Abstract
Aims
The purpose of this prospective cohort study was to examine the association of Life Essential 8 (LE8), the recently updated algorithm for quantifying cardiovascular health (CVH) by the American Heart Association (AHA), with the risk of mortality in Chronic Obstructive Pulmonary Disease (COPD) patients.
Methods
Data from the National Health and Nutrition Examination Survey (NHANES) 2007–2012 and the National Death Index mortality data up to December 31, 2019, were included in this cohort analysis. To characterize the relationship between LE8 and CVD and all-cause mortality as well as assess any potential nonlinear relationships, the limited cubic spline mixed with the Cox proportional hazards model was used.
Results
The final analysis included 785 subjects. The weighted mean age of the study population was 59 years, and 479 were male. In the overall population, every 10-point increase in the LE8 score was individuals with associated with reduced risks of 4% for CVD mortality; moderate CVH had a 23% lower risk of COPD, while high CVH was linked to a 40% lower risk compared to low CVH. Among the COPD individuals, every 10-point increase in the LE8 score was associated with reduced risks of 4% for all-cause mortality,8% for CLRD mortality, and 12% for cancer mortality.
Conclusion
This study demonstrates that low levels of LE8 were associated with increased risks of all-cause and cause-specific mortality in COPD individuals.
Keywords: chronic obstructive pulmonary disease, life essential 8, cardiovascular disease, mortality
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a significant public health issue marked by ongoing and worsening airflow restriction, along with chronic respiratory symptoms like shortness of breath, coughing, sputum production, and acute flare-ups. The incidence and mortality rates of COPD have been increasing each year, making it the third leading cause of death worldwide.1 An analysis by the Centers for Disease Control and Prevention (CDC) in 2019, using data from the Global Burden of Disease (GBD) database from 1990 to 2017, revealed that COPD was the fourth leading cause of years of life lost (YLLs) among the Chinese population in 2017.2 Additionally, a separate META study indicated that the annual direct medical expenses for COPD patients in various regions of China ranged from US$72 to US$3565 per person, which represents 33.33% to 118.09% of the average annual income in those areas.3 Clearly, COPD not only greatly diminishes a patient’s quality of life but also imposes a substantial burden on overall health.
Studies have shown that people with COPD face an increased risk of cardiovascular disease compared to those without the condition, and that the mortality rate from heart disease is higher in COPD patients than from respiratory issues. A large cohort study found that, after three years, COPD patients were 2 to 4 times more likely to die from cardiovascular disease than individuals without COPD.4 Anthonisen NR carried out a randomized controlled trial which revealed that over a follow-up period of 14.5 years, there were 731 deaths among 5887 smokers with mild to moderate airway obstruction but no symptoms. Of these deaths, only 7.8% were linked to non-cancerous respiratory conditions, while 22% were attributed to cardiovascular diseases.5 These results highlight the significance of managing cardiovascular disease (CVD) risk in patients with chronic obstructive pulmonary disease (COPD), indicating the need for a comprehensive strategy to prevent COPD and enhance the reduction of mortality risk associated with it.
Studies show that higher Life’s Simple 7 (LS7) scores, as recommended by the American Heart Association (AHA), are associated with a reduced risk of all-cause mortality related to COPD.6 Moreover, study has shown that the LS7 is a reliable instrument for evaluating cardiovascular health.7 The LS7 has several limitations, including a limited scope of health behaviors (notably omitting sleep health), inaccurate weighted scores for its indicators, and a low ability to detect variations both within individuals and between different individuals. In response to these issues, the AHA created a new scoring system known as the Life Essential 8 (LE8), which enhances and refines the scoring methods of the original seven components and incorporates sleep as a factor.8 Studies have shown that LE8 is more closely related to cardiovascular disease than LS7.9
There are limited studies that explore the link between the LE8 score and mortality risk in COPD. Consequently, this research seeks to analyze the connection between updated LE8 metrics and mortality from cardiovascular disease, all causes, chronic lower respiratory disease, and cancer in COPD patients. The main objective of this study is to enhance the survival rates of individuals with COPD.
Materials and Methods
Study Design and Population
The study using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2007 to 2012.NHANES is a comprehensive, ongoing, and representative periodic health survey that focuses on the nutritional and general health of non-institutionalized American people. The survey has been gathering data on the demographics, socioeconomic status, diet, and health of a nationally representative sample of the US population on a two-year cycle since 1999 using a stratified, multistage, and probabilistic clustering design. The NCHS Institutional Ethics Review Board authorized the survey, which was conducted by the National Center for Health Statistics (NCHS), and each participant completed an informed consent form before taking part in Study10.
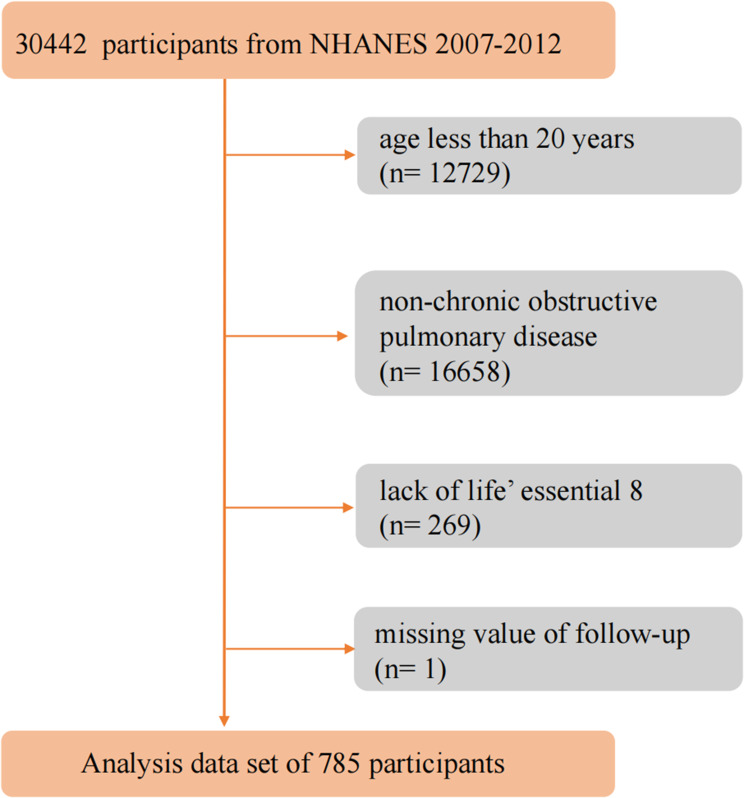
This cohort analysis utilized data from NHANES spanning 2007 to 2012, along with mortality data from the National Death Index (NDI) up to December 31, 2019. Participants were excluded if they met any of the following criteria: 1) they were under 20 years old; 2) they did not have COPD; 3) there was no data on LE8 scores; or 4) there was no information on their death status. Ultimately, the study comprised 785 adult participants. (displayed in Figure 1)
Figure 1.
