Abstract
Several reports have described the existence of synergy between neutralizing monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 (HIV-1). Synergy between human MAbs b12, 2G12, 2F5, and 4E10 in neutralization of primary isolates is of particular interest. Neutralization synergy of these MAbs, however, has not been studied extensively, and the mechanism of synergy remains unclear. We investigated neutralization synergy among this human antibody set by using the classical approach of titrating antibodies mixed at a fixed ratio as well as by an alternative, variable ratio approach in which the neutralization curve of one MAb is assessed in the presence and absence of a fixed, weakly neutralizing concentration of a second antibody. The advantage of this second approach is that it does not require mathematical analysis to establish synergy. No neutralization enhancement of any of the MAb combinations tested was detected for the T-cell-line-adapted molecular HIV-1 clone HxB2 using both assay formats. Studies of primary isolates (89.6, SF162, and JR-CSF) showed neutralization synergy which was relatively weak, with a maximum of two- to fourfold enhancement between antibody pairs, thereby increasing neutralization titers about 10-fold in triple and quadruple antibody combinations. Analysis of b12 and 2G12 binding to oligomeric envelope glycoprotein by using flow cytometry failed to demonstrate cooperativity in binding between these two antibodies. The mechanism by which these antibodies synergize is, therefore, not yet understood. The results lend some support to the notion that an HIV-1 vaccine that elicits moderate neutralizing antibodies to multiple epitopes may be more effective than hereto supposed, although considerable caution in extrapolating to a vaccine situation is required.
The induction of broadly neutralizing antibodies directed against conserved and accessible regions on the human immunodeficiency virus type 1 (HIV-1) envelope spike is a highly desirable property of a vaccine against HIV-1. Four relatively conserved epitopes have been defined by a set of five neutralizing human monoclonal antibodies (MAbs). Two antibodies recognize epitopes located on the gp120 surface unit of the envelope spike: MAb b12 is directed against an epitope overlapping the CD4 binding site (7) and MAb 2G12 recognizes a unique epitope in a carbohydrate-rich region on the outer domain of gp120 (54). Three antibodies recognize epitopes located on the membrane-proximal external region of the gp41 transmembrane protein: MAb 2F5 has been mapped to a region overlapping the conserved sequence ELDKWA (30) and MAb Z13 and 4E10 recognize an epitope involving the sequence NWF(D/N)IT located carboxy terminal of the 2F5 epitope (4, 58).
Passive transfer studies using MAbs b12, 2F5, and 2G12 have shown that these antibodies protect against HIV-1 challenge in animal models when present at sufficient concentrations prior to or shortly after exposure (2, 13, 17, 24, 26, 33, 36). Significantly, it has been demonstrated that, when administered systemically, the antibodies can effectively protect against mucosal challenge (2, 26, 36). A strong correlation is observed between neutralization in vitro and protection with sterile protection generally occurring at serum neutralizing antibody titers greater than approximately 1:100 (32, 35, 36). This correlation between neutralization and protection appears to hold independent of the animal model, challenge route, or HIV-1 challenge virus used (36). It should be noted that an exception has been found in a passive transfer study with anti-gp120 MAb 2G12 in which protection against vaginal challenge with a simian-human immunodeficiency virus (SHIV), containing a primary isolate env gene, occurred at a more modest neutralizing antibody serum titer (26). Overall, however, most of the macaque data indicate that sterile protection against SHIVs corresponds to complete antibody neutralization of the challenge virus (24, 36, 47). Similar conclusions were reached for HIV-1 challenge of hu-PBL-SCID mice (18, 33) and SHIV challenge of macaques (2) by using viruses containing the env genes of T-cell-line-adapted viruses.
A well-known characteristic of the HIV-1 envelope glycoprotein is its extreme variability. It has thus been recognized that even relatively conserved epitopes on HIV-1, such as the CD4 binding site, show some variability between different isolates (31, 40, 56). An antibody targeted to one of these conserved sites can then be expected to pay some price for its breadth of reactivity by a loss in affinity for the envelope spike of any one particular isolate. Indeed, the moderate neutralizing ability of these MAbs (typically of the order of 10 to 50 μg/ml) for many isolates suggest this is probably so. These moderate neutralizing activities translate into relatively high MAb concentrations for sterile protection; typically serum concentrations of the order of 1 to 5 mg/ml must be achieved (36). To expect that sustained antibody concentrations of this magnitude could be induced by a vaccine is unrealistic. However, antibody responses elicited by a vaccine would be polyclonal, not monoclonal, and would ideally target a number of broadly neutralizing epitopes. The protection threshold could then indeed be achieved at lower antibody concentrations if the antibodies in the cocktail or polyclonal serum act cooperatively or synergistically to increase their effective neutralization titers. Synergy in antibody neutralization of HIV-1, however, is controversial, and no mechanism has yet been demonstrated.
A number of studies have addressed the neutralization properties of antibody combinations against HIV-1, mostly using neutralization sensitive T-cell-line-adapted viruses. Moderate synergy for neutralization of an SHIV containing the envelope of a T-cell-line-adapted virus has been described for 2F5 and 2G12 (22, 23), 2G12 and b12, and b12 and 2F5 (22). Synergistic neutralization of T-cell-line-adapted HIV-1 has also been described for antibodies against the CD4 binding site and V2 loop or V3 loop (21, 27, 42, 52, 55). Synergy in neutralization of primary HIV-1 isolates has been less extensively studied (24, 25, 42). A mathematical method to determine dose-effect relationships of drug combinations has been developed based on the median effect principle defined by Chou and Talalay (11); a combination index (CI) is calculated to serve as an indicator of synergy (11). This method has been applied in a number of studies to analyze HIV-1 neutralization by antibody combinations (1, 21–23, 27, 42, 52). The CI does not, however, always predict synergy by HIV-1 neutralizing antibodies accurately (42). The magnitude of synergy predicted by the CI furthermore appears to vary with the concentration of certain antibody combinations or indicates, counterintuitively, antagonism at low antibody concentrations (1, 22, 52). The latter is unexpected and may be due to limitations of the mathematical model but, if correct, might pose difficulties for vaccination in which the magnitude of antibody concentrations cannot be effectively controlled. A correlation between synergy and virus heterogeneity has furthermore been suggested (55). Another, less widely used mathematical model (5) was applied in one study to demonstrate neutralization synergy between MAbs 2F5 and 2G12 (25).
Here we show that moderate synergy exists between combinations of broadly neutralizing antibodies in primary isolate neutralization assays by using two assay formats and using biologically as well as molecularly cloned virus. Neutralization synergy, in contrast, was not apparent with a molecular clone of a T-cell-line-adapted virus.
MATERIALS AND METHODS
Viruses.
The HIV-1 primary isolates were obtained from the National Institutes of Health AIDS Research and Reagent Reference Program (ARRRP), including HIV-1JR-CSF (contributed by Irvin Chen) (20), HIV-1SF162 (contributed by Jay Levy) (8), and HIV-189.6 (contributed by Ronald Collman) (12). U87.CD4.CCR5 cells (ARRRP) were contributed by HongKui Deng and Dan Littman (3). The HxB2 stock was kindly provided by Abraham Pinter and Shermaine Tilley; it was prepared by transfecting the HxB2 molecular clone of HIV-1IIIB into H9 cells followed by a few passages in H9 cells to prepare the viral stock used (55). The recombinant vaccinia virus expressing HIV-189.6 envelope glycoprotein was kindly provided by Bob Doms (14).
Antibodies.
