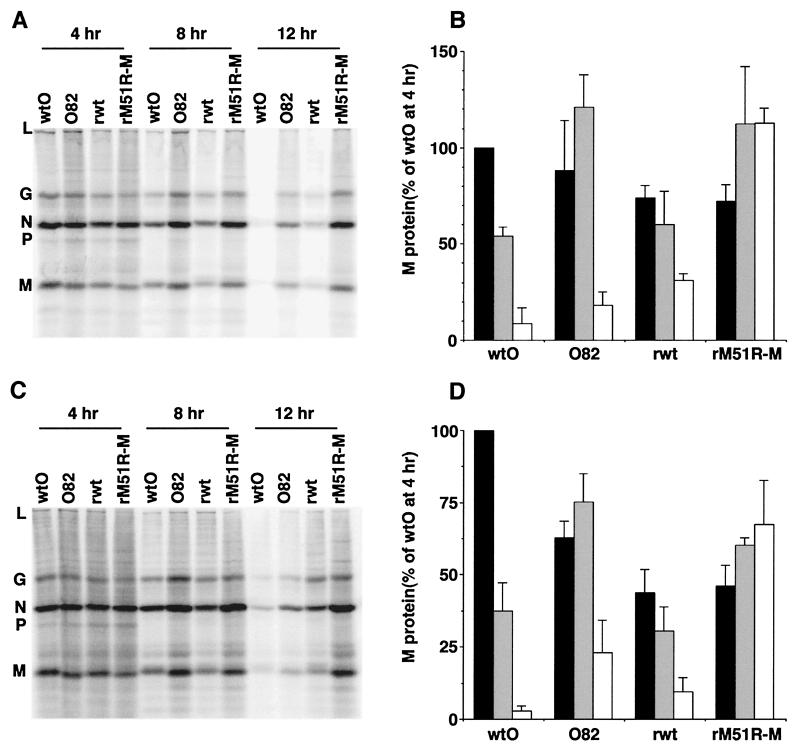
The levels of viral protein synthesis in cells infected with M protein mutants and the corresponding wt viruses are shown in Fig. 9. HeLa or BHK cells were infected with each of the four viruses for 4, 8, or 12 h and then pulsed with [35S]methionine for 10 min. The proteins were solubilized and analyzed by SDS-PAGE and phosphorescence imaging. Figure 9A and C show representative images obtained with HeLa and BHK cells, respectively. Figure 9B and D show quantitation of the radioactivity of the M protein bands from three separate experiments expressed as a percentage of the amount of M protein synthesis at 4 h postinfection with the wtO virus, which was near the maximum rate. Rates of M protein synthesis in cells infected with the wtO and rwt viruses declined over the 12-h time course in both cell types due to the dramatic inhibition of both viral and host protein synthesis that is typical of VSV-infected cells. rwt virus synthesized less M protein at 4 h postinfection than did the wtO virus, particularly in BHK cells. This may contribute to the differences in virus growth seen in Fig. 8 and to the differences between these two virus strains in the induction of apoptosis. However, M protein synthesis by the tsO82 and rM51R-M viruses was similar to that of the respective wt controls at 4 h postinfection (black bars) and actually increased between 4 and 8 h postinfection (gray bars). By 12 h postinfection (white bars), M protein synthesis by the tsO82 virus was reduced, indicating that viral protein synthesis was inhibited in these cells at this time point. M protein synthesis in cells infected with rM51R-M virus increased over the 12-h time course, indicating that viral protein synthesis in these cells was not inhibited at these times postinfection. Most importantly, the level of M protein expressed by the mutant viruses was not less than the level of M protein expressed by the corresponding wt viruses. These data indicate that the M protein mutation does not lead to reduced levels of M protein expression. Thus, the effects of the M protein mutation on the induction of apoptosis seen in Fig. 5 and 6 are due to changes in the activity of M protein in cytopathogenesis rather than to changes in the level of M protein expression.
FIG. 9.
Effects of M gene mutations on rates of protein synthesis in VSV-infected cells. HeLa cells (A and B) or BHK cells (C and D) were infected with one of the four viruses indicated. At 4, 8, or 12 h postinfection, cells were labeled with [35S]methionine for 10 min and analyzed by SDS-PAGE. Radioactivity was determined by phosphorescence imaging. Representative images are shown in panels A and C. The viral proteins are indicated to the left of each gel. The radioactivity of the M protein bands was quantified and is shown in panels B and D as the average ± the standard deviation of three experiments. The amount of M protein is expressed as a percentage of the amount of M protein synthesized by wtO virus at 4 h postinfection. The amounts of M protein synthesized at 4 h (black bars), 8 h (gray bars), and 12 h (white bars) are grouped together for each virus.