Flow chart of the identification of the study population. There were 785 patients with chronic obstructive pulmonary disease mellitus involved in the final analysis.
Definitions of LE8 Metrics
Two surveys were utilized to assess dietary habits: the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean Eating Pattern for Americans (MEPA). A score of 15 to 16 on the MEPA or at least the 95th percentile on the DASH was assigned a full score of 100 points for that item. For scoring, 100 points were given for an MEPA score of 15 to 16 or a DASH score in the 95th percentile or higher.
Physical activity refers to the time spent on moderate to vigorous activities, such as housework, walking, cycling, and recreational pursuits, within a week. Any time spent exceeding 150 minutes earns a score of 100 points on the physical activity measure.
Self-reported cigarette smoking or use of nicotine delivery systems was considered as a measure of nicotine exposure, along with living with active indoor smokers. Smoking status was categorized into three groups: never smokers (those who have smoked fewer than 100 cigarettes in their lifetime), former smokers (those who have smoked more than 100 cigarettes but no longer smoke), and current smokers (those who have smoked more than 100 cigarettes and still smoke, either occasionally or daily). A score of 100 indicates a person has never smoked, while scores of 0, 25, 50, and 75 correspond to current smokers, those who quit less than a year ago, those who quit one to five years ago, and those who quit more than five years ago, respectively.
A score of 100 was given for an optimal sleep duration of 7 to 9 hours per night. Body Mass Index (BMI) was calculated by dividing weight (kg) by height squared (m²), with a score of 100 assigned for a BMI of less than 25 kg/m².
For non-HDL cholesterol, a score of 100 is given for levels below 130 mg/dL, with an additional deduction of 20 points if the individual is on lipid-lowering medication. Non-HDL cholesterol is calculated by subtracting HDL cholesterol from the total cholesterol level.
Blood pressure was scored based on systolic and diastolic readings, with a score of 100 for readings of <120/<80 mm Hg. An additional 20 points were deducted if the individual was taking antihypertensive medication at the time of assessment.
The blood glucose index was evaluated using various factors, including glycated hemoglobin, fasting blood glucose, and diabetes history. A person with no diabetes history and a fasting glucose level below 100 mg/dL received a score of 100.
The overall LE8 score was categorized into three levels: low cardiovascular health (CVH) (0–420), moderate CVH (421–530), and high CVH (531–780).
COPD Diagnostic Criteria
-
1)
(FEV1) / forced vital capacity (FVC) of less than 70% following bronchodilator inhalation;2) ever told you had emphysema;3) age above 40, with smoke history or chronic bronchitis, use drug: selective phosphodiesterase-4 inhibitors, or mast cell stabilizers, or leukotriene modifiers, or inhaled corticosteroids.
Assessment of Outcomes
The main outcomes of the study were mortality rates from all causes, chronic lower respiratory disease (CLRD), cardiovascular disease (CVD), and cancer. The LE8 score served as the independent variable, while mortality from all causes, CLRD, CVD, and cancer were the dependent variables. We collected and analyzed data on all-cause mortality as well as mortality related to CVD, cancer, and CLRD, using the International Classification of Diseases, 10th Revision (ICD-10). CVD-related mortality was categorized under heart diseases (I00–I09, I11, I13, I20–I51), cancer-related mortality was classified as malignant neoplasms (C00–C97), and CLRD was defined as chronic lower respiratory disease (J40-J47).11.
Covariates
The study included several covariates such as age, gender, race/ethnicity, education level, poverty incidence rate (PIR), history of cardiovascular disease (CVD), and alcohol consumption status. Race was categorized into non-Hispanic white, non-Hispanic black, Hispanic, and other races. Education levels were classified as having a bachelor’s degree or higher, holding a high school diploma, or having less than a year of education. The PIR was calculated by dividing monthly household income into three groups: low income (<=1), moderate income (1–3), and high income (>3). The history of CVD was categorized into heart failure, coronary heart disease, angina pectoris, and myocardial infarction. Alcohol consumption was classified into three groups: never drank, previously drank, and currently drinking.
Statistical Analysis
The sample weight in this multistage, stratified NHANES design was calculated by multiplying 1/10 by the 2-Year MEC Weight from the cycles between 2007 and 2012. Continuous variables are presented as means ± standard error (SE), while categorical variables are displayed as counts and percentages. To compare the groups for continuous and categorical variables, one-way analysis of variance and chi-square tests were utilized, respectively. The statistical analyses were conducted using R software (version 4.2.2), with a two-sided P-value of less than 0.05 indicating statistical significance. After adjusting for covariates, Weighted Cox proportional hazards regression was performed to investigate the relationships between LE8 and all-cause, CLRD, CVD, and cancer mortality in COPD patients, using three models: model 1 was the unadjusted model, model 2 adjusted for age (continuous), sex (categorical), and ethnicity (categorical), and model 3 further adjusted for education (categorical), family income-poverty ratio (categorical), and drinking status (categorical). LE8 levels were categorized into tertiles, with the lowest tertile serving as the reference group in the Cox logistic regression. The Kaplan-Meier survival curve, analyzed using the weighted Log rank test, illustrated the probability of all-cause mortality across different CVH groups.