The antibodies used in this study have all been described in detail previously. Immunoglobulin G1 (IgG1) b12 is a human antibody directed against an epitope overlapping the CD4 binding site on gp120 (6, 7, 31, 36). MAb 2G12 is directed against a unique epitope, consisting at least in part of carbohydrate chains and located at the junction of the silent and neutralizing faces of gp120 (4, 54). MAb 2F5 is directed against a conserved epitope at the C-terminal part of the extracellular domain on gp41 (30, 43). MAb 4E10 is directed to a conserved membrane-proximal epitope on gp41 located C terminal of the 2F5 epitope (4, 58). MAbs b12, 2G12, 2F5, and 4E10 each have been shown to neutralize a broad range of HIV-1 primary isolates (7, 38, 53, 58).
HIV-1 neutralization assays.
A number of different HIV-1 neutralization assay formats were used. The first assay is based on the infection of HeLa cells expressing human CD4 and the HIV-1 long terminal repeat fused to the β-galactosidase gene (obtained from the ARRRP [19], contributed by M. Emerman) and which were described previously (37, 39). Briefly, HIV-1HxB2 was preincubated with dilutions of the MAbs in Dulbecco's modified Eagle medium (DMEM)–10% fetal calf serum (FCS) for 1 h at 37°C in a total volume of 50 μl. HeLa cells were plated in flat-bottomed microtiter plates one day prior to the assay at a concentration of about 5 × 105 cells/ml. The medium was aspirated, and the virus-antibody mixtures were added. After 1 h of incubation at 37°C the cells were washed with DMEM–10% FCS and cultured for a further 36 to 48 h. The medium was aspirated and the cells were lysed in a solution of phosphate-buffered saline (PBS) containing 0.5% NP-40. An equal volume of a solution containing 16 mM chlorophenol red β-d-galactopyranoside (Boehringer Mannheim, Indianapolis, Ind.) was added and incubated for 30 min at 37°C. The absorbance at 550 nm was read, and the percentage of neutralization was calculated.
A second assay format used was to challenge H9 cells by HIV-1HxB2, or phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMC) by the primary isolates, in the presence or absence of MAb followed by detection of p24 antigen in enzyme-linked immunosorbent assays (ELISA) to assess HIV-1 replication as described previously (58). PBMC (from three CCR5 wild-type donors) were isolated and stimulated with PHA (5 μg/ml) for 48 h followed by PHA and interleukin 2 (40 U/ml) for 72 h in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine. The antibodies were diluted and 50 μl per well were pipetted in round-bottom microtiter plates, after which an equal volume containing 100 50% tissue culture infective doses of HIV-1 stock was added. The antibody-virus mixture was incubated for 1 h at 37°C. Next, 100 μl of PHA-activated PBMC (5 × 105/ml for primary isolates) or H9 cells (2 × 105/ml for HIV-1HxB2) were added to each well. The calculated neutralization titers refer to the antibody concentration present during this incubation step. After an overnight incubation the cells were washed two times with tissue culture medium. On day 7 the supernatants were collected and treated with 1% (vol/vol) Empigen (Calbiochem). Triplicate samples were then tested for p24 content by using an in-house ELISA as originally described by Moore et al. (28). In brief, sheep anti-p24 antibody D7320 (Aalto Bioreagents) was coated overnight on 96-well polystyrene enzyme immunoassay plates (Costar) in 100 mM NaHCO3, pH 8.5. The plates were washed in PBS, and p24 was captured from serial dilutions of the HIV-1 containing samples in PBS–0.1% Empigen. After a 3-h incubation, unbound p24 was washed away and bound p24 was detected with an alkaline phosphatase-labeled antibody, BC1071 (International Enzymes), diluted 1:3,000 in PBS containing 20% sheep serum and 2% nonfat dry milk. After a 1-h incubation the plates were washed and developed with an AMPAK kit (Dako Diagnostics) as recommended by the manufacturer. Production of p24 antigen in the antibody-containing cultures was compared to p24 production in cultures without antibody run in the same assay, and the antibody concentrations resulting in 90% reduction in p24 content were determined.
In the third assay format a single-round infectious molecular clone, JR-CSF, produced by envelope complementation, was used. Virus competent for a single round of replication was produced by cotransfection of pSVIIIexE7–JR-CSF and pNL4-3.luc.R-E- (provided by Nathaniel Landau). The pSVIIIenv vector (provided by Joseph Sodroski [50]) was modified in order to introduce the HIV-1JR-CSF env gene. One XhoI site located immediately upstream of the HIV long terminal repeat was knocked out. The JR-CSF env gene was amplified by PCR from the pYK-JR-CSF molecular clone (obtained from the ARRRP; contributed by Irvin Chen and Yoshio Koyanagi) and then subcloned in the modified vector using KpnI and XhoI cloning sites. The degree of virus neutralization by antibody was achieved by measuring luciferase activity. Briefly, 2 × 104 U87.CD4.CCR5 cells in 100 μl of medium (DMEM containing 15% FBS, 1 μg of puromycin/ml, 300 μg of G418/ml, glutamine, and penicillin-streptomycin) were added to microplate wells (96-well flat-bottom; Corning Inc., Corning, N.Y.) and incubated for 24 h at 37°C in 5% CO2. One hundred microliters of medium containing an amount of virus previously determined to yield ∼100,000 counts (see below) was mixed with various amounts of antibody, incubated for 1 h at 37°C, added to the cells, and incubated for a further 3 days. The wells were aspirated and washed once with PBS, and 60 μl of luciferase cell culture lysis reagent (Promega, Madison, Wis.) was added. The wells were scraped and the lysate was mixed by pipetting, 50 μl were transferred to a round-bottom plate (Corning), and the plate was centrifuged at 1,800 × g for 10 min at 4°C. Twenty microliters were transferred to an opaque assay plate (Corning), and the luciferase activity was measured on a luminometer (EG&G Berthold LB 96V; Perkin Elmer, Gaithersburg, Md.) by using luciferase assay reagent (Promega).
Determination of neutralization synergy.
The level of neutralization enhancement by neutralizing antibody combinations was assessed using two different approaches. First, the classical approach was used in which antibodies were mixed at a constant ratio that was determined on the basis of their relative neutralization potency (90% inhibitory dose, or ID90). Dose-response curves were then determined, in the same assay, for the antibody mixture and each of the antibodies in the mixture alone. The presence or absence of synergy was assessed by comparing the ID90 values for each of the antibodies and their mixture. The presence or absence of synergy was also assessed by using the computer program CalcuSyn (Biosoft, Ferguson, Mo.). The program automatically calculates the CI, with values of less than 1, equal to 1, and greater than 1 indicating synergy, additive effect, and antagonism, respectively. To determine the CIs, we ensured that all the basic requirements were met as recommended (10).
A second approach with variable antibody ratio design was also used to determine neutralization synergy. Here, we titrated one antibody in the combination and then added a fixed amount of a second neutralizing antibody at a weakly neutralizing concentration (to standardize the amount of that antibody bound to the virus). The presence of neutralization enhancement was assessed by comparing the ID90s achieved to those obtained with single antibody titration performed in the same test.
Analysis of antibody binding by flow cytometry.
H9 cells obtained from the ARRRP (contributed by Robert Gallo) (41) were cultured in growth medium (RPMI 1640 containing 10% FCS, 100 U of penicillin/ml, and 100 μg of streptomycin/ml) at 5% CO2. Infection of H9 cells with HIV-1HxB2 was performed by adding the virus at a multiplicity of infection (MOI) of 0.001 for 2 h at 37°C. After being washed the cells were cultured for an additional 8 to 10 days. At this time 100% of the cells expressed viral envelope glycoprotein as determined by indirect immunofluorescent staining with anti-gp120 MAbs but not with CD4, as detected by MAbs to the first and fourth domains of CD4, carried out as previously described (45).
BHK-21 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Va.). The cells were cultured in growth medium (as above) in 6-well culture plates and were grown to approximately 80% confluency. The growth medium was removed, and trypsin-treated recombinant vaccinia virus diluted in 1 ml of growth medium was added to each well at an MOI of 10 to 20 and incubated at 37°C with occasional shaking. After 2 h, 3 ml of growth medium was added, after which the cells were grown for an additional 24 h. The cells were harvested with a cell scraper, resuspended, and used in flow cytometry.