Also apparent in Fig. 9A and C are the differences in the viruses' abilities to inhibit host protein synthesis, as seen by differences in the levels of the nonviral protein bands (e.g., the region between viral proteins L and G). The wtO and rwt viruses were both effective at inhibiting host protein synthesis, as is typical during a VSV infection. The tsO82 virus was less effective than the wtO virus, and the rM51R-M virus was less effective than the rwt virus at inhibiting host protein synthesis over this time course examined. This indicates that the M51R M protein mutation reduced the ability of VSV to inhibit translation of host proteins in infected cells.
DISCUSSION
Our previous experiments and those of other laboratories have established that the VSV M protein plays a major role in cytopathogenesis by VSV (5, 7, 17). M protein is a structural component of the virion and has several important functions in virus assembly (23). However, M protein plays an important role in viral cytopathogenesis that is genetically separable from its function in virus assembly (6, 26). M protein is a potent inhibitor of host gene expression, both in virus-infected cells and when expressed in transfected cells in the absence of other viral gene products (5, 12, 30). M protein also inhibits cytoskeletal and cell adhesion functions, leading to cell rounding (7, 26, 29). The purpose of the experiments presented here was to determine whether M protein is also responsible for the induction of apoptosis in VSV-infected cells. The data presented here show that M protein induces cell death when expressed in the absence of other viral components (Fig. 1, 2, and 3). Furthermore, cell death occurred by induction of apoptosis rather than necrosis, as shown by induction of the morphological changes that are characteristic of apoptosis and by the activation of caspase-3. M protein mutants demonstrated that the induction of apoptosis by M protein expression is genetically correlated with its ability to inhibit host gene expression and not with its viral assembly function. The ability of M protein to induce apoptosis was supported by results obtained with HeLa cells infected with the recombinant virus containing the M51R M protein mutation, which renders M protein defective in the ability to inhibit host gene expression and in the ability to induce apoptosis. HeLa cells infected with the rM51R-M virus entered apoptosis more slowly than did cells infected with the rwt virus (Fig. 5A, 6A, and 7A), supporting a role for wt M protein in the induction of apoptosis in VSV-infected HeLa cells.
However, there is clearly another viral component that contributes to induction of apoptosis in VSV-infected cells in addition to M protein. The most striking argument for the presence of another viral inducer of apoptosis comes from the results obtained with BHK cells (Fig. 5C, 6C, and 7C). Most BHK cells infected with either rM51R-M or tsO82 virus entered apoptosis faster than did cells infected with the corresponding wt viruses. A similar result was obtained previously by using another mutant virus containing the M51R mutation in its M protein (L. Poliquin and D. D. Dunigan, Abstr. 15th Annu. Meet. Am. Soc. Virol., abstr. W20-7, 1996). Since the M51R M protein is defective in the ability to induce apoptosis (Fig. 2), these results indicate that another viral component is the principal inducer of apoptosis in BHK cells infected with VSV. The effect of wt M protein was to delay VSV-induced apoptosis in BHK cells. These results can be explained by a model in which the induction of apoptosis by another viral component requires new host gene expression in BHK cells (25). In this model, wt M protein inhibits the expression of host gene products necessary for the induction of apoptosis. Thus, the effect of wt M protein would be to delay the induction of apoptosis by VSV in BHK cells. In cells infected with the M protein mutant viruses, the expression of proapoptotic host gene products would not be inhibited and apoptosis would be induced more rapidly than in cells infected with wt viruses.
The existence of another viral component besides M protein that is involved in the induction of apoptosis could account for the difference between the tsO82 and rM51R-M viruses in the induction of apoptosis. Both of these viruses contain the M51R mutation in their M proteins, yet tsO82 virus induced apoptosis more rapidly than did rM51R-M virus in both HeLa and BHK cells (Fig. 5, 6, and 7). It is likely that sequence differences between these viruses in genes other than M account for this difference in their rates of induction of apoptosis. The sequences of the M proteins of these viruses do differ in 6 out of 229 amino acids (15), since the M protein of tsO82 virus is derived from the Orsay strain of VSV while that of the recombinant virus is derived from the San Juan strain (37). It is possible that these sequence differences in their M proteins are responsible for the difference in the induction of apoptosis. However, there is no detectable difference between the San Juan and Orsay M proteins in the ability to inhibit host gene expression (unpublished results). Thus, it is more likely that sequence differences in a viral component other than M protein are responsible for the difference in induction of apoptosis by these two viruses.