Results
Characteristics of the Participants
In a study involving 785 individuals with COPD from the NHANES dataset covering 2007 to 2012, the average age was 59 years, with males making up 58.37% of the group. Among the 782 participants in this analysis, 83.6% (538) identified as non-Hispanic White, 6.8% (128) as non-Hispanic Black, 1.4% (38) as Mexican-American, and 6.1% (81) as belonging to other racial groups. Educational attainment showed that 54.4% (350) had completed college or higher, 24.9% (192) had finished high school, and 21.7% (24) were under one year old. In terms of smoking status, 44% (350) were trying to quit, 35.6% (283) were current smokers, and 20.1% (152) had never smoked. Regarding alcohol consumption, 25.7% (233) were quitting, 65.6% (474) were current drinkers, and 6.5% (53) had never consumed alcohol. Among these participants, 53.4% (352) were high school graduates, and 21.7% (24) were under one year old. Income levels were reported as 11.4% (156) low income, 35.4% (320) middle income, and 45.1% (251) high income. Health conditions included 53.8% (469) with hypertension, 83.9% (648) with hyperlipidemia, and 20.3% (205) with cardiovascular disease.
When comparing participants with low LE8 to those with high LE8, the 256 individuals in the high LE8 group exhibited higher levels of economic and educational achievement, as well as better FEV1/FVC ratios. They were also less likely to have hyperlipidemia and cardiovascular disease. Additionally, lower levels of blood pressure, fasting blood glucose, HbA1c, low-density lipoprotein cholesterol, and BMI were associated with those in the higher tertiles of LE8. A statistically significant difference was observed in the ratios of drinking to smoking (all P < 0.01). Furthermore, individuals with higher scores were more likely to have previously smoked but had since quit.(as seen in Table 1).
Table 1.
Characteristics of Participants Categorized by Tertiles of Life’ Essential 8
| Variables | Total | Life’ Essential 8, score | P value | ||
|---|---|---|---|---|---|
| Tertile 1(≤420) | Tertile 2(421–530) | Tertile 3(531–780) | |||
| Participants | 785 | 270 | 259 | 256 | – |
| Age, years | 59.444±0.462 | 60.302±0.568 | 58.973±0.903 | 59.215±0.889 | 0.471 |
| Sex, % | 0.85 | ||||
| Women | 306(41.633) | 109(39.980) | 102(42.951) | 95(41.695) | |
| Men | 479(58.367) | 161(60.020) | 157(57.049) | 161(58.305) | |
| Ethnicity, % | 0.154 | ||||
| Non-Hispanic White | 538(85.634) | 167(82.620) | 184(84.594) | 187(88.940) | |
| Non-Hispanic Black | 128(6.832) | 58(10.148) | 40(6.505) | 30(4.561) | |
| Mexican American | 38(1.421) | 15(1.865) | 13(1.661) | 10(0.853) | |
| Other | 81(6.113) | 30(5.366) | 22(7.240) | 29(5.646) | |
| Education, % | < 0.0001 | ||||
| Some college or above | 352(53.433) | 93(39.161) | 107(52.500) | 152(65.384) | |
| High school graduate | 192(24.888) | 66(27.464) | 70(25.670) | 56(22.161) | |
| Less than 12th | 241(21.679) | 111(33.375) | 82(21.831) | 48(12.455) | |
| Smoking, % | < 0.0001 | ||||
| Never | 152(20.075) | 15(4.161) | 50(16.801) | 87(35.473) | |
| Former | 350(44.364) | 105(36.230) | 116(45.699) | 129(49.441) | |
| Now | 283(35.561) | 150(59.609) | 93(37.499) | 40(15.086) | |
| Drinking, % | < 0.0001 | ||||
| Never | 53(6.490) | 17(4.449) | 23(11.483) | 13(3.847) | |
| Former | 233 (25.742) | 107 (39.917) | 69 (22.738) | 57 (19.110) | |
| Now | 474 (65.561) | 137 (55.634) | 157 (65.779) | 180 (77.043) | |
| Family income-poverty ratio, % | < 0.0001 | ||||
| ≤1 | 156 (11.406) | 73 (20.260) | 54 (12.767) | 29 (6.178) | |
| 1–3 | 320 (35.375) | 119 (45.627) | 111 (39.854) | 90 (31.886) | |
| >3 | 251 (45.104) | 57 (34.114) | 78 (47.379) | 116 (61.936) | |
| Hypertension, % | 0.002 | ||||
| No | 316 (46.185) | 77 (36.888) | 100 (42.120) | 139 (57.180) | |
| Yes | 469 (53.815) | 193(63.112) | 159(57.880) | 117(42.820) | |
| Hyperlipidemia, % | < 0.0001 | ||||
| No | 137(16.080) | 16(3.597) | 43(13.282) | 78(28.371) | |
| Yes | 648(83.920) | 254(96.403) | 216(86.718) | 178(71.629) | |
| Cardiovascular disease, % | < 0.0001 | ||||
| No | 580(79.719) | 172(67.499) | 191(80.012) | 217(88.936) | |
| Yes | 205(20.281) | 98(32.501) | 68(19.988) | 39(11.064) | |
| BMI, Kg/m2 | 28.895±0.286 | 32.267±0.607 | 28.558±0.508 | 26.589±0.296 | < 0.0001 |
| Blood pressure, mmHg | < 0.0001 | ||||
| Systolic | 125.262±0.655 | 128.447±1.184 | 126.711±1.363 | 121.443±0.964 | |
| Diastolic | 70.473±0.605 | 71.252±0.910 | 70.849±0.925 | 69.518±0.805 | |
| TC, mmol/L | 5.204±0.043 | 5.498±0.089 | 5.191±0.082 | 4.986±0.052 | < 0.0001 |
| TG, mmol/L | 1.544±0.058 | 1.906±0.137 | 1.637±0.103 | 1.224±0.068 | < 0.001 |
| HDL-C, mmol/L | 1.365±0.024 | 1.194±0.023 | 1.362±0.030 | 1.500±0.042 | < 0.0001 |
| LDL-C, mmol/L | 3.082±0.062 | 3.367±0.115 | 3.057±0.135 | 2.904±0.072 | 0.006 |
| FBG, mmol/L | 5.941±0.074 | 6.284±0.215 | 6.064±0.092 | 5.613±0.059 | < 0.001 |
| HbA1c, % | 5.799±0.040 | 6.268±0.107 | 5.700±0.036 | 5.525±0.029 | < 0.0001 |
| FEV1/FVC | 0.642±0.003 | 0.643±0.004 | 0.627±0.008 | 0.654±0.004 | 0.016 |
Note: Bold text signifies that the differences in participant characteristics between those with high LE8 and those with low LE8 are statistically significant.
