Abstract
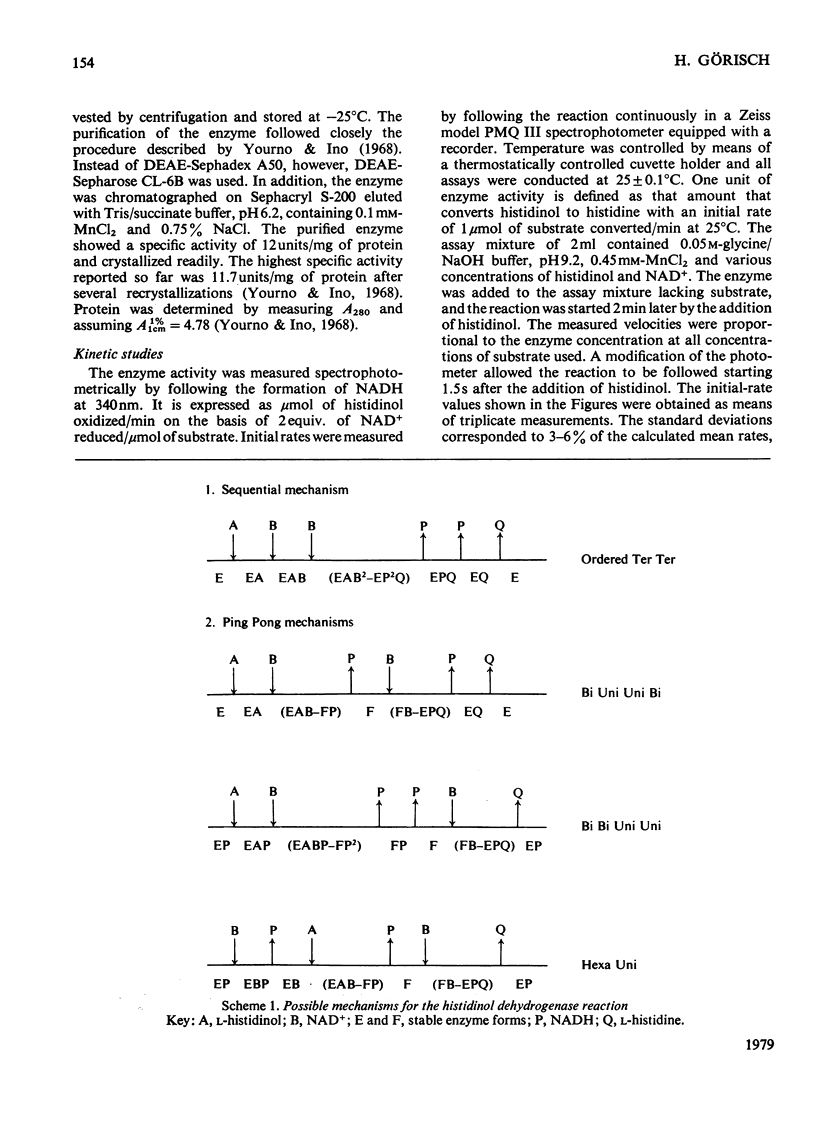
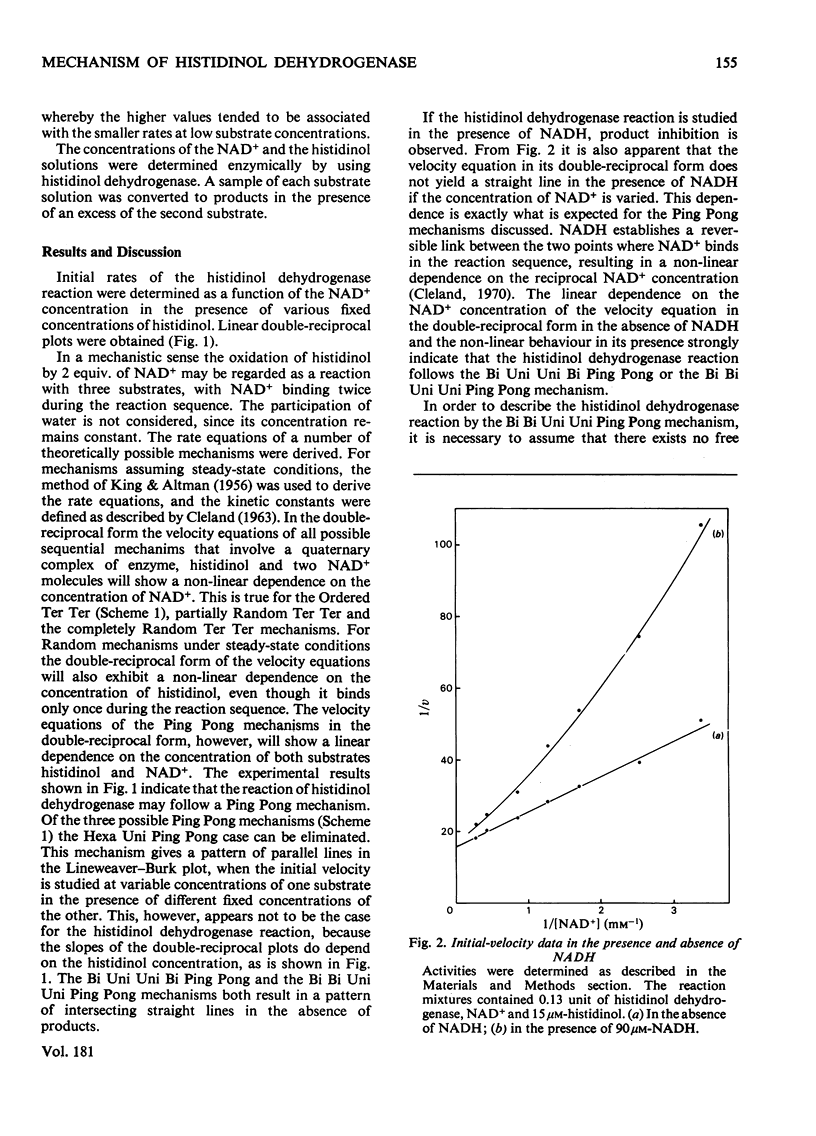
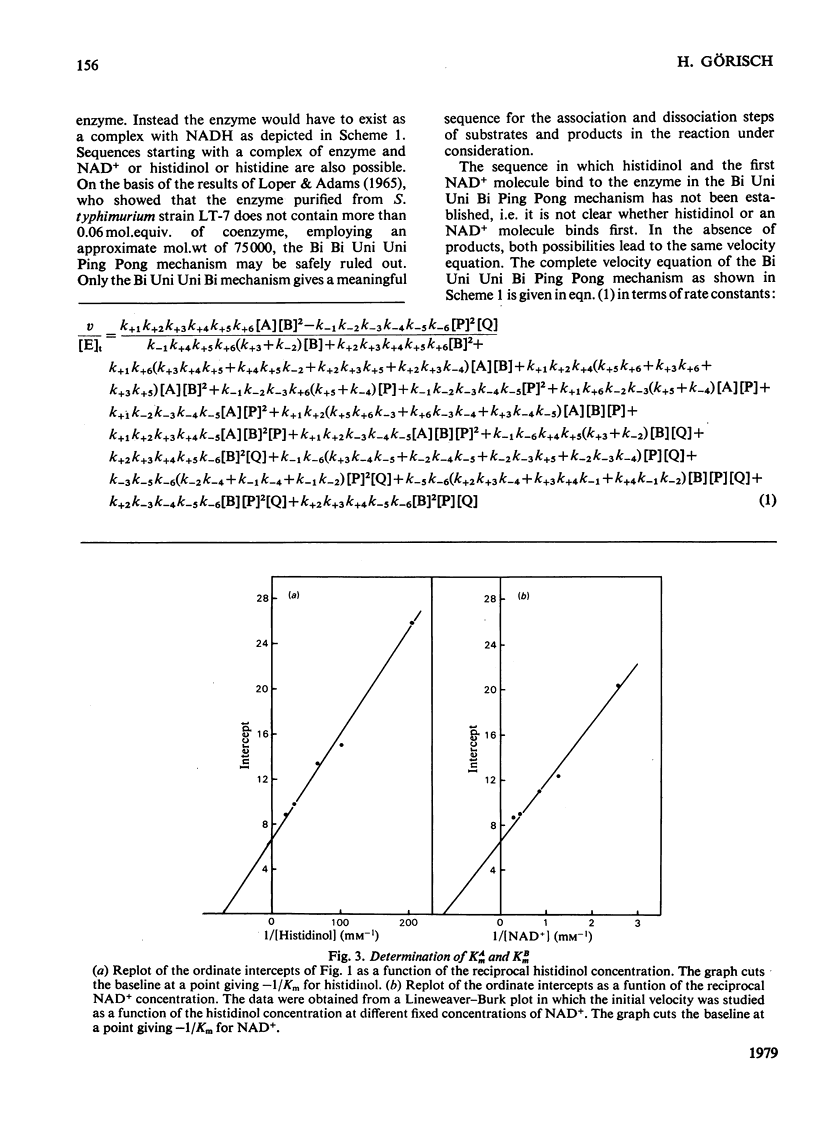
Initial velocities of the histidinol dehydrogenase reaction (EC 1.1.1.23) were measured as a function of the concentrations of the substrates histidinol and NAD+ and in the presence and absence of the product NADH. The data are consistent with a Bi Uni Uni Bi Ping Pong mechanism. The kinetic constants of this mechanism were determined; Km for histidinol was found to be 14 microM and for NAD+ 0.7 mV; Ki for NAD+ was 0.4 mM.
