Abstract
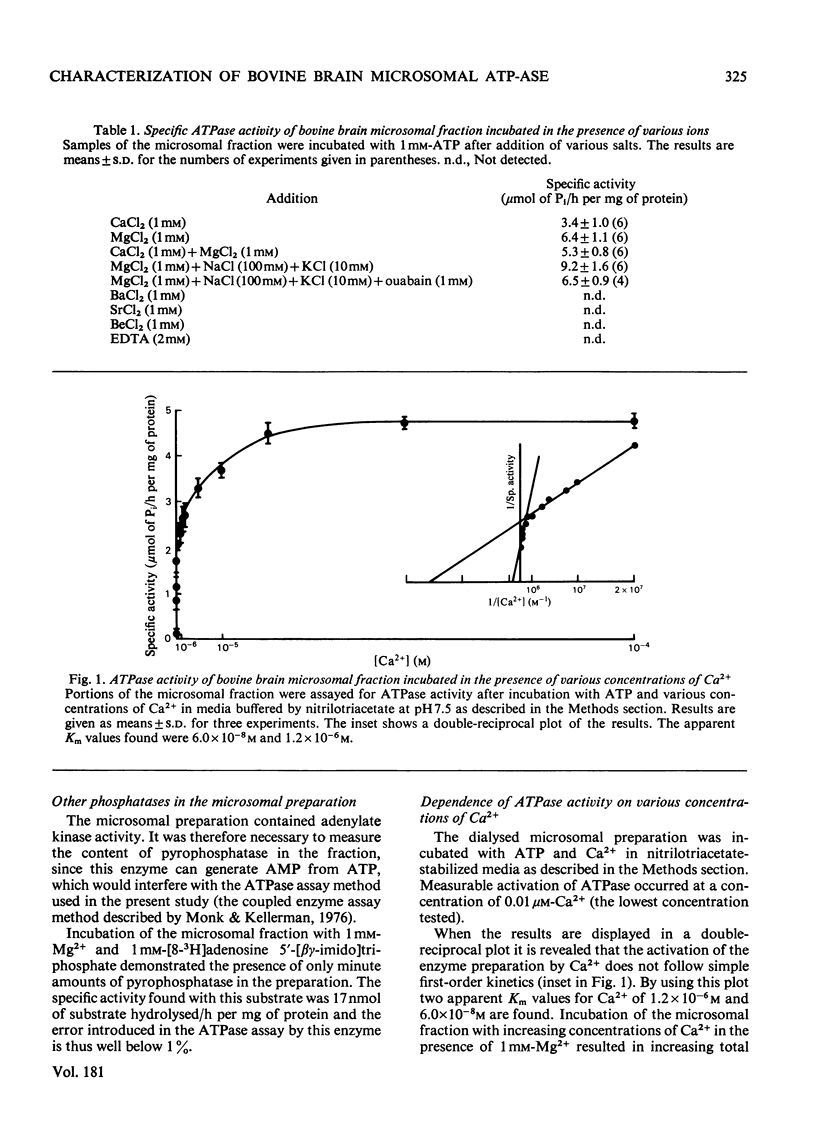
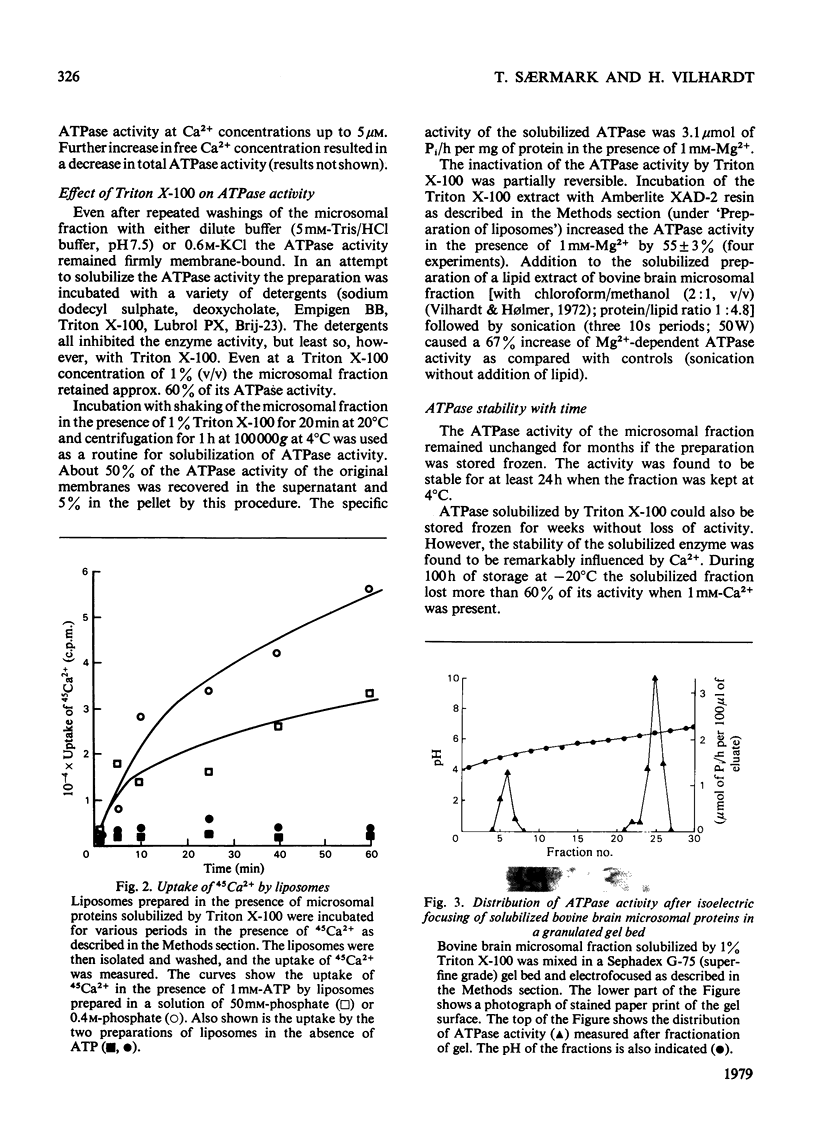
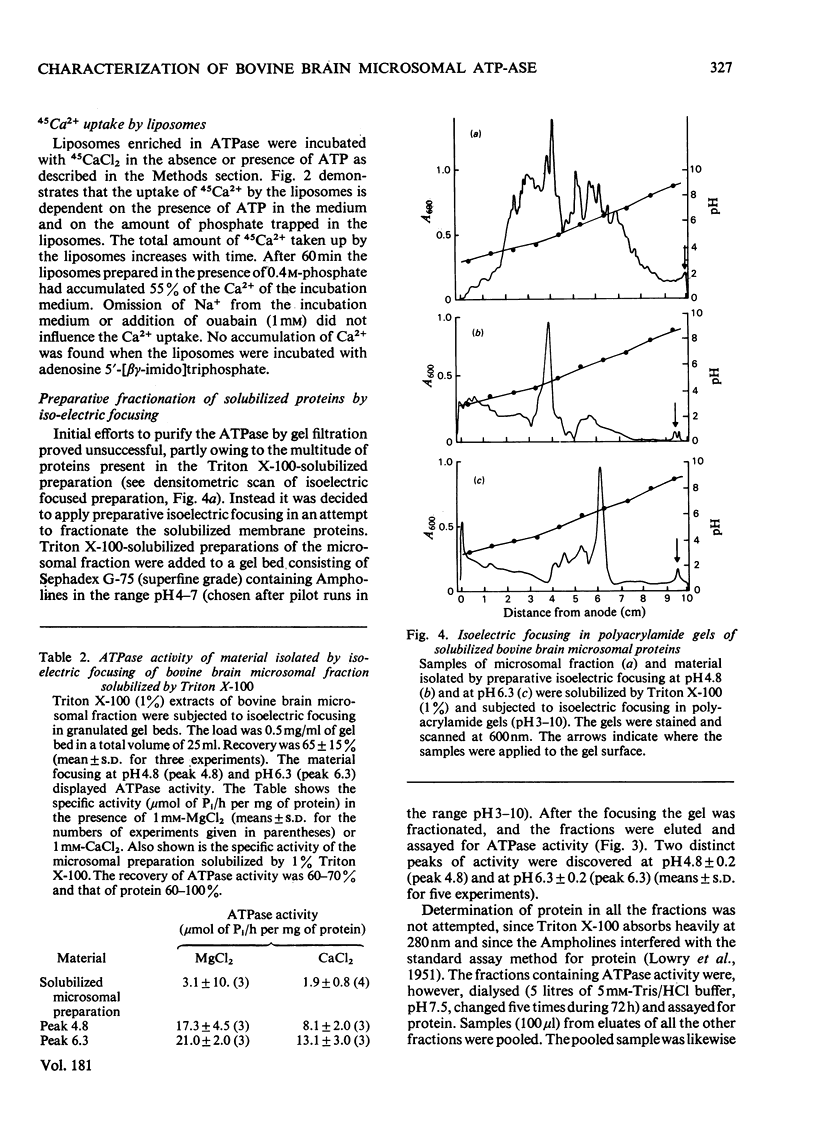
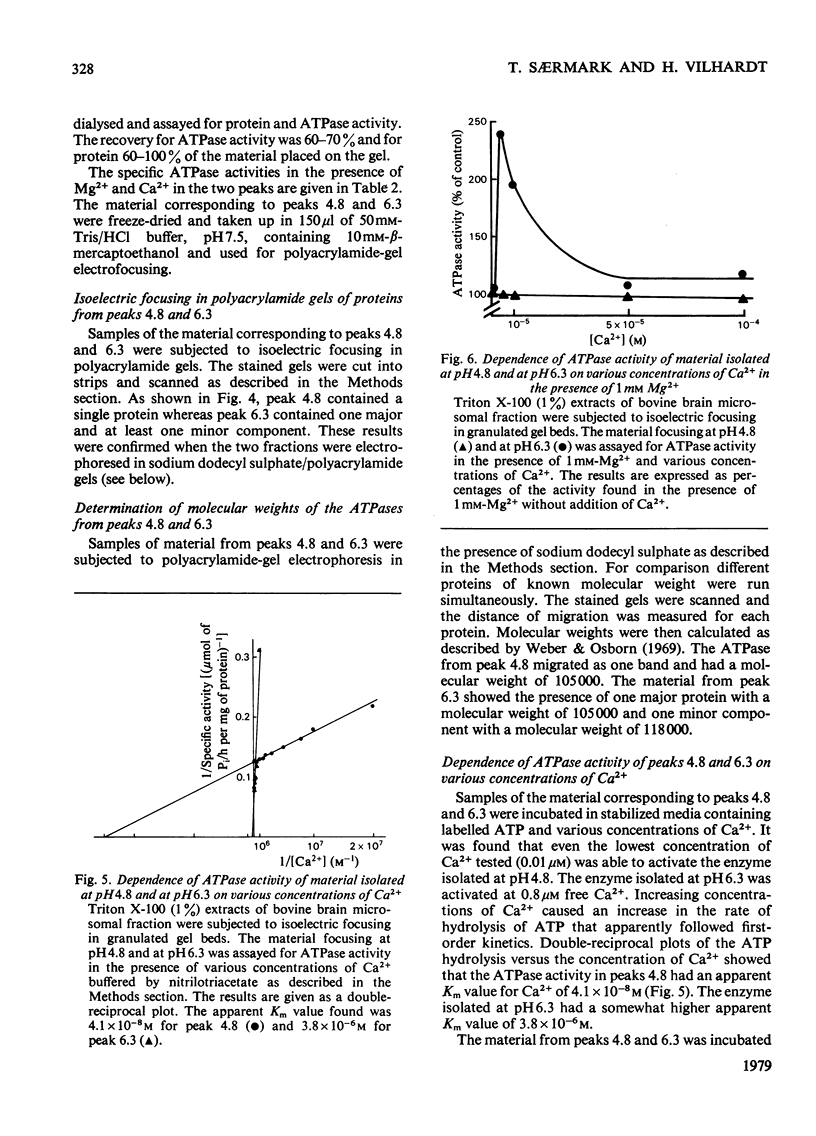
Microsomal fraction was prepared by ultracentrifugation of homogenates of cortical tissue from bovine brains. The preparation displayed ATPase (adenosine triphosphatase) activity in the presence of Mg2+ (6.4μmol of Pi/h per mg of protein) and Ca2+ (3.4μmol of Pi/h per mg of protein). Kinetic analysis of the activation of the enzyme preparation by Ca2+ resulted in the demonstration of two apparent Km values for Ca2+ (6.0×10−8m and 1.2×10−6m). Treatment of the microsomal membranes with Triton X-100 resulted in solubilization of the ATPase, though with some loss of activity. The solubilized microsomal proteins were incorporated into liposomes. By incubation of the liposomes in media containing 45Ca2+ an ATP-dependent uptake of Ca2+ was demonstrated. The solubilized preparation was subjected to preparative isoelectric focusing in granulated gel beds. Two distinct peaks of Mg2+- and Ca2+-dependent ATPase activity were observed at pH4.8 (peak 4.8) and at pH6.3 (peak 6.3). The material isolated in peaks 4.8 and 6.3 was focused in polyacrylamide gel with pH gradients. The material corresponding to peak 4.8 consisted of a single protein, whereas peak 6.3 contained one major and at least one minor protein. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis confirmed these results and indicated that the major component of peak 4.8 and the protein of peak 6.3 both had a molecular weight of 105000. The material in peaks 4.8 and 6.3 was assayed for ATPase activity in the presence of various concentrations of Ca2+. Kinetic analysis of the results for peak 4.8 demonstrated an apparent Km value for Ca2+ of 4.1×10−8m. The enzyme isolated at pH6.3 had an apparent Km value of 3.8×10−6m. However, when the material from peak 4.8 was incubated in the presence of 1mm-Mg2+ the ATPase could not be activated by Ca2+.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Carrier-mediated sodium-dependent and calcium-dependent calcium efflux from pinched-off presynaptic nerve terminals (synaptosomes) in vitro. Biochim Biophys Acta. 1976 Jan 21;419(2):295–308. doi: 10.1016/0005-2736(76)90355-2. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Ishida A., Poisner A. M. The effect of metabolic inhibitors on the release of vasopressin from the isolated neurohypophysis. J Physiol. 1965 Dec;181(4):753–759. doi: 10.1113/jphysiol.1965.sp007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J. Properties of the Ca(2+)-ATPase activity of mammalian synaptic membrane preparations. J Neurochem. 1976 Nov;27(5):1277–1279. doi: 10.1111/j.1471-4159.1976.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Flodgaard H., Fleron P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of (Mg2+), (K+), and ionic strength determined from equilibrium studies of the reaction. J Biol Chem. 1974 Jun 10;249(11):3465–3474. [PubMed] [Google Scholar]