Abbreviations: BMI, body mass index; TC, total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; FEV1/FVC, forced expiratory volume in one second /forced vital capacity.
Demographic Factors Between COPD Survivors and Non-Survivors
In this research, there were 245 fatalities, with an average age of 67 years and a significant age difference (P<0.0001). Among the deceased, 40.4% had an education level above university, 63 individuals had completed high school, making up 24.1%, and 35.5% had graduated less than a year prior. There were 133 former smokers, representing 50.9%, 88 current smokers, accounting for 39.1%, and 24 individuals who had never smoked, which constituted 10%, with significant differences in smoking behavior (P<0.0001). Additionally, 110 individuals (45.3%) had quit drinking, 112 (48.1%) were current drinkers, and 15 (6.6%) had never consumed alcohol, with a statistically significant difference in drinking behavior composition (P<0.0001). In terms of income, 51 individuals (16.5%) were classified as low-income, 131 (56.6%) as middle-income, and 46 (26.9%) as high-income, with a significant variation in the economic composition ratio (P<0.0001). (as seen in Table 2)
Table 2.
Demographic Factors Between Survivors and Non-Survivors
| Variables | Alive | Deceased | P value |
|---|---|---|---|
| Participants | 540 | 245 | |
| Age, years | 57.001±0.622 | 67.159±0.953 | < 0.0001 |
| Sex, % | 0.741 | ||
| Women | 218(41.351) | 88(42.525) | |
| Men | 322(58.649) | 157(57.475) | |
| Ethnicity, % | 0.338 | ||
| Non-Hispanic White | 357(84.988) | 181(87.674) | |
| Non-Hispanic Black | 91(6.772) | 37(7.023) | |
| Mexican American | 32(1.601) | 6(0.855) | |
| Other | 60(6.640) | 21(4.448) | |
| Education, % | < 0.0001 | ||
| Some college or above | 266(57.575) | 86(40.355) | |
| High school graduate | 129(25.133) | 63(24.113) | |
| Less than 12th | 145(17.292) | 96(35.532) | |
| Smoking, % | 0.021 | ||
| Never | 128(23.259) | 24(10.022) | |
| Former | 217(42.285) | 133(50.928) | |
| Now | 195(34.456) | 88(39.050) | |
| Drinking, % | < 0.0001 | ||
| Never | 38(6.633) | 15(6.646) | |
| Former | 123(20.327) | 110(45.271) | |
| Now | 362(73.040) | 112(48.082) | |
| Family income-poverty ratio, % | < 0.0001 | ||
| ≤1 | 105(11.158) | 51(16.508) | |
| 1–3 | 189(32.943) | 131(56.632) | |
| >3 | 205(55.899) | 46(26.860) |
Note: Bold text indicates that the difference in these demographic factors between survivors and non-survivors is statistically significant.
Clinical Factors Between COPD Survivors and Non-Survivors
In this study involving 245 deaths, 172 individuals, representing 64.8% of the group, had hypertension, while 73 individuals, or 35.2%, did not have hypertension. The difference in the proportion of those with hypertension is statistically significant (P<0.05). Additionally, 210 individuals, or 86.3%, had hyperlipidemia, compared to 35 individuals, or 13.7%, who did not; this difference is also statistically significant (P<0.0001). Furthermore, 100 individuals, or 36.8%, had cardiovascular diseases, while 145 individuals, or 63.2%, did not, with this difference being statistically significant as well (P<0.0001). The deceased individuals exhibited higher levels of glycated hemoglobin, while their triglyceride and LDL cholesterol levels were lower, with a statistically significant difference (P<0.05). (as seen in Table 3)
Table 3.
Clinical Factors Between Survivors and Non-Survivors
| Variables | Alive | Deceased | P value |
|---|---|---|---|
| Participants | 540 | 245 | |
| Hypertension, % | 0.006 | ||
| No | 243(49.663) | 73(35.204) | |
| Yes | 297(50.337) | 172(64.796) | |
| Dyslipidemia, % | 0.374 | ||
| No | 102(16.847) | 35(13.656) | |
| Yes | 438(83.153) | 210(86.344) | |
| Cardiovascular disease, % | < 0.0001 | ||
| No | 435(84.953) | 145(63.190) | |
| Yes | 105(15.047) | 100(36.810) | |
| BMI, Kg/m2 | 28.733±0.292 | 29.406±0.630 | 0.312 |
| Blood pressure, mmHg | |||
| Systolic | 124.105±0.743 | 128.917±1.425 | 0.004 |
| Diastolic | 71.956±0.651 | 65.780±0.963 | < 0.0001 |
| TC, mmol/L | 5.316±0.056 | 4.850±0.068 | < 0.0001 |
| TG, mmol/L | 1.540±0.066 | 1.554±0.085 | 0.888 |
| HDL-C, mmol/L | 1.370±0.025 | 1.347±0.048 | 0.637 |
| LDL-C, mmol/L | 3.203±0.084 | 2.711±0.069 | < 0.0001 |
| FBG, mmol/L | 5.893±0.080 | 6.088±0.144 | 0.218 |
| HbA1c, % | 5.738±0.043 | 5.989±0.080 | 0.005 |
| FEV1/FVC | 0.644±0.003 | 0.627±0.008 | 0.095 |
Note: Bold text indicates that the difference in these clinical factors between survivors and non-survivors is statistically significant.