Full text
PDF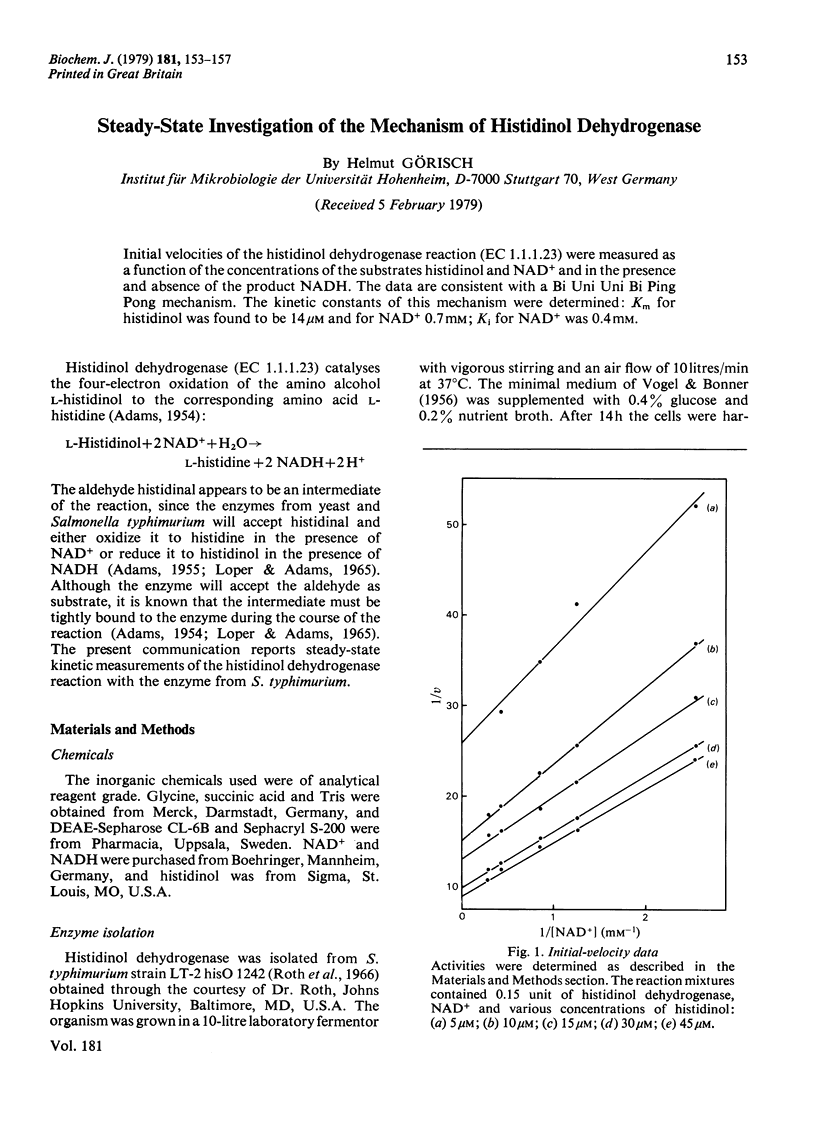
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. L-Histidinal, a biosynthetic precursor of histidine. J Biol Chem. 1955 Nov;217(1):325–344. [PubMed] [Google Scholar]
- ADAMS E. The enzymatic synthesis of histidine from histidinol. J Biol Chem. 1954 Aug;209(2):829–846. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- LOPER J. C., ADAMS E. PURIFICATION AND PROPERTIES OF HISTIDINOL DEHYDROGENASE FROM SALMONELLA TYPHIMURIUM. J Biol Chem. 1965 Feb;240:788–795. [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yourno J., Ino I. Purification and crystallization of histidinol dehydrogenase from Salmonella typhimurium LT-2. J Biol Chem. 1968 Jun 25;243(12):3273–3276. [PubMed] [Google Scholar]


