Abstract
The aim of this prospective, international multicenter, pseudorandomized study comparing RICT HCT to standard-of-care chemotherapy in intermediate- or high-risk AML patients 50–70 years using the donor versus no-donor concept. Part 1 included only patients with potential family donors (RD) at the date of HLA-typing of the first potential sibling or CR-date, if later. Part 2 allowed the inclusion of patients without a possible sibling donor using the start of an unrelated donor (URD) search as inclusion date. 360 patients were registered and 309 analyzed. The median follow-up was 47 months (1–168). There was no difference in overall survival (OS) between the RD (n = 124) and the Control (n = 77) groups (p = 0.50, 3-year OS RD: 0.41(95% CI; 0.32–0.50); Controls: 0.49 (95% CI; 0.37–0.59)). The main cause of death was relapse (67% RD; 88% Controls). In Part 2, the 3-year OS was 0.60 (95% CI 0.50–0.70) for URD-HCT (n = 86) and 0.37 (95% CI 0.13–0.62) for Controls (n = 20), respectively (p = 0.10). When analyzing transplanted patients (Part 2), the OS at 3-years was higher for URD-HCT than RD-HCT (0.67 (0.55–0.76) vs. 0.42 (0.26–0.57; p = 0.005). This study doesn’t support elderly HLA-identical siblings as donors for older AML patients undergoing a RICT allogeneic HCT in first CR.
Subject terms: Stem-cell therapies, Acute myeloid leukaemia
Introduction
The curative potential of allo-HCT is due to the preparative chemotherapy, and the immunological graft-versus-leukemia (GvL) effect exerted by donor T-cells. The introduction of reduced intensity and thereby lower toxicity conditioning before allo-HCT (RICT) has expanded the transplant option to elderly patients and patients with co-morbidities [1, 2], with the aim of retaining GvL whilst reducing non-relapse mortality (NRM).
Different approaches were used to compare allo-HCT with chemotherapy. These were mainly retrospective studies utilizing registry data [3–5]. A well-recognized problem is the difficulty to compare in a controlled setting allo-HCT vs. standard-of-care since stringent randomized studies are very difficult to perform. Imitating randomization, HCT vs chemotherapy studies have been performed using biological allocation (presence or absence of an HLA-identical sibling donor) in patients undergoing myeloablative HCT [6]. Zittoun et al found in a prospective study, where treatment allocation was done in first complete remission (CR1), an improvement after both auto- and allo-HCT as compared to chemotherapy [7]. In a similarly designed prospective study, Cassileth et al found a slight advantage of chemotherapy compared to auto- or allo-HCT [8]. In contrast, Cornelissen et al found an improvement in disease free survival by donor availability with transplants performed with myeloablative conditioning in patients <55 years having an intermediate or high-risk profile [9]. In this study, the aim was to use comparable starting points for the two treatments using the time for start of donor search for either a family or unrelated donor but also requiring that the patients had entered first complete remission.
Methods
Study design
The design of this study used the pseudorandomized donor versus no donor concept with the aim to compare RICT to standard-of-care chemotherapy in AML patients between 50 and 70 years. When the study was initiated (2003) only patients having a potential HLA-identical sibling (RD) were eligible for the study (Part 1). Due to an increased use of unrelated donors, the study design was changed to allow inclusion of patients without potential HLA-identical siblings (Part 2). Patients could be registered into the study at any time after diagnosis but were only eligible for study inclusion after having entered CR1. The date for study inclusion was the date the first sample was obtained for HLA-typing of a sibling (Parts 1 and 2) or start of unrelated donor (URD) search (Part 2). If either of these occurred before CR, the date of CR was set as the inclusion date. Patients never entering CR were excluded.
Study Part 1 (2003–2012)
The primary objective both for first part of the study and the whole study was to evaluate in an intention-to-treat setting the OS after RICT from HLA-identical sibling donors compared to standard-of-care chemotherapy. Additional objectives were to assess relapse-free survival (RFS), NRM, and relapse incidence (RI) between the two groups.