Abbreviations: BMI, body mass index; TC, total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol;
FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; FEV1/FVC, forced expiratory volume in one second /forced vital capacity.
Correlation Between LE8 and All-Cause Mortality in COPD Patients
Table 4 presents the hazard ratios (HR) for LE8 scores in relation to all-cause mortality among COPD patients across three different models. In model 1, an increase in LE8 levels over time was associated with a significant reduction in all-cause mortality for COPD patients (unadjusted HR: 0.95; 95% CI, 0.94–0.97; P < 0.001). Conversely, in model 3, even after adjusting for factors such as age, sex, race, alcohol consumption, education level, and poverty rate, a low all-cause mortality rate in COPD patients remained statistically associated with high LE8 levels.(adjusted HR: 0.96; 95% CI, 0.95–0. 97; P < 0.001).
Table 4.
Association of Life’ Essential 8 with Mortality from the NHANES2001–2011
| No. of Participants | No. of deaths | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| All-cause mortality | ||||||||
| Tertile 1 | 270 | 116 | Reference | - | Reference | - | Reference | - |
| Tertile 2 | 259 | 77 | 0.54(0.38,0.77) | <0.001 | 0.53(0.39,0.73) | <0.0001 | 0.73(0.55,0.99) | 0.041 |
| Tertile 3 | 256 | 52 | 0.33(0.22,0.51) | <0.0001 | 0.30(0.20,0.43) | <0.0001 | 0.40(0.25,0.63) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||||
| Per 10 score increment | 0.95(0.94,0.97) | <0.001 | 0.95(0.93,0.96) | <0.001 | 0.96(0.95,0.97) | <0.001 | ||
| CLRD mortality | ||||||||
| Tertile 1 | 178 | 24 | Reference | - | Reference | - | Reference | - |
| Tertile 2 | 202 | 20 | 0.41(0.17,0.97) | 0.042 | 0.35 (0.17,0.75) | 0.007 | 0.57 (0.29,1.13) | 0.004 |
| Tertile 3 | 216 | 12 | 0.22(0.07,0.74) | 0.014 | 0.17 (0.06,0.48) | <0.001 | 0.22 (0.08,0.61) | <0.0001 |
| P for trend | 0.017 | <0.001 | 0.002 | |||||
| Per 10 score increment | 0.94(0.9,0.99) | 0.021 | 0.93 (0.89,0.98) | 0.004 | 0.96(0.93, 0.99) | 0.016 | ||
| CVD mortality | ||||||||
| Tertile 1 | 184 | 30 | Reference | Reference | - | Reference | - | |
| Tertile 2 | 192 | 10 | 0.37 (0.18,0.77) | 0.008 | 0.35 (0.16,0.78) | 0.011 | 0.38 (0.18,0.84) | |
| Tertile 3 | 214 | 10 | 0.21 (0.01,0.49) | <0.001 | 0.18 (0.08,0.4) | <0.0001 | 0.21 (0.01,0.45) | |
| P for trend | <0.001 | <0.001 | <0.001 | |||||
| Per 10 score increment | 0.93 (0.9,0.96) | <0.0001 | 0.92(0.9,0.95) | <0.0001 | ||||
| Cancer mortality | ||||||||
| Tertile 1 | 182 | 28 | Reference | Reference | - | Reference | - | |
| Tertile 2 | 206 | 24 | 0.7(0.36,1.36) | 0.3 | 0.61(0.34,1.12) | 0.11 | 0.83 (0.43,1.59) | 0.573 |
| Tertile 3 | 217 | 13 | 0.3(0.13,0.15) | 0.01 | 0.25(0.1,0.64) | 0.39 (0.13,1.12) | 0.08 | |
| P for trend | 0.005 | 0.002 | 0.06 | |||||
| Per 10 score increment | 0.94(0.96,0.98) | <0.0001 | 0.95(0.93,0.97) | <0.0001 | 0.97(0.94, 0.99) | |||
Notes: Model 1: Unadjusted. Model 2: adjusts for age, sex, and ethnicity. Model 3: adjusts for age, sex, ethnicity, education, family income-poverty ratio, and drinking status. Bold text signifies that 1. the variations in all-cause mortality, CLRD mortality, and CVD mortality among models 1, 2, and 3 are statistically significant. 2. the differences in all-cause mortality, CLRD mortality, CVD mortality, and cancer mortality between Tertile 1, 2, and 3 are also statistically significant.
Abbreviations: CI, confidence interval; HR, hazard ratio; CLRD, Chronic lower respiratory disease; CVD, cardiovascular disease.