Flow cytometry was essentially performed as described previously (37). H9 cells infected with HIV-1HxB2 or vaccinia virus-infected BHK cells were washed twice in RPMI 1640–10% FCS and resuspended at a concentration of 2 × 106 cells per ml. Fifty microliters of MAb previously diluted in PBS–1% FCS–0.05% sodium azide (wash buffer, or WB) was added to 50 μl of cell suspension in a round-bottom, 96-well microtiter plate and were incubated with gentle agitation at 37°C for 2 h. The cells were washed three times in WB and then fixed overnight at 4°C in WB containing 1% formaldehyde. After two further washes in WB, 50 μl of F(ab′)2 fragment of goat anti-human IgG F(ab′)2-fluorescein isothiocyanate conjugate (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) diluted 1:100 in WB was added, and the cells were incubated for 1 h at 4°C. The cells were washed twice and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) with CellQuest software (version 3.1f). A total of 10,000 events were measured per sample. Values are reported in mean fluorescent intensity units.
RESULTS
Absence of synergy in MAb neutralization of HIV-1HxB2.
We began by evaluating possible synergy of the broadly neutralizing MAbs b12, 2G12, and 2F5 in combination against a molecular clone of the T-cell-line-adapted virus HIV-1HxB2. The classical method used to assess neutralization synergy is to mix antibodies in a fixed ratio and compare the dose response with that from neutralization assays performed with the individual antibodies. This was the approach adopted here. The T-cell line H9 was used as a target cell, and detection of p24 by ELISA was used as a reporter assay. No synergy was evident by comparison of the ID90s of each of the antibodies individually with the value for the triple combination (Table 1). We then analyzed the data using the CalcuSyn software package based on the median effect principle as formulated by Chou and Hayball (10). This analysis confirmed the observations based on the ID90 comparison. The median effect dose was in the range of 0.15 to 0.2 μg/ml for each individual antibody and was 0.17 μg/ml for the combination. At the ID90 the CI equaled 1 and the dose reduction indices suggested that threefold lower concentrations of each antibody were required in the triple combination; both the CI and dose reduction index, therefore, indicate additivity.
TABLE 1.
Neutralization of HIV-1HxB2 by individual antibodies or in combinationa
| MAb | ID90 (μg/ml)b | Dm (μg/ml)c | rd | CIe at
|
Dose reduction indexf at
|
||
|---|---|---|---|---|---|---|---|
| ID50 | ID90 | ID50 | ID90 | ||||
| IgG1 b12 | 0.25 | 0.2 | 0.99 | 1.1 | 2.9 | ||
| 2F5 | 0.5 | 0.15 | 0.99 | 0.9 | 2.3 | ||
| 2G12 | 0.5 | 0.2 | 0.96 | 1.2 | 2.7 | ||
| Combination (1:1:1) | 0.125 (each) | 0.17 (each) | 0.97 | >1 | 1 | ||
Neutralization synergy of antibody combinations for HIV-1HxB2 was assessed by using the classical approach to analyzing synergy in which dose-response curves were determined for each of the antibodies alone and for antibody combinations mixed at a constant ratio. The ratio at which the antibodies were mixed was based on their relative neutralization potency (ID90). The presence or absence of synergy was assessed by comparing ID90 values and by using the computer program CalcuSyn. Dm, r, CI, and dose reduction index values were determined using the CalcuSyn computer program (10).
ID90s were calculated by estimating the 90% neutralization titer from the neutralization curves.
Dm, median effect dose; antibody concentration at half-maximal neutralization.
r, linear correlation coefficient.
According to Chou, Talalay, and Hayball, CIs of 0.3 to 0.7 indicate synergism, 0.7 to 0.85 indicate moderate synergism, 0.85 to 0.9 indicate slight synergism, 0.9 to 1.1 indicate additivity, and greater CIs indicate antagonism (10, 11).
Dose reduction index, the ratio of doses required for each antibody to reach the indicated degree of neutralization (9, 10).
Analysis of neutralization synergy of primary isolates HIV-1 JR-CSF and HIV-1 SF162 using the classical (fixed antibody ratio) approach.
We next studied neutralization of two primary isolates also using the classical approach to synergy. PHA-activated human PBMC were used as target cells, and detection of p24 was used as a reporter assay. We attempted to mix the antibodies in a fixed ratio that would reflect their relative potency. This was not always achieved in these experiments due to variations in the biological assay. The median effect analysis, however, calculates synergy independent of the ID90 ratios achieved. Determination of synergy on the basis of ID90s is difficult if the antibodies are tested at unequal ratios. Here, we therefore primarily relied on the median effect analysis to determine synergy (Tables 2 and 3). The CIs at the ID50 and ID90 are 0.8 and 0.6 for b12, 2G12, and 2F5 neutralization of HIV-1JR-CSF and 0.6 and 0.8 for b12 and 2F5 neutralization of HIV-1SF162, respectively (2G12 in the latter case was not tested, as it neutralizes HIV-1SF162 very poorly). These CIs suggest intermediate to moderate synergy.
TABLE 2.
Neutralization of HIV-1JR-CSF by individual antibodies or in combinationa
| MAb | ID90 (μg/ml)b | Dm (μg/ml)c | rd | CIe at
|
Dose reduction indexf at
|
||
|---|---|---|---|---|---|---|---|
| ID50 | ID90 | ID50 | ID90 | ||||
| IgG1 b12 | 20 | 16 | 0.99 | 25 | 29 | ||
| 2F5 | 50 | 20 | 0.99 | 1.5 | 2 | ||
| 2G12 | 200 | 72 | 0.91 | 11 | 33 | ||
| Combination (1:20:10) | 1.3, 25, 12.5 g | 0.65, 13, 6.5 g | 0.99 | 0.8 | 0.6 | ||
Neutralization synergy of antibody combinations for HIV-1JR-CSF was assessed by using the classical approach to analyzing synergy in which dose-response curves were determined for each of the antibodies alone and for antibody combinations mixed at a constant ratio. The ratio at which the antibodies were mixed was based on their relative neutralization potency (ID90). The presence or absence of synergy was assessed by comparing ID90 values and by using the computer program CalcuSyn. Dm, r, CI, and dose reduction index values were determined using the CalcuSyn computer program (10).
ID90s were calculated by estimating the 90% neutralization titer from the neutralization curves.
Dm, median effect dose; antibody concentration at half-maximal neutralization.
r, linear correlation coefficient.
According to Chou, Talalay, and Hayball, CIs of 0.3 to 0.7 indicate synergism, 0.7 to 0.85 indicate moderate synergism, 0.85 to 0.9 indicate slight synergism, 0.9 to 1.1 indicate additivity, and greater CIs indicate antagonism (10, 11).
Dose reduction index, the ratio of doses required for each antibody to reach the indicated degree of neutralization (9, 10).
Concentrations of b12, 2F5, and 2G12 in the mixture, respectively.
TABLE 3.
Neutralization of HIV-1SF162 by individual antibodies or in combinationa
| MAb | ID90 (μg/ml)b | Dm (μg/ml)c | rd | CIe at
|
Dose reduction indexf at
|
||
|---|---|---|---|---|---|---|---|
| ID50 | ID90 | ID50 | ID90 | ||||
| IgG1 b12 | 2.5 | 2.2 | 0.99 | 19 | 13 | ||
| 2F5 | 100 | 40.3 | 0.99 | 1.7 | 1.5 | ||
| Combination (1:200) | 0.5, 100 g | 0.11, 23.2 g | 0.99 | 0.6 | 0.8 | ||
Neutralization synergy of antibody combinations for HIV-1SF162 was assessed by using the classical approach to analyzing synergy in which dose-response curves were determined for each of the antibodies alone and for antibody combinations mixed at a constant ratio. The ratio at which the antibodies were mixed was based on their relative neutralization potency (ID90). The presence or absence of synergy was assessed by comparing ID90 values and by using the computer program CalcuSyn. Dm, r, CI, and dose reduction index values were determined using the CalcuSyn computer program (10).