After entering CR1, patients with at least one sibling were informed about the study and after signed consent included into the study. If the sibling(s) were willing and had no contraindication for stem cell donation, HLA-typing was performed, and patients were assigned to one of two groups; RD (donor identified) or Controls (no donor).
Study Part 2 (2012–2016)
The protocol was updated due to slow accrual and the increasing use of URD allowing search for a suitable URD either after failed RD search or upfront in patients without an available sibling. Depending on possible donor types, the patients were grouped into three strata (Fig. 1). HLA typing of potential related donors and unrelated donor search was permitted after registration but before confirmation of CR to decrease the time between diagnosis and transplant. Patients without a donor were allocated to one of the Control groups. The additional objectives of this part of the study, besides the original objective of comparing RD vs. Controls, were to assess if patients receiving RICT from URD had superior OS compared with Controls (no RD, no URD) or to RICT from RD. Other objectives were to analyze RFS, NRM, and RI after URD RICT compared to Controls.
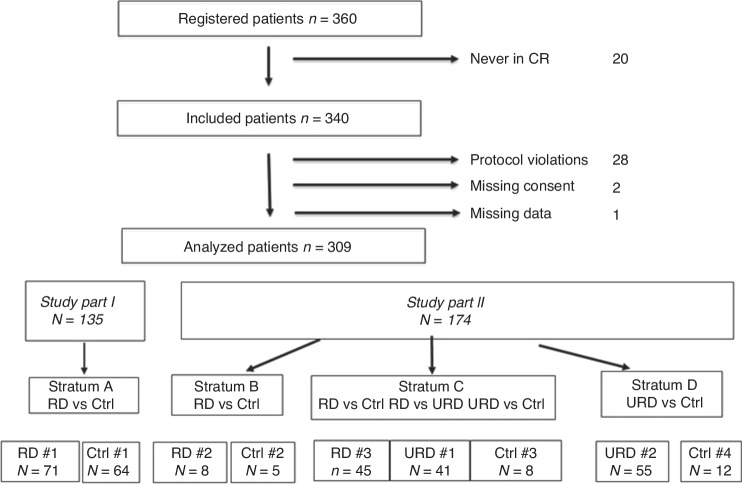
Fig. 1. Study flow chart including reasons for exclusion from analysis.
Flow chart describing patient inclusion and reasons for exclusions from analysis in the two different study periods.
Study procedures
Patients ages 50–70 years with intermediate- or high-risk AML in CR1 and who were judged to be able to tolerate further therapy including RIC-HCT could be included. Important exclusion criteria were favorable risk AML as defined by cytogenetics and as of study part 2 by presence of mutation in NPM1 as sole molecular abnormality, abnormal renal (s-creatinine >2× ULN) or liver function (AST or ALT > 3× ULN), or severe concurrent illness preventing additional post-remission therapy.
Treatments
Induction and consolidation therapy were given according to standard-of-care at participating centers, which varied between countries. The protocol recommended as conditioning regimens either the use of fludarabine 5–6 days (150–180 mg/sqm) combined with busulphan either given orally (total dose 8–11 mg/kg) or iv (Busulphex®; total dose 6.4 mg/kg) or a combination of fludarabine, carmustin, and melphalan. As graft-versus-host disease (GVHD) prophylaxis before URD HCT either anti-thymocyte globuline (ATG) or alemtuzumab was allowed. Recommended immunosuppression was ciclosporin (CyA) combined either with methotrexate (used in 63% of the patients) or mycophenolate mofetil (MMF).
Cytogenetic and disease risk features
Two independent reviewers categorized the patients as high or intermediate risk according to the European Leukemia Net 2017 genetic risk classification [10, 11]. Interpretable reports from diagnostic bone marrow samples were available for 262 patients (85%). Patients with favorable risk cytogenetics were excluded including those with only NPM1 mutation.