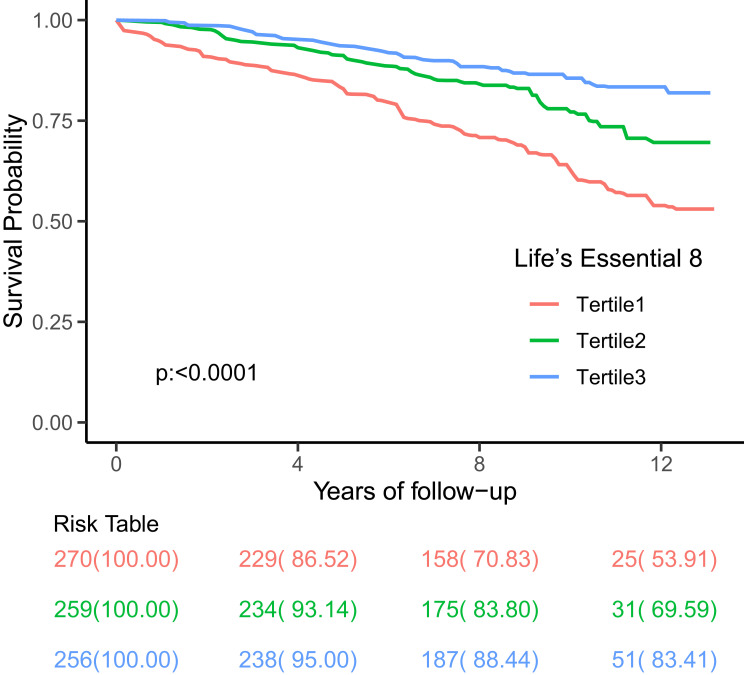
We also examined the connection between the LE8 score and mortality rates from cardiovascular diseases, cancer, and lower respiratory diseases in patients with COPD. In model 1, the LE8 score was associated with reduced mortality from respiratory diseases (unadjusted HR: 0.94; 95% CI, 0.90–0.99; P < 0.05), cardiovascular diseases (unadjusted HR: 0.93; 95% CI, 0.90–0.96; P < 0.0001), and cancer (unadjusted HR: 0.94; 95% CI, 0.96–0.98; P < 0.0001) among COPD patients, indicating that mortality rates decreased as LE8 levels increased. In model 2, patients with low LE8 scores showed a statistically significant association with mortality from respiratory diseases (adjusted HR: 0.93; 95% CI, 0.89–0.98; P < 0.05), cardiovascular diseases (adjusted HR: 0.92; 95% CI, 0.89–0.95; P < 0.0001), and cancer (adjusted HR: 0.95; 95% CI, 0.93–0.97; P < 0.0001) even after adjusting for confounding factors such as age, sex, and race. After further adjustments for factors like alcohol consumption, education level, and poverty rate in model 2, the LE8 score was significantly linked to lower respiratory disease (adjusted HR: 0.96; 95% CI, 0.93–0.99; P < 0.05) and cardiovascular disease mortality (adjusted HR: 0.92; 95% CI, 0.90–0.95; P < 0.0001). However, different LE8 levels did not show a significant correlation with cancer mortality (adjusted HR: 0.97; 95% CI, 0.94–0.99; P > 0.05). The survival curve analysis further indicates that individuals with a high LE8 score have a better survival rate and longer lifespan compared to those with a low LE8 score.(Figure 2 displays the Kaplan-Meier survival curves for all-cause mortality in people with COPD).
Figure 2.
All-cause Mortality in Subjects with COPD by LE8 Metrics, p<0.0001. LE8=Life Essential 8. Tertile 1,2,3 are the same as low cardiovascular health (0–420), moderate cardiovascular health (421–530), and high cardiovascular health (531–780), respectively.
Discussion
As far as we know, this is the inaugural research examining the relationship between the LE8 score, a new metric for assessing cardiovascular health (CVH), and overall and cardiovascular disease (CVD) mortality in US adults with chronic obstructive pulmonary disease (COPD). Additionally, individuals with a high LE8 score had adjusted risks for all-cause mortality that were 60% lower (p<0.001) and 79% lower for CVD mortality (p<0.001) compared to those with a low LE8 score.
There are currently a number of methods available for COPD patients to estimate their mortality, including ADO, BODE, BODEX, CODEX, DOSE, DECAF,12 Diffusion capacity,13 APACHE II,14 FI-Lab,15 PNI.16 In recent years, various other measures have also been shown to provide a reference point for predicting the prognosis of patients with COPD.
Our findings indicate that around 80% of the participants were either current or former smokers. Prolonged exposure to cigarette smoke can cause ongoing inflammation and oxidative stress in the lungs, leading to continuous lung repair and remodeling.17 And Long-term cigarette smoking’s primary effect is airflow restriction in both the big and small airways.18 It is reported that Quit smoking is useful to improve symptoms, respiratory function and metabolic parameters in the short term.19 So it is unusual that our study found smoking status is strongly related to mortality among the participants with COPD.
Many epidemiological studies suggest that dietary patterns similar to the Mediterranean diet, which emphasize high consumption of fruits, vegetables, whole grains, and dietary fiber, are associated with a reduced risk of developing the disease. In contrast, Western dietary patterns that include high amounts of meat or processed meat, saturated fats, and desserts are associated with a higher risk of COPD.20 In addition, a meta-analysis of patients with moderate to severe COPD revealed a reduction in the frequency of acute exacerbations following vitamin D administration.21 Additionally, research shows that heavy drinkers experience a notable decline in lung function, while those who drink low to moderate amounts tend to have higher FEV1 levels.22 Consistently, We found that approximately 90% of participants were current or former drinkers.
Research indicates that body mass index (BMI) can influence pneumonia, inflammation, and related biological markers. A prospective study with 1659 patients suffering from COPD revealed that various BMI categories corresponded to distinct clinical symptoms and conjunctivitis in these patients. The lowest BMI group exhibited the highest mortality risk, primarily due to respiratory-related deaths.23. Similarly, a META analysis also found that a low BMI was a risk factor for accelerating the decline in lung function in COPD patients, while a high BMI had a protective effect. A cohort study found a decrease in BMI associated with more than 10 days of hospitalization for COPD patients.24 In our research, we found that managing BMI did not correlate with mortality rates in patients with COPD. There is a scarcity of information concerning the relationship between BMI and mortality risk in individuals with COPD.
Various epidemiological studies indicate that diabetes is more common among COPD patients compared to the control group and is associated with poorer outcomes for the condition.25 Studies show that type 2 diabetes, linked to various harmful physiological changes such as oxidative-antioxidant imbalance, insulin resistance, alterations in fat metabolism, and inflammatory responses, is likely to be triggered by COPD.26 In advanced COPD, a lack of oxygen is common and can lead to increased fat breakdown, reduced insulin sensitivity, and resistance to sugar.27 However, it is still unknown how corticosteroid medication affects blood sugar levels in COPD patients.28 Nonetheless, individuals with type 2 diabetes are more likely to have COPD compared to the reference group, and they face a greater risk of mortality from respiratory and pulmonary diseases than those without COPD.29 Our study found Blood glucose is reversely related to mortality among the participants with COPD.