ID90s were calculated by estimating the 90% neutralization titer from the neutralization curves.
Dm, median effect dose; antibody concentration at half-maximal neutralization.
r, linear correlation coefficient.
According to Chou, Talalay, and Hayball, CIs of 0.3 to 0.7 indicate synergism, 0.7 to 0.85 indicate moderate synergism, 0.85 to 0.9 indicate slight synergism, 0.9 to 1.1 indicate additivity, and greater CIs indicate antagonism (10, 11).
Dose reduction index, the ratio of doses required for each antibody to reach the indicated degree of neutralization (9, 10).
Concentrations of b12 and 2F5 in the mixture, respectively.
Analysis of neutralization synergy using an alternative approach in which one antibody concentration is fixed.
In the classical approach used above neutralizing antibodies are combined at a fixed ratio, and therefore the amounts of each antibody bound to envelope spikes are all expected to vary upon titration. Mathematical modeling is required to determine synergy. In an alternative approach using a varying antibody ratio design we studied neutralization synergy by varying the concentration of one neutralizing antibody in the combination (antibody 1), after which we added a fixed amount of a second antibody (antibody 2) at a weakly neutralizing antibody concentration. An enhancement of neutralization can then simply be observed as a significant increase of neutralization by the combination compared to the titration curve of the first antibody alone. This type of assay was performed for the HIV-1HxB2 molecular clone (Table 4) and the primary isolates HIV-1JR-CSF (Table 5), HIV-189.6 (Table 6), and HIV-1SF162 (Table 7).
TABLE 4.
Neutralization of HIV-1HxB2 by MAbs alone or in combinationa
| Antibody 2b | ID90 of antibody 1 alone or in combination with antibody 2c
|
|||
|---|---|---|---|---|
| IgG1 b12 | F(ab′)2 b12 | 2G12 | 2F5 | |
| None | 1 | 1 | 10 | 10 |
| IgG1 b12 (0.02) | 10 | 10 | ||
| F(ab′)2 b12 (0.05) | 10 | NDd | ||
| 2G12 (0.2) | 1 | 1 | 10 | |
| 2F5 (2) | 1 | ND | 10 | |
Neutralization synergy of antibody combinations for HIV-1HxB2 was assessed by using an alternative approach to determining synergy in which the neutralization dose-response curve of one MAb (antibody 1) was assessed in the presence or absence of a fixed, weakly neutralizing concentration of a second MAb (antibody 2).
The fixed concentration of antibody 2 is indicated in micrograms per milliliter in parenthesis.
ID90s are shown in micrograms of antibody 1 per milliliter alone or in combination with a weakly neutralizing concentration of antibody 2. A decrease in the ID90 of antibody 1 indicates synergy.
d ND, not done.
TABLE 5.
Neutralization of HIV-1JR-CSF by MAbs alone or in combinationa
| Antibody 2b | ID90 of antibody 1 alone or in combination with antibody 2c
|
||
|---|---|---|---|
| IgG1 b12 | 2G12 | 2F5 | |
| None | 25 | 200 | 100 |
| IgG1 b12 (10) | 25 | >50 | |
| 2G12 (50) | 12.5 | 25 | |
| 2F5 (50) | 12.5 | 50 | |
Neutralization synergy of antibody combinations for HIV-1JR-CSF was assessed by using an alternative approach to determining synergy in which the neutralization dose-response curve of one MAb (antibody 1) was assessed in the presence or absence of a fixed, weakly neutralizing concentration of a second MAb (antibody 2).
The fixed concentration of antibody 2 is indicated in micrograms per milliliter in parenthesis.
ID90s are shown in micrograms of antibody 1 per milliliter alone or in combination with a weakly neutralizing concentration of antibody 2. A decrease in the ID90 of antibody 1 indicates synergy.
TABLE 6.
Neutralization of HIV-189.6 by MAbs alone or in combinationa
| Antibody 2b | ID90 of antibody 1 alone or in combination with antibody 2c
|
|||
|---|---|---|---|---|
| IgG1 b12 | F(ab′)2 b12 | 2G12 | 2F5 | |
| None | 6.25 | 3.1 | 100 | 75 |
| IgG1 b12 (1) | 25 | 50 | ||
| F(ab′)2 b12 (1) | 50 | 50 | ||
| 2G12 (50) | 1.25 | NDd | 50 | |
| 2F5 (10) | 6.25 | ND | 50 | |
Neutralization synergy of antibody combinations for HIV-189.6 was assessed by using an alternative approach to determining synergy in which the neutralization dose-response curve of one MAb (antibody 1) was assessed in the presence or absence of a fixed, weakly neutralizing concentration of a second MAb (antibody 2).
The fixed concentration of antibody 2 is indicated in micrograms per milliliter in parenthesis.
ID90s are shown in micrograms of antibody 1 per milliliter alone or in combination with a weakly neutralizing concentration of antibody 2. A decrease in the ID90 of antibody 1 indicates synergy.
ND, not done.
TABLE 7.
Neutralization of HIV-1SF162 by MAbs alone or in combinationa
| Antibody 2b | ID90 of antibody 1 alone or in combination with antibody 2c
|
||
|---|---|---|---|
| IgG1 b12 | 2G12 | 2F5 | |
| None | 5 | >200 | 100 |
| IgG1 b12 (0.5) | >200 | 100 | |
| 2G12 (50) | 2.5 | 50 | |
| 2F5 (50) | 2.5 | 200 | |
Neutralization synergy of antibody combinations for HIV-1SF162 was assessed by using an alternative approach to determining synergy in which the neutralization dose-response curve of one MAb (antibody 1) was assessed in the presence or absence of a fixed, weakly neutralizing concentration of a second MAb (antibody 2).
The fixed concentration of antibody 2 is indicated in micrograms per milliliter in parenthesis.
ID90s are shown in micrograms of antibody 1 per milliliter alone or in combination with a weakly neutralizing concentration of antibody 2. A decrease in the ID90 of antibody 1 indicates synergy.
The results shown in Table 4 for neutralization of HIV-1HxB2 with b12, 2G12, 2F5, and their combinations confirm the results shown in Table 1. CD4-expressing HeLa cells containing the HIV-1 long terminal repeat fused to the β-galactosidase gene were used as target cells, and detection of β-galactosidase activity was used as a reporter assay (19, 37). The mixing of b12, 2G12, or 2F5 in all possible two-antibody combinations did not alter the neutralization titers observed. We also assessed the combinations of b12 F(ab′)2 fragments in combination with 2G12; the F(ab′)2 fragments behaved as intact b12. The results with the primary isolates were somewhat different (Tables 5, 6, and 7). Here we found moderate enhancements of neutralization, in particular between combinations of b12 and 2G12. The addition of subneutralizing concentrations of 2G12 to b12 and vice versa reduced ID90s approximately two- to fourfold for all three primary isolates tested.
Analysis of neutralization synergy by using recombinant primary HIV-1 isolates.
We reasoned that the enhancement of neutralization by antibody combinations of the primary isolate but not the molecularly cloned T-cell-line-adapted virus could be the result of virus heterogeneity, or alternatively it could be a characteristic of primary isolate envelope. To investigate this in more detail we performed neutralization assays with recombinant HIV-1JR-CSF and HIV-189.6 in an envelope complementation format using luciferase activity as a reporter assay. In addition to the b12, 2G12, 2F5 antibody combination, we also assessed a combination including the recently described broadly HIV-1 neutralizing human antibody 4E10 (58). The ID90s in the envelope complementation assay are somewhat lower than those observed with the corresponding primary isolates tested in the PHA-activated PBMC-based assay. We find this to be a typical phenomenon of this assay that may be due to a slightly greater neutralization sensitivity of the recombinant virions or, alternatively, the absence of virus heterogeneity.