The data presented here raise the question of how M protein and another viral component induce apoptosis in VSV-infected cells. Our hypothesis is that the induction of apoptosis by M protein is a consequence of the inhibition of host gene expression. The VSV M protein is remarkably potent as an inhibitor of host gene expression. For example, the levels of M protein required to inhibit gene expression in transfected cells are 1,000-fold lower than the levels of M protein expressed in VSV-infected cells (5, 27). The inhibition of host gene expression by M protein occurs at the level of transcription and at the level of nuclear-cytoplasmic transport of host RNAs (25). Inhibition of such fundamental cellular processes is inconsistent with cell survival, and it is likely that these activities of M protein are responsible for the induction of apoptosis. Alternatively, M protein may induce apoptosis by inhibiting other cellular processes, such as cytoskeletal function, or M protein may have an apoptosis-inducing activity that is independent of its other cytopathic effects.
Understanding the mechanism by which another viral component induces apoptosis in VSV-infected cells depends, of course, on the identity of the other viral component. A likely candidate is viral double stranded RNA (10, 22). This would be consistent with recent evidence implicating the activation of protein kinase R in the induction of apoptosis by VSV (3). Double-stranded RNA is a by-product of viral replication not normally present in uninfected cells and is a major activator of protein kinase R (2, 35). Another possibility is that viral leader RNA contributes to the induction of apoptosis of VSV-infected cells. Leader RNA is the first gene product transcribed from the viral genome, but it does not encode a protein. Leader RNA has been implicated in the virus-induced inhibition of host transcription, but protein synthesis is necessary for the inhibition of host RNA synthesis during a VSV infection, indicating that leader RNA could not be solely responsible for the inhibition (11, 16, 28, 32). Nonetheless, leader RNA might contribute to the cytopathic effects of VSV infection and could play a role in the induction of apoptosis. The viral glycoprotein (G protein) is another candidate that may contribute to induction of apoptosis of VSV-infected cells. For example, G protein might bind to cellular receptors that activate pathways involved in the induction of apoptosis. Stable cell lines have been made that express the VSV G protein, indicating that expression of G protein is not inherently toxic to cells (14). Thus, G protein probably cannot induce apoptosis in the absence of other viral components. However, it is possible that during a viral infection, G protein interacts with other viral components to activate cell death receptors. A goal of our future experiments will be to identify the viral components other than M protein that contribute to the induction of apoptosis by VSV.
Induction of apoptosis in virus-infected cells is usually considered a host antiviral response to limit the amount of viral progeny. Inhibition of host gene expression by M protein appears to be a mechanism by which to prevent expression of host antiviral gene products, such as interferons and other antiviral proteins (25). Thus, the delay caused by wt M protein in the onset of VSV-induced apoptosis in BHK cells would be consistent with this role of M protein in the inhibition of the host antiviral response. However, a possible consequence of VSV's encoding an M protein that promotes apoptosis is that in some cell types, premature cell death may result in a lower number of viral particles released from each infected cell. HeLa cells infected with rM51R-M virus survived much longer than cells infected with rwt virus, and rM51R-M virus grew to slightly higher titers in HeLa cells (Fig. 5, 6, 7, and 8), supporting the idea that premature induction of apoptosis in cells infected with rwt virus limits virus growth. However, there are clearly other factors that determine the level of virus growth, since both the wtO and tsO82 viruses grew to high titers in HeLa cells (Fig. 8), despite the fact that they rapidly induce apoptosis in infected cells (Fig. 5, 6, and 7). Thus, it is likely that the premature induction of apoptosis by M protein is only a minor disadvantage for VSV compared to the advantages derived from the M protein-induced inhibition of the host antiviral response.
In many cases of viral disease, including VSV, virus-induced cell death is the primary cause of disease symptoms in host organisms (18). In the case of Sindbis virus, mortality in infected mice is dramatically reduced when apoptosis is suppressed by Bcl-2 protein expressed by the virus (24). Differential sensitivity of cells in different tissues to induction of apoptosis could play a role in determining which tissues give rise to disease symptoms following virus infection. Our results showing that HeLa and BHK cells have dramatically different responses to the activity of M protein following VSV infection, suggest that M protein plays a role in determining which cell types are most affected by virus-induced apoptosis in the intact host. Thus, these results provide a basis for exploration of the differential responsiveness among cell types to viral inducers of apoptosis and the implications of this differential responsiveness for viral pathogenesis in intact hosts.
ACKNOWLEDGMENTS
We thank Griffith Parks and Maryam Ahmed for helpful advice and comments on the manuscript. We also thank Margie McKenzie for isolating virus from the infectious clones.