Studies have shown that lipid molecules and their metabolic processes experience significant changes in COPD, which ultimately play a role in the advancement of the disease by increasing the production of inflammatory substances, immune regulation, or cell death.30 Research has demonstrated that the inflammation pathway is disturbed and that there is an increase in inflammatory lipid media in the lung tissue of COPD patients.31 A cohort study found that individuals with hypoglycemia had a 1.48 times greater risk of developing COPD compared to those in the non-hypoglycemic control group32(95% CI 1.44–1.53, p<0.001). Our research indicates that participants with COPD who have high total cholesterol (TC) but low low-density lipoprotein cholesterol (LDL-C) levels are associated with increased mortality. However, it remains uncertain whether this heightened risk is a result of disease progression or disruptions in cholesterol regulation.
Individuals with COPD may suffer from ongoing low oxygen levels as well as intermittent oxygen shortages. A lack of oxygen can increase sympathetic nerve activity, which can subsequently influence heart rate and average arterial pressure in various ways.33 The study found that COPD patients showed significantly greater contraction and hypertension, along with altered pulse characteristics, when compared to the control group.34 Likewise, Esther I. Schwarz and her team found that patients with moderate to severe COPD showed notable increases in heart rate and blood pressure when subjected to low-pressure oxygen deficiency at medium altitude, in contrast to the healthy control group.35 These alterations were linked to a reduction in stress reflex sensitivity and a rise in blood pressure variability. A prospective study involving 1728 participants revealed that having COPD in patients with elevated blood pressure notably heightened the risk of stroke, heart attack, and serious cardiovascular incidents.36 In our study, 54% of the participants have a history of hypertension, which is associated with mortality among them.
The LE8 framework covers various systems, including cardiovascular, endocrine-metabolic, gastrointestinal, pulmonary, neuropsychiatric, and others, meeting the clinical need for a thorough assessment of COPD, which is a complex condition. This research indicated that patients with a high LE8 score had a 60% reduced risk of dying from any cause compared to those with a low LE8 score. Additionally, we explored the connection between LE8 and mortality in COPD patients due to respiratory, cancer, and cardiovascular diseases. Our findings revealed that in COPD patients, higher LE8 scores were also associated with a lower risk of death from respiratory and cardiovascular diseases: compared to COPD patients with low LE8 scores, those with high LE8 scores had an adjusted hazard ratio (HR) of 0.22 (95% CI, 0.08–0.61) for respiratory disease deaths and an adjusted HR of 0.21 (95% CI, 0.01–0.45) for cardiovascular disease deaths.
It is crucial to consider the limitations of this study. Firstly, our overall sample size was reduced because not all individuals from the NHANES 2007–2012 dataset had both spirometry and LE8 measurements. Additionally, there was a lower representation of Asian participants, indicating a need for more research on Asian COPD patients and LE8. Secondly, self-report questionnaires are the main source of measurement errors in LE8, making them unsuitable for assessing health behavior indicators. Moreover, personal characteristics and health factors may also contain inaccuracies, similar to many epidemiological studies. This could lead us to overestimate the actual risks present in the situation due to the unpredictability and constraints of these factors. Lastly, the data for this cross-sectional study was derived from an observational survey, future longitudinal studies are needed to further explore the potential relationship between LE8 and COPD mortality.
As a result, we conclude that there is a link between higher LE8 scores in COPD patients and improved health outcomes. Unlike other scoring systems, the LE8 score provides important insights into the cardiovascular health of those with COPD. It is particularly useful for predicting the prognosis of patients with mild to moderate COPD, which influences their future preventive and diagnostic approaches. Additionally, we affirm that adequate sleep duration plays a positive role in reducing mortality risk among COPD patients. Research indicates that both sleep duration and quality are related to various chronic respiratory conditions, yet they are not currently considered in evaluating the functional status of COPD patients. This is a new aspect of the LE8 score. This finding has important implications for public health, emphasizing the necessity of regularly monitoring lifestyle and cardiovascular disease risk factors in COPD patients. This includes reducing smoking, staying active, following a healthy diet, and managing blood pressure and glucose levels.
Acknowledgments
We are grateful to Dr. Jing Zhang for her assistance in revising the article presented in this paper. This work was supported by Grants from the Natural Science Foundation of China (No. 81970084).
Data Sharing Statement
Publicly accessible data from the National Health and Nutrition Examination Survey (NHANES) can be found at https://wwwn.cdc.gov/nchs/nhanes.Chang Sun can be contacted with any additional questions.
Statement of Ethics
The study procedure was approved by the Ethics Review Board of the National Center for Health Statistics and every participant signed informed consent. All methods were performed in accordance with the 1964 helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval to conduct studies using publicly available databases is exempt under the following legislation:
Items 1 and 2 of Article 32 of “the Measures for Ethical Review of Life Science and Medical Research Involving Human Subjects”, which was reviewed by the National Science and Technology Ethics Committee, approved by the State Council of China, and jointly promulgated by the National Health Commission, the Ministry of Education, the Ministry of Science and Technology and the State Administration of Traditional Chinese Medicine on Feb. 18, 2023.
Author Contributions
Chang Sun and Xinxin Zhang contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1. © 2022, 2023 Global initiative for chronic obstructive lung disease, available from global initiative for chronic obstructive lung disease - global initiative for chronic obstructive lung disease - GOLD (goldcopd.org), published in Deer Park, IL, USA.
- 2.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi: 10.2147/COPD.S161555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D;.American Heart Association Strategic Planning Task Force and Statistics Committee, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 7.Crisan LL, Lee HM, Fan W, et al. Association of cardiovascular health with mortality among COPD patients: national health and nutrition examination survey III. Respir Med Res. 2021;80:100860. doi: 10.1016/j.resmer.2021.100860 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: a presidential advisory from the American heart association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Ma X, Lin P, et al. Predictive value of cardiovascular health score for health outcomes in patients with PCI: comparison between life’s simple 7 and life’s essential 8. Int J Environ Res Public Health. 2023;20(4):3084. doi: 10.3390/ijerph20043084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention. National health and nutrition examination survey: questionnaires, datasets, and related documentation, national center for health statistics. Available from: wwwn.cdc.gov/nchs/nhanes/Nhanes3/Default.aspx. Accessed November 25, 2024.