Potential synergy was determined in assays using the classical approach with antibodies combined at a fixed ratio. Interestingly, we observed a moderate enhancement of recombinant HIV-1JR-SCF neutralization which was comparable to that observed in the HIV-1JR-CSF primary isolate neutralization assays described above (Tables 2 and 5). Significant synergy was apparent at the level of 90% neutralization. The increase in neutralization observed is, again, of the order of two- to fourfold, although it is difficult to assess this number in this assay, as discussed above. However, the amounts of b12, 2F5, and 2G12 required at 90% neutralization were each reduced about two- to fourfold compared to the ID90 of each antibody alone. Addition of MAb 4E10 to this mixture then reduced the amount of b12, 2F5, and 2G12 required another twofold, making an overall enhancement of about 10-fold (Table 8).
TABLE 8.
Neutralization of recombinant HIV-1JR-CSF by individual antibodies or in combinationa
| MAb | ID90 (μg/ml) | Dm (μg/ml) | r | CI at
|
Dose reduction index at
|
||
|---|---|---|---|---|---|---|---|
| ID50 | ID90 | ID50 | ID90 | ||||
| IgG1 b12 | 2 | 0.4 | 0.97 | 3 | 4.7 | ||
| 2F5 | 40 | 8.9 | 0.95 | 7.4 | 13.6 | ||
| 2G12 | 7.5 | 0.4 | 0.98 | 1.4 | 13.8 | ||
| Combination (1:10:2) | 0.9, 9, 1.8b | 0.1, 1.2, 0.3b | 0.99 | 1.2 | 0.4 | ||
| IgG1 b12 | 2 | 0.4 | 0.97 | 6.1 | 8.5 | ||
| 2F5 | 40 | 8.9 | 0.95 | 14 | 25 | ||
| 2G12 | 7.5 | 0.4 | 0.98 | 2.7 | 25 | ||
| 4E10 | 200 | 18.5 | 0.97 | 0.7 | 0.3 | 22 | 75 |
| Combination (1:10:2:13.3) | 0.4, 4, 0.8, 5.3c | 0.06, 0.6, 0.1, 0.9c | |||||
Neutralization synergy of antibody combinations for HIV-1JR-CSF was assessed by using the classical approach to analyzing synergy. The viruses tested are recombinant primary isolates, and neutralization was assessed in an envelope complementation format. The presence or absence of synergy was assessed by comparing ID90 values and by using the computer program CalcuSyn (10). Dm, median effect dose; r, linear correlation coefficient. Dose reduction index, the ratios of doses required for each antibody to reach the indicated degree of neutralization (9, 10).
Concentrations of b12, 2F5, and 2G12 in the mixture, respectively.
Concentrations of b12, 2F5, 2G12, and 4E10 in the mixture, respectively.
The findings with recombinant HIV-189.6 were similar. In this case, however, the combination of MAbs b12, 2G12, and 2F5 enhanced neutralization approximately 10-fold. The magnitude of this enhancement is in agreement with the HIV-189.6 primary isolate neutralization assays by antibody combinations and determined by the alternative approach to assess synergy as described above (Table 6). In contrast to HIV-1JR-CSF, however, addition of MAb 4E10 did not increase neutralization of the antibody cocktail any further (Table 9). Neutralizing antibody combinations therefore induced moderate neutralization synergies of similar magnitude in assays with biological and molecular clones of the primary viruses tested.
TABLE 9.
Neutralization of recombinant HIV-189.6 by individual antibodies or in combinationa
| MAb | ID90 (μg/ml) | Dm (μg/ml) | r | CI at
|
Dose reduction index at
|
||
|---|---|---|---|---|---|---|---|
| ID50 | ID90 | ID50 | ID90 | ||||
| IgG1 b12 | 2.5 | 0.1 | 0.92 | 6.3 | 7.0 | ||
| 2F5 | 50 | 2.3 | 0.89 | 7.0 | 51.0 | ||
| 2G12 | 40 | 0.3 | 0.99 | 0.6 | 12.0 | ||
| Combination (1:16:20) | 0.16, 2.6, 3.2b | 0.02, 0.4, 0.4b | 0.95 | 2.1 | 0.3 | ||
| IgG1 b12 | 2.5 | 0.1 | 0.92 | 6.9 | 7.2 | ||
| 2F5 | 50 | 2.3 | 0.89 | 0.7 | 52.5 | ||
| 2G12 | 40 | 0.2 | 0.99 | 7.5 | 12.3 | ||
| 4E10 | 100 | 7.0 | 0.91 | 1.8 | 0.3 | 8.3 | 21.0 |
| Combination (1:16:20:40) | 0.16, 2.6, 3.2, 6.4c | 0.02, 0.3, 0.4, 0.7c | |||||
Neutralization synergy of antibody combinations for HIV-189.6 was assessed by using the classical approach to analyzing synergy. The viruses tested are recombinant primary isolates, and neutralization was assessed in an envelope complementation format. The presence or absence of synergy was assessed by comparing ID90 values and by using the computer program CalcuSyn (10). Dm, median effect dose; r, linear correlation coefficient. Dose reduction index, the ratios of doses required for each antibody to reach the indicated degree of neutralization (9, 10).
Concentrations of b12, 2F5, and 2G12 in the mixture, respectively.
Concentrations of b12, 2F5, 2G12, and 4E10 in the mixture, respectively.
Absence of cooperativity of MAb binding to HIV-1 envelope spikes.
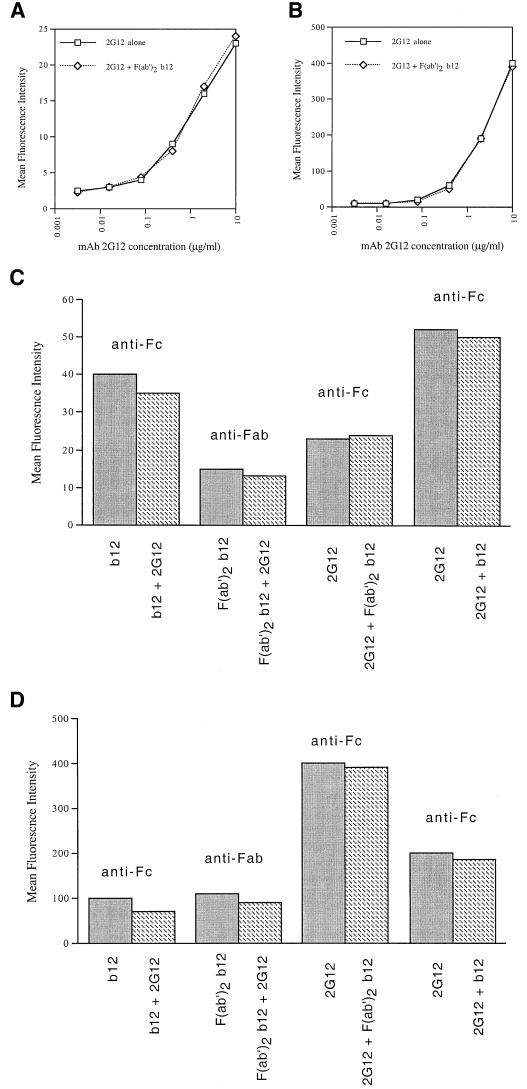
There is a strong correlation between antibody binding to the mature envelope spike and neutralization, at least for T-cell-line-adapted viruses (37). Synergy in neutralization might, therefore, reflect cooperative MAb binding to HIV-1 envelope spikes. To assess the binding characteristics of anti-gp120 antibodies, and combinations thereof, to envelope spikes we examined their binding to HIV-1HxB2-infected H9 cells and BHK cells infected with a recombinant vaccinia virus expressing HIV-189.6 envelope glycoprotein by flow cytometry. In the experiment shown in Fig. 1A and B, we titrated the 2G12 antibody alone or in the presence of a subsaturating amount (resulting in 75% of maximal binding) of F(ab′)2 fragments of MAb b12. The binding of MAb 2G12 to HxB2 or 89.6 envelope was then detected with an antibody against the human Fc fragment; bound F(ab′)2 b12 fragments were, therefore, not detected. For both the HxB2 as well as the 89.6 envelope glycoprotein expressed on cells, no differences in binding were observed. Although F(ab′)2 fragments and whole b12 behaved similarly in neutralization assays (see above), it cannot be excluded that cooperativity of binding requires the presence of the antibody Fc fragment. We therefore also performed binding experiments using whole b12 and 2G12 antibodies. In these experiments titrations of b12 and 2G12 were mixed with a fixed amount of the appropriate complementary antibody, as shown for HxB2 and 89.6 in Fig. 1C and D, respectively. The experiments with the b12 F(ab′)2 fragments discussed above are also shown for comparison. Bound antibody was detected by using antibody Fc or Fab fragment-specific reagents as indicated. In the experiment using mixtures of whole antibodies we corrected the fluorescence signal by subtracting the signal corresponding to the antibody added at the fixed amount. In the first two bars shown in Fig. 1C, for example, MAb b12 alone gave a signal of 39 fluorescence units at 10 μg/ml (dark bar). The same amount of b12 combined with MAb 2G12 (added at 2 μg/ml, corresponding to 20 fluorescent units) gave a signal of 55 fluorescence units, which resulted in a corrected signal for the amount of bound b12 of 35 U (hatched bar). We did not observe any significant changes in binding of b12 or 2G12 in any of the assay formats analyzed. We therefore did not find evidence for cooperativity of binding between 2G12 and b12 for the T-cell-line-adapted and primary isolate envelopes tested.