- Flodgaard H., Torp-Pedersen C. A calcium ion-dependent adenosine triphosphate pyrophosphohydrolase in plasma membrane from rat liver. Demonstration that the adenosine triphosphate analogues adenosine 5'-[betagamma-imido]triphosphate and adenosine 5'-[betagamma-methylene]-triphosphate are substrates for the enzyme. Biochem J. 1978 Jun 1;171(3):817–820. doi: 10.1042/bj1710817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby B., Capito K., Egeberg J., Hedeskov C. J. Ca-activated ATPase activity in subcellular fractions of mouse pancreatic islets. Am J Physiol. 1976 Feb;230(2):441–448. doi: 10.1152/ajplegacy.1976.230.2.441. [DOI] [PubMed] [Google Scholar]
- Germain M., Proulx P. Adenosinetriphosphatase activity in synaptic vesicles of rat brain. Biochem Pharmacol. 1965 Dec;14(12):1815–1819. doi: 10.1016/0006-2952(65)90271-6. [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Meligeni J. A. Neurotransmitter accumulation and calcium-dependent release from different regions of rat brain. Life Sci. 1977 Dec 15;21(12):1837–1843. doi: 10.1016/0024-3205(77)90166-7. [DOI] [PubMed] [Google Scholar]
- Hosie R. J. The localization of adenosine triphosphatases in morphologically characterized subcellular fractions of guinea-pig brain. Biochem J. 1965 Aug;96(2):404–412. doi: 10.1042/bj0960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles A. F., Racker E. Properties of a reconstituted calcium pump. J Biol Chem. 1975 May 10;250(9):3538–3544. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Monk B. C., Kellerman G. M. A rapid method for the assay of mitochondrial ATPase activity. Anal Biochem. 1976 May 21;73(1):187–191. doi: 10.1016/0003-2697(76)90153-6. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Kosakai M., Konishi K. Some properties of brain microsome adenosine triphosphatases activated by magnesium and calcium. Arch Biochem Biophys. 1967 Apr;120(1):15–21. doi: 10.1016/0003-9861(67)90592-9. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Douglas W. W. A possible mechanism of release of posterior pituitary hormones involving adenosine triphosphate and an adenosine triphosphatase in the neurosecretory granules. Mol Pharmacol. 1968 Sep;4(5):531–540. [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. I. Thin-layer isoelectric focusing of proteins. Biochim Biophys Acta. 1973 Feb 21;295(2):412–428. doi: 10.1016/0005-2795(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. (Ca + Mg)-stimulated ATPase activity of a rat brain microsomal preparation. Arch Biochem Biophys. 1976 Sep;176(1):366–374. doi: 10.1016/0003-9861(76)90176-4. [DOI] [PubMed] [Google Scholar]