This work was supported by Public Health Service grant AI 32983 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahmed M, Lyles D S. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J Virol. 1998;72:8413–8419. doi: 10.1128/jvi.72.10.8413-8419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran S, Kim C N, Yeh W C, Mak T W, Bhalla K, Barber G N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran S, Roberts P C, Kipperman T, Bhalla K N, Compans R W, Archer D R, Barber G N. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black B L, Brewer G, Lyles D S. Effect of vesicular stomatitis virus matrix protein on host-directed translation in vivo. J Virol. 1994;68:555–560. doi: 10.1128/jvi.68.1.555-560.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black B L, Lyles D S. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J Virol. 1992;66:4058–4064. doi: 10.1128/jvi.66.7.4058-4064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black B L, Rhodes R B, McKenzie M, Lyles D S. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondel D, Harmison G G, Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990;64:1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins J A, Schandi C A, Young K K, Vesely J, Willingham M C. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45:923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- 9.Coulon P, Deutsch V, Lafay F, Martinet-Edelist C, Wyers F, Herman R C, Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J Gen Virol. 1990;71:991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- 10.Der S D, Yang Y L, Weissmann C, Williams B R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunigan D D, Baird S, Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986;150:231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- 12.Ferran M C, Lucas-Lenard J M. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J Virol. 1997;71:371–377. doi: 10.1128/jvi.71.1.371-377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamand A. Genetic study of vesicular stomatitis virus: classification of spontaneous thermosensitive mutants into complementation groups. J Gen Virol. 1970;8:187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- 14.Florkiewicz R Z, Smith A, Bergmann J E, Rose J K. Isolation of stable mouse cell lines that express cell surface and secreted forms of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1983;97:1381–1388. doi: 10.1083/jcb.97.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopalakrishna Y, Lenard J. Sequence alterations in temperature-sensitive M-protein mutants (complementation group III) of vesicular stomatitis virus. J Virol. 1985;56:655–659. doi: 10.1128/jvi.56.3.655-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinnell B W, Wagner R R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983;48:88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Her L S, Lund E, Dahlberg J E. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- 18.Huneycutt B S, Bi Z, Aoki C J, Reiss C S. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptur P E, McKenzie M O, Wertz G W, Lyles D S. Assembly functions of vesicular stomatitis virus matrix protein are not disrupted by mutations at major sites of phosphorylation. Virology. 1995;206:894–903. doi: 10.1006/viro.1995.1012. [DOI] [PubMed] [Google Scholar]
- 20.Koyama A H. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 1995;37:285–290. doi: 10.1016/0168-1702(95)00026-m. [DOI] [PubMed] [Google Scholar]
- 21.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 23.Lenard J. Negative-strand virus M and retrovirus MA proteins: all in a family? Virology. 1996;216:289–298. doi: 10.1006/viro.1996.0064. [DOI] [PubMed] [Google Scholar]
- 24.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyles D S. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol Mol Biol Rev. 2000;64:709–724. doi: 10.1128/mmbr.64.4.709-724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyles D S, McKenzie M O. Activity of vesicular stomatitis virus M protein mutants in cell rounding is correlated with the ability to inhibit host gene expression and is not correlated with virus assembly function. Virology. 1997;299:77–89. doi: 10.1006/viro.1996.8415. [DOI] [PubMed] [Google Scholar]
- 27.Lyles D S, McKenzie M O, Ahmed M, Woolwine S C. Potency of wild-type and temperature-sensitive vesicular stomatitis virus matrix protein in the inhibition of host-directed gene expression. Virology. 1996;225:172–180. doi: 10.1006/viro.1996.0585. [DOI] [PubMed] [Google Scholar]
- 28.McGowan J J, Emerson S U, Wagner R R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982;28:325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- 29.Melki R, Gaudin Y, Blondel D. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology. 1994;202:339–347. doi: 10.1006/viro.1994.1350. [DOI] [PubMed] [Google Scholar]
- 30.Paik S Y, Banerjea A C, Harmison G G, Chen C J, Schubert M. Inducible and conditional inhibition of human immunodeficiency virus proviral expression by vesicular stomatitis virus matrix protein. J Virol. 1995;69:3529–3537. doi: 10.1128/jvi.69.6.3529-3537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen J M, Her L S, Varvel V, Lund E, Dahlberg J E. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol. 2000;20:8590–8601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirot M K, Schnitzlein W M, Reichmann M E. The requirement of protein synthesis for VSV inhibition of host cell RNA synthesis. Virology. 1985;140:91–101. doi: 10.1016/0042-6822(85)90448-9. [DOI] [PubMed] [Google Scholar]
- 33.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar G, Sommer S S. The “megaprimer” method of site-directed mutagenesis. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 35.Thomis D C, Samuel C E. Mechanism of interferon action: characterization of the intermolecular autophosphorylation of PKR, the interferon-inducible, RNA-dependent protein kinase. J Virol. 1995;69:5195–5198. doi: 10.1128/jvi.69.8.5195-5198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Kobbe C, van Deursen J M, Rodrigues J P, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo-Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin nup98. Mol Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 37.Whelan S P, Ball L A, Barr J N, Wertz G T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan H, Puckett S, Lyles D S. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J Virol. 2001;75:4453–4458. doi: 10.1128/JVI.75.9.4453-4458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Yoza B K, Lyles D S. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251:383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]