FIG. 1.
Analysis of cooperativity of MAb binding to HIV-1 envelope spikes. Binding of MAb 2G12 to HIV-1 envelope expressed on the surface of HIV-1HxB2-infected H9 cells (A) or BHK cells infected with a recombinant vaccinia virus expressing HIV-189.6 envelope (B) was detected by using flow cytometry. Dose-response curves of MAb 2G12 binding were assessed in the absence or presence of a subsaturating amount of F(ab′)2 fragments of MAb b12. Detection of bound antibody was performed with a fluorochrome-labeled antibody against Fc (A and B). (C and D) Binding of MAb b12, MAb 2G12, and F(ab′)2 b12 and their combinations to HIV-1HxB2-infected H9 cells and BHK cells infected with a recombinant vaccinia virus expressing HIV-189.6 envelope, respectively. Antibody binding was detected with a fluorochrome-labeled antibody against the Fc or Fab fragment as indicated above the bars. The primary antibody (dark bars, or indicated first in the antibody pair described below the hatched bars) was tested at 10 μg/ml, whereas the secondary antibody (shown as the second antibody below the hatched bars) was tested at a subsaturating concentration (resulting in 50 to 75% of maximal binding). The results in the third pair of bars correspond to the data in panels A and B and are shown for comparison.
DISCUSSION
Increased neutralization of HIV-1 isolates by antibody combinations could be explained by at least two mechanisms. First, the antibodies may act on the same virion particles. An interaction of antibodies against nonoverlapping epitopes might lead to cooperative binding and enhanced neutralization. In addition, increased affinity for envelope spikes as a result of cooperative binding might also result in a broadening of the range of HIV-1 isolates recognized. Second, the antibodies may act on different virion particles. The recognition of a broader range of HIV-1 quasispecies by an antibody combination may then result in increased neutralization of heterogeneous HIV-1 isolates containing multiple quasispecies. An HIV-1 isolate, for example, might contain quasispecies which are neutralized by antibody 1 but resistant to antibody 2 and vice versa. A combination of these two neutralizing antibodies may then achieve a significantly greater reduction in virus growth than that predicted from assays performed with the two antibodies alone. Whereas both mechanisms may explain enhanced neutralization by a broadening of the response, only the first mechanism would be influenced by changes in antibody affinity due to binding cooperativity of antibody combinations. In addition to this, other mechanisms not directly related to antibody binding may apply.
In the primary isolate neutralization assays with constant antibody ratio design, we calculated CIs in a range of 0.6 to 0.8. Chou, Talalay, and Hayball have previously determined that a CI of 0.3 to 0.7 indicates synergism, 0.7 to 0.85 indicates moderate synergism, 0.85 to 0.9 indicates slight synergism, and 0.9 to 1.1 indicates additivity (9–11). The synergism observed in neutralization of HIV-1JR-CSF and HIV-1SF162 by combinations of b12, 2G12, and 2F5 (CI = 0.6 to 0.8) is similar to that reported for these antibodies previously in a study by Li et al. In the latter study, the CIs reported were in the range 0.6 to 1.0 for all three possible double combinations for neutralization of an SHIV containing the envelope glycoprotein of the T-cell-line-adapted strain HIV-1IIIB (22). Mascola et al. examined the neutralization of SHIV89.6PD by using 2G12 and 2F5 (24). Some neutralization enhancement by the 2F5-2G12 combination was observed, although the neutralization of this virus by MAb 2G12 was very weak. The enhancement at the ID90 was about fourfold, which is in the range we have observed for neutralization of HIV-1 JR-CSF, 89.6, and SF162. In another study, neutralization synergy of the same order of magnitude was reported by Mascola and colleagues for neutralization of a subset of clade B HIV-1 primary isolates by combinations of MAb 2F5 and 2G12 (25). Our data are also in agreement with a study by Potts et al. in which neutralization synergies between anti-CD4 and anti-V3 loop MAbs for T-cell-line-adapted viruses were assessed (42). Although CIs suggesting intermediate to strong synergy were calculated, it was found that there was only a limited (two- to fourfold) increase in neutralization potency in most cases (42). Laal et al. also reported apparently significant CIs, while neutralization only increased two- to fourfold (21). Similarly, dose reduction indices calculated with the mathematical model in our study often appeared overly optimistic and suggested greater dose reductions than were observed (Tables 2, 8, and 9). We carried out neutralization using different target cells (H9, U87, PHA-activated PBMC), reporter systems (p24 ELISA, β-galactosidase, and luciferase expression), and viruses (T-cell-line-adapted viruses, primary viruses, and recombinant viruses) in this study to make certain that the effects observed were reproducible under different neutralization assay conditions. The good agreement between the results in the various neutralization assay formats indicates that this is indeed the case.
A study by Vijh-Warrier et al. suggested a correlation between virus heterogeneity and synergy (55). In that study, a combination of chimpanzee antibodies against the V2 and V3 loop, for example, appeared to neutralize HIV-1IIIB synergistically (CI at ID90 of 0.5), whereas the same antibodies were suggested to be only additive against HIV-1HxB2, a molecular clone of IIIB (CI at ID90 of 0.8 to 1.0) (55). In a study by Thali et al. (51), using molecular HIV-1 clones, cooperativity in neutralization by antibodies against the CD4 binding site and V3 loop were examined in HIV-1 envelope glycoprotein complementation assays. An enhancement of neutralization using neutralizing antibody pairs was found only sporadically. The effects observed were also weak (twofold or less) and could not be predicted by antibody binding to envelope glycoprotein expressed on the surface of COS cells (51).
The studies above suggest that the estimation of synergy is difficult and its magnitude may be overestimated when using the mathematical model. An alternative approach to assess synergy is to vary the concentration of one neutralizing antibody in the test while adding a second neutralizing antibody at a fixed concentration at or just below its neutralization threshold (variable antibody ratio design). The level of occupancy of the second antibody on HIV-1 envelope spikes is thus expected to be fixed, and a smaller number of parameters are varied in the assay. Significant changes in the ID90 that might indicate synergy are assessed more easily compared to results from assays based on the classical approach to determine synergy. By using this alternative strategy, a moderate two- to fourfold enhancement of primary isolate neutralization by b12, 2G12, and 2F5 combinations became apparent.