- Russell J. T., Thorn N. A. Isolation and purification of calcium-binding proteins from bovine neurohypophyses. Biochim Biophys Acta. 1977 Apr 25;491(2):398–408. doi: 10.1016/0005-2795(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Trotta E. E., de Meis L. ATP-dependent calcium accumulation in brain microsomes. Enhancement by phosphate and oxalate. Biochim Biophys Acta. 1975 Jun 25;394(2):239–247. doi: 10.1016/0005-2736(75)90262-x. [DOI] [PubMed] [Google Scholar]
- Vilhardt H., Baker R. V., Hope D. B. Isolation and protein composition of membranes of neurosecretory vesicles and plasma membranes from the neural lobe of the bovine pituitary gland. Biochem J. 1975 Apr;148(1):57–65. doi: 10.1042/bj1480057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhardt H., Holmer G. Lipid composition of membranes of secretory granules and plasma membranes from bovine neurohypophyses. Acta Endocrinol (Copenh) 1972 Dec;71(4):638–648. doi: 10.1530/acta.0.0710638. [DOI] [PubMed] [Google Scholar]
- Vilhardt H., Hope D. B. Adenosine triphosphatase activity in the neural lobe of the bovine pituitary gland. Biochem J. 1974 Oct;143(1):181–190. doi: 10.1042/bj1430181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- de Meis L., Rubin-Altschul M., Machado R. D. Comparative data of Ca2+ transport in brain and skeletal muscle microsomes. J Biol Chem. 1970 Apr 25;245(8):1883–1889. [PubMed] [Google Scholar]