- 11.National Center for Health Statistics Division of Analysis and Epidemiology. Continuous NHANES Public-Use Linked Mortality Files 2019. 2019. [cited 2023 Jul 5,]. Available from: https://www.cdc.gov/nchs/404.htm. Accessed November 25, 2024.
- 12.Owusuaa C, Dijkland SA, Nieboer D, et al. Predictors of mortality in chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med. 2022;22(1):125. doi: 10.1186/s12890-022-01911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian A, Putcha N, MacIntyre NR, et al. Diffusing capacity and mortality in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2023;20(1):38–46. doi: 10.1513/AnnalsATS.202203-226OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja W, Ahmed N, Rizvi NA, et al. Comparison of DECAF (dysponea, eosinopenia, consolidation, acidaemia, and atrial fibrillation) and APACHE II (acute physiology and chronic health evaluation ii) scoring system to predict mortality among patients with acute exacerbation of chronic obstructive pulmonary disease. J Pak Med Assoc. 2021;71(8):1935–1939. doi: 10.47391/JPMA.618 [DOI] [PubMed] [Google Scholar]
- 15.Gu JJ, Liu Q, Zheng LJ. A frailty assessment tool to predict in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1093–1100. doi: 10.2147/COPD.S300980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng JC, Nie F, Li YJ, et al. Prognostic nutritional index as a predictor of 30-day mortality among patients admitted to intensive care unit with acute exacerbation of chronic obstructive pulmonary disease: a single-center retrospective cohort study. Med Sci Monit. 2022;28:e934687. doi: 10.12659/MSM.934687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strzelak A, Ratajczak A, Adamiec A, et al. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi: 10.3390/ijerph15051033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Q, Zhao YY, Zeng YQ, et al. The characteristics of airflow limitation and future exacerbations in different GOLD groups of COPD patients. Int J Chronic Obstr Pulm Dis. 2021;16:1401–1412. doi: 10.2147/COPD.S309267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzuto A, Ricci A, D’Ascanio M, et al. Short-term benefits of smoking cessation improve respiratory function and metabolism in smokers. Int J Chron Obstruct Pulmon Dis. 2023;18:2861–2865. doi: 10.2147/COPD.S423148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng PF, Shu L, Si CJ, et al. Dietary patterns and chronic obstructive pulmonary disease: a meta-analysis. COPD. 2016;13(4):515–522. doi: 10.3109/15412555.2015.1098606 [DOI] [PubMed] [Google Scholar]
- 21.Jolliffe DA, Camargo CA Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. doi: 10.1016/S2213-8587(21)00051-6 [DOI] [PubMed] [Google Scholar]
- 22.Kaluza J, Harris HR, Linden A, et al. Alcohol consumption and risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Am J Epidemiol. 2019;188(5):907–916. doi: 10.1093/aje/kwz020 [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine. 2016;95(28):e4225. doi: 10.1097/MD.0000000000004225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. doi: 10.1186/s12931-019-1209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogliani P, Lucà G, Lauro D. Chronic obstructive pulmonary disease and diabetes. COPD Res Pract. 2015;1(1):3. doi: 10.1186/s40749-015-0005-y [DOI] [Google Scholar]
- 26.Wells CE, Baker EH. Metabolic syndrome and diabetes mellitus in COPD. In: Rabe KF, Wedzicha JA, Wouters EFM, editors. COPD and Comorbidity. Sheffield, UK: European Respiratory Society; 2013:117–134. [Google Scholar]
- 27.Cazzola M, Rogliani P, Calzetta L, et al. Targeting mechanisms linking COPD to type 2 diabetes mellitus. Trends Pharmacol Sci. 2017;38(10):940–951. doi: 10.1016/j.tips.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Patel R, Naqvi SA, Griffiths C, et al. Systemic adverse effects from inhaled corticosteroid use in asthma: a systematic review. BMJ Open Respir Res. 2020;7(1):e000756. doi: 10.1136/bmjresp-2020-000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caughey GE, Roughead EE, A.i V, et al. Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabet Res Clin Pract. 2010;87(3):385–393. doi: 10.1016/j.diabres.2009.10.019 [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Li Z, Dong L, et al. Lipid metabolism in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1009–1018. doi: 10.2147/COPD.S196210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pena KB, Ramos CO, Soares NP, et al. The administration of a high refined carbohydrate diet promoted an increase in pulmonary inflammation and oxidative stress in mice exposed to cigarette smoke. Int J Chron Obstruct Pulmon Dis. 2016;11:3207–3217. doi: 10.2147/COPD.S119485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HY, Hu LY, Chen HJ, et al. Increased risk of chronic obstructive pulmonary disease in patients with hyperlipidemia: a nationwide population-based cohort study. Int J Environ Res Public Health. 2022;19(19):12331. doi: 10.3390/ijerph191912331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leuenberger UA, Brubaker D, Quraishi SA, et al. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121(1–2):87–93. doi: 10.1016/j.autneu.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 34.Arslan S, Yildiz G, Özdemir L, et al. Association between blood pressure, inflammation and spirometry parameters in chronic obstructive pulmonary disease. Korean J Intern Med. 2019;34(1):108–115. doi: 10.3904/kjim.2017.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz EI, Latshang TD, Furian M, et al. Blood pressure response to exposure to moderate altitude in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:659–666. doi: 10.2147/COPD.S194426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chazova IE, Lazareva NV, Oshchepkova EV. Arterial hypertension and chronic obstructive pulmonary disease: clinical characteristics and treatment efficacy (according to the national register of arterial hypertension). Ter Arkh. 2019;91(3):4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly accessible data from the National Health and Nutrition Examination Survey (NHANES) can be found at https://wwwn.cdc.gov/nchs/nhanes.Chang Sun can be contacted with any additional questions.