The absence of neutralization enhancement by antibody combinations in assays using the molecular clone HIV-1HxB2 suggested that virus heterogeneity may play a role in the moderate neutralization enhancement observed with HIV-1 isolates JR-CSF, 89.6, and SF162. To address this question we assessed neutralization with the molecularly cloned envelopes of HIV-1JR-CSF and HIV-189.6 in envelope complementation assays. We found a similar enhancement of neutralization by the b12, 2G12, and 2F5 combination for the cloned envelope compared to results from assays using HIV-1 grown by several passages through PBMC. Therefore, heterogeneity as a result of the presence of HIV-1 quasispecies is not an apparent explanation for the neutralization synergy observed. Primary isolates may differ in this aspect from T-cell-line-adapted viruses. Using the molecular clone HIV-1HxB2, we did not observe any enhancement of neutralization by antibody combinations in both combination assay formats used. This is in agreement with the trend observed by Vijh-Warrier et al. (55).
As discussed above, increased neutralization by antibody combinations could be the result of cooperative binding of antibodies to envelope spikes. Binding of b12 and 2G12 to envelope glycoprotein expressed on the surface of cells was therefore assessed in flow cytometry studies. Similar studies using MAbs 2F5 and 4E10 are difficult, as these antibodies bind poorly to envelope expressed on the surface of cells (46, 58). No cooperativity of binding between b12 and 2G12 was apparent for binding to HxB2 as well as 89.6 envelope. For HIV-1HxB2 this is expected, since neutralization by b12 and 2G12 is additive in both assay formats discussed above. However, there is an apparent conflict between the b12-2G12 neutralization data and the envelope-binding assay (Fig. 1, Tables 6 and 9). Whereas b12 and 2G12 enhance each other approximately fourfold in neutralization, they do not appear to affect each other's binding to 89.6 envelope expressed on recombinant vaccinia virus-infected cells. This may indicate that the enhanced neutralization observed is not directly related to binding. It should be noted, however, that neutralization curves are typically steep and small increases in binding therefore may result in strong increases in neutralization. A two- to fourfold increase in neutralization may therefore be due to an increase in binding which would be difficult to assess in this type of assay.
A good correlation between antibody binding and neutralization has been observed in studies with T-cell-line-adapted strains of HIV-1, such as the HxB2 molecular clone of HIV-1 used above (37, 44, 45). As it is presently unclear whether such analyses extend to primary isolates of HIV-1, we analyzed antibody binding to BHK cells expressing the envelope glycoprotein of a recombinant primary isolate. It should be noted, however, that recombinant envelope expressed on such cells may be present in molecular forms distinct from those present on the primary virion. Thus, recombinant vaccinia virus-expressing cells may express unprocessed gp160 as well as mature envelope spikes on their surface. Studies in which binding of Fab b12 to the recombinant 89.6-expressing BHK cells was compared to binding of the nonneutralizing CD4 binding site antibody Fab b6 (which binds gp160 strongly but binds mature envelope poorly [34]), however, showed that Fab b12 bound ∼10 times more strongly than Fab b6 (data not shown). This suggests that the majority of HIV-189.6 envelope on the surface of these BHK cells is present as mature envelope.
In a recent study an attempt was made to demonstrate and explain synergy by assessing b12, 2G12, and 2F5 binding to recombinant monomeric gp120 and multimeric gp160 by using surface plasmon resonance (57). It was found that MAb 2G12 binding to gp160 interfered with the binding of MAb b12. This is in contrast to observations with monomeric gp120 (29) and our observations on b12 and 2G12 binding to envelope spikes on infected cells. In the study mentioned above, MAb 2G12 furthermore enhanced 2F5 binding to oligomeric gp160 (57). A number of studies, however, have suggested that unprocessed gp160 may have a different conformation from that of mature envelope spikes on the surface of the virus and infected cells (15, 16, 48, 49), and the study may therefore have little predictive value for binding of antibodies to envelope spikes.
The observations in our study have significance for the development of a humoral component of a vaccine against HIV-1. The results should be interpreted with the appropriate caution, however. It should be noted that although the observed synergy results in neutralization of HIV-1 isolates at decreased concentration of individual neutralizing antibodies, the total neutralizing antibody concentration increases in most cases. The dose of MAb b12 required to neutralize HIV-1SF162, for example, may be reduced 5-fold by combining it with MAb 2F5; the total antibody concentration (b12 and 2F5 combined) required to neutralize HIV-1SF162 by the cocktail, however, was about 40-fold increased (compared to neutralization by b12 alone) (Table 3). Similarly, HIV-1JR-CSF is neutralized by the quadruple (b12, 2F5, 2G12, and 4E10) combination when each of the components is present at reduced concentrations, with individual dose reductions ranging from 5- (b12) to 38-fold (4E10). The total antibody concentration required to neutralize HIV-1JR-CSF by the cocktail, however, is increased by about eightfold (compared to that for neutralization by b12 alone) (Table 8). There are only very limited data on this issue from in vivo studies. Single antibodies and antibody combinations have been compared in passive antibody transfer, SHIV challenge studies by Mascola et al. (24, 26), but the data are in agreement with the above discussion. MAbs 2G12 and 2F5, for example, displayed moderate neutralization synergy against the isolate tested (SHIV89.6PD) (24). Whereas 2G12 protected 2 out of 4 animals at a plasma concentration of ∼200 μg/ml, 2 out of 5 animals were protected by the 2F5-2G12 antibody cocktail, with a (higher) combined concentration of ∼400 μg/ml (26).
It has been of concern for HIV-1 vaccine design that neutralizing antibody concentrations required to protect against HIV-1 infection are high and exceed levels which can likely be reached and sustained by vaccination (26, 36). Neutralization synergy of antibody combinations therefore may seem promising. As discussed, however, neutralization synergy may not lead to reduction of the total HIV-1-specific antibody concentration required for neutralization. It can be argued that it may be easier to induce intermediate antibody titers against multiple epitopes by a vaccine than to induce high titers against a single epitope, even though the effective antibody concentration in the multiple-epitope vaccine may be higher. This information, however, is presently unavailable and is therefore an important question to address to further develop knowledge-based approaches to vaccine design.
In summary, our data suggest that neutralization enhancement may be observed in HIV-1 neutralization assays with combinations of broadly neutralizing antibodies. Studies on primary isolates (89.6, SF162, and JR-CSF) showed an enhancement of neutralization which was relatively weak between antibody pairs, with a maximum enhancement of two- to fourfold. A significantly greater enhancement was observed by triple and quadruple antibody combinations which, depending on the isolate tested, increased neutralization titers by up to about 10-fold. This observation encourages enthusiasm for the development of a humoral component of a vaccine against HIV-1, as individual antibodies might be able to provide sterile protection or benefit at lower levels than suggested by passive transfer studies using single antibodies or double antibody combinations (24, 26, 36). The possible implications of neutralization synergy for vaccine development, however, are presently unclear and require further investigation.
ACKNOWLEDGMENTS
We thank Maxime Moulard for help with flow cytometry experiments and Edwin Golez for help with assessing HIV-1 neutralization using the luciferase reporter assay. We acknowledge the assistance of the General Clinical Research Center of The Scripps Research Institute with obtaining blood (M01 RR00833).
M.B.Z. was supported by a fellowship from the Natural Science and Engineering Research Council of Canada (NSERC). This work was supported by grants from the National Institutes of Health to P.W.H.I.P. (AI40377), P.P. (AI45357), and D.R.B. (AI33292).
REFERENCES
- 1.Allaway G P, Ryder A M, Beaudry G A, Maddon P J. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res Hum Retrovir. 1993;9:581–587. doi: 10.1089/aid.1993.9.581. [DOI] [PubMed] [Google Scholar]
- 2.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 3.Bjorndal A, Deng H, Jansson M, Fiore R R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E-M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauder A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 5.Bunow B, Weinstein J N. COMBO: a new approach to the analysis of drug combinations in vitro. Ann NY Acad Sci. 1990;616:490–494. doi: 10.1111/j.1749-6632.1990.tb17857.x. [DOI] [PubMed] [Google Scholar]
- 6.Burton D R, Barbas C F, III, Persson M A A, Koenig S, Chanock R M, Lerner R A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Levy J A. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol. 1988;23(Suppl.):S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 9.Chou T-C, editor. Synergism and antagonism in chemotherapy. San Diego, Calif: Academic Press; 1991. The median-effect principle and the combination index for quantification of synergism and antagonism; pp. 61–102. [Google Scholar]
- 10.Chou T-C, Hayball M P. CalcuSyn, windows software for dose effect analysis. Ferguson, Mo: Biosoft; 1996. [Google Scholar]
- 11.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley A J, Kessler II J A, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark G E I, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S, Parmentier M, Collman R G, Doms R W. A dual-tropic, primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-2b as fusion cofactors. Cell. 1996;86:1149–1159. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Earl P L, Broder C C, Doms R W, Moss B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J Virol. 1997;71:2674–2684. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauduin M C, Parren P W H I, Weir R, Barbas III C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 18.Gauduin M C, Safrit J T, Weir R, Fung M S, Koup R A. Pre- and post-exposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J Infect Dis. 1995;171:1203–1209. doi: 10.1093/infdis/171.5.1203. [DOI] [PubMed] [Google Scholar]
- 19.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 21.Laal S, Burda S, Gorny M K, Karwowska S, Buchbinder A, Zolla-Pazner S. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J Virol. 1994;68:4001–4008. doi: 10.1128/jvi.68.6.4001-4008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li A, Baba T W, Sodroski J, Zolla-Pazner S, Gorny M K, Robinson J, Posner M R, Katinger H, Barbas III C F, Burton D R, Chou T C, Ruprecht R M. Synergistic neutralization of a chimeric SIV/HIV-1 virus with combinations of human anti-HIV-1 envelope monoclonal antibodies or hyperimmune globulins. AIDS Res Hum Retrovir. 1997;13:647–656. doi: 10.1089/aid.1997.13.647. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Katinger H, Posner M R, Cavacini L, Zolla-Pazner S, Gorny M K, Sodroski J, Chou T C, Baba T W, Ruprecht R M. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodefieciency virus type 1 immunoglobulins. J Virol. 1998;72:3235–3240. doi: 10.1128/jvi.72.4.3235-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic SHIV-89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 27.McKeating J A, Cordell J, Dean C J, Balfe P. Synergistic interaction between ligands binding to the CD4 binding site and V3 domain of human immunodeficiency virus type 1 gp120. Virology. 1992;191:732–742. doi: 10.1016/0042-6822(92)90249-o. [DOI] [PubMed] [Google Scholar]
- 28.Moore J P, McKeating J A, Weiss R A, Sattentau Q J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 29.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ollmann Saphire E, Parren P W H I, Pantophlet R, Zwick M B, Morris G M, Rudd P M, Dwek R A, Stanfield R L, Burton D R, Wilson I A. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 32.Parren P W H I, Burton D R. The anti-viral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parren P W H I, Ditzel H J, Gulizia R J, Binley J M, Barbas III C F, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Parren P W H I, Fisicaro P, Labrijn A F, Binley J M, Yang W P, Ditzel H J, Barbas III C F, Burton D R. In vitro antigen challenge of human antibody libraries for vaccine evaluation: the human immunodeficiency virus type I envelope. J Virol. 1996;70:9046–9050. doi: 10.1128/jvi.70.12.9046-9050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren P W H I, Gauduin M C, Koup R A, Poignard P, Sattentau Q J, Fisicaro P, Burton D R. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;58:125–132. doi: 10.1016/s0165-2478(97)00109-0. [DOI] [PubMed] [Google Scholar]
- 36.Parren P W H I, Marx P A, Hessell A J, Luckay A, Harouse J, Cheng-Mayer C, Moore J P, Burton D R. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of HIV-1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13(Suppl. A):S137–S162. [PubMed] [Google Scholar]
- 39.Poignard P, Fouts T, Naniche D, Moore J P, Sattentau Q J. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J Exp Med. 1996;183:473–484. doi: 10.1084/jem.183.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poignard P, Ollmann Saphire E, Parren P W H I, Burton D R. GP120: biologic aspects of structural features. Annu Rev Immunol. 2001;19:253–274. doi: 10.1146/annurev.immunol.19.1.253. [DOI] [PubMed] [Google Scholar]
- 41.Popovic M, Read-Conole E, Gallo R C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984;ii:1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- 42.Potts B J, Field K G, Wu Y, Posner M, Cavacini L, White-Scharf M. Synergistic inhibition of HIV-1 by CD4 binding domain reagents and V3-directed monoclonal antibodies. Virology. 1993;197:415–419. doi: 10.1006/viro.1993.1604. [DOI] [PubMed] [Google Scholar]
- 43.Purtscher M, Trkola A, Grassauer A, Schulz P M, Klima A, Dropper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Roben P, Moore J P, Thali M, Sodroski J, Barbas III C F, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sattentau Q J, Zolla-Pazner S, Poignard P. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 47.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 48.Stamatatos L, Lim M, Cheng-Mayer C. Generation and structural analysis of soluble oligomeric gp140 envelope glycoprotein derived from neutralization-resistant and neutralization-susceptible primary HIV type 1 isolates. AIDS Res Hum Retrovir. 2001;16:981–994. doi: 10.1089/08892220050058407. [DOI] [PubMed] [Google Scholar]
- 49.Staropoli I, Chanel C, Girard M, Altmeyer R. Processing, stability, and receptor binding properties of oligomeric envelope glycoprotein from a primary HIV-1 isolate. J Biol Chem. 2000;275:35137–35145. doi: 10.1074/jbc.M003868200. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan N, Sun Y, Binley J, Lee J, Barbas III C F, Parren P W H I, Burton D R, Sodroski J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thali M, Furman C, Wahren B, Posner M, Ho D D, Robinson J, Sodroski J. Cooperativity of neutralizing antibodies directed against the V3 and CD4 binding regions of the human immunodeficiency virus gp120 envelope glycoprotein. J Acquir Immune Defic Syndr. 1992;5:591–599. [PubMed] [Google Scholar]
- 52.Tilley S A, Honnen W J, Racho M E, Chou T C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4 binding site of gp120. AIDS Res Hum Retrovir. 1992;8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 53.Trkola A, Pomales A P, Yuan H, Korber B, Maddon P J, Allaway G, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type I. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vijh-Warrier S, Pinter A, Honnen W J, Tilley S A. Synergistic neutralization of human immunodeficiency virus type 1 by a chimpanzee monoclonal antibody against the V2 domain of gp120 in combination with monoclonal antibodies against the V3 loop and the CD4-binding site. J Virol. 1996;70:4466–4473. doi: 10.1128/jvi.70.7.4466-4473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 57.Zeder-Lutz G, Hoebeke J, Van Regenmortel M H V. Differential recognition of epitopes present on monomeric and oligomeric forms of gp160 glycoprotein of human immunodeficiency virus type 1 by human monoclonal antibodies. Eur J Biochem. 2001;268:2856–2866. doi: 10.1046/j.1432-1327.2001.02167.x. [DOI] [PubMed] [Google Scholar]
- 58.Zwick M B, Labrijn A F, Wang M, Spenlehauer C, Ollmann Saphire E, Binley J M, Moore J P, Stiegler G, Katinger H, Burton D R, Parren P W H I. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]