Clinical high-risk features were defined as more than two inductions to reach CR1, secondary AML, and blasts ≥15% after first induction. Patients with any high-risk feature were assigned to the high-risk group. Risk factors in the different cohorts are presented in Supplementary table 1.
Ethics approval
The study protocol and ensuing amendments were approved by the Ethical Committee at University of Gothenburg for Swedish patients (S 240-04, S 288-03, S-266-03, S 272-03, S-231-12), and by the corresponding authorities in participating centers and countries. All patients signed informed consent. The study was performed in accordance with all relevant regulations and guidelines in the participating countries.
Administrative information
The study was registered at ClinicalTrial.gov (CTN #00342316).
Statistics
Additional information about statistical analysis is given in Supplementary information.
The primary endpoint was OS in an intention to treat (ITT) setting. Baseline and treatment data were compared between ITT groups by Fisher´s exact tests and Wilcoxon´s rank-sum tests. Kaplan–Meier plots were used for illustration of time-to event endpoints. All endpoints (OS, RFS, NRM and RI) were determined from date of inclusion. The patients were grouped into strata A-D according to Fig. 1 based on the partition of the study in Part 1 and Part 2 and possible type of donor. In the ITT analyses, it was assumed that treatment allocation within strata was random. OS and RFS were compared by stratified log-rank tests and NRM and RI by stratified Gray´s tests considering competing risks. Three-year values with 95% confidence intervals are also presented. Hazard ratios (HR) were determined by means of stratified Cox regression. Proportions of patients with acute and chronic GVHD were analyzed with Fisher´s Exact test. Stata version 14 and R version 4.1.0 were used in the statistical analyses.
Results
Patient population and distribution
The study population is described in Fig. 1. 360 patients were registered into the study. Twenty patients never reached CR and were therefore not included. Thus, 340 patients were included in the study population. 31 were excluded due to donor search initiation before inclusion (n = 19), low-risk cytogenetics (n = 9), missing consent (n = 2), and missing data (n = 1). Moreover, three patients withdrew consent within the first three months after study inclusion. These are included in the analysis until date of consent withdrawal.
Thus, the analyzed study cohort included 309 patients of which 135 were included in Part 1 (2003–April 2012), and 174 in Part 2 (May 2012–2016) of the study. The age of patients (median 62 vs 64 years) was similar in the two parts. The median unrelated donor age was 25 (18–68) years (Part 2), whereas sibling donors were older; 60 (48–76) years; p < 0.001; Parts 1 and 2). The first patient was included December 18, 2003, and the last patient was included July 19, 2016. Data was analyzed as of August 1, 2018.
RD versus Controls; study parts 1 and 2 combined
The patient and donor distributions in the different study parts are shown in Fig. 1. 71 patients were assigned to the RD/donor and 64 to the Control/no donor group in Part 1 of the study (Fig. 1; Stratum A). Some study sites continued during the 2nd part of the study including only patients fulfilling the criteria of study Part 1 (Stratum B: RD; n = 8, and Controls; n = 5).
In study Part 2, 45 patients had RD and patients without either RD or URD (n = 8) were considered as controls for both the RD and URD comparisons (Stratum C). Thus, the total number of patients with RD was 124 (71 + 8 + 45) and the number of Controls was 77 (64 + 5 + 8). In the Control group, 8/77 (8%) patients were treated off-protocol by allografting from alternative donors. These patients were included in the intention to treat (ITT) analysis as Controls.
URD versus Controls; study part 2
An URD was identified for 96 patients. Twenty patients without an identified donor were Controls to the URD group (Fig. 1; Strata C and D). Six patients underwent transplants from either cord blood or haploidentical donors. These patients were included as Controls in the ITT analysis. Two of these patients (from Stratum C) were controls also for the RD versus Controls comparison.
RD versus Control (Part 1 and 2)
201 patients were included in the RD versus Control ITT comparison, 138 (69%) of whom died during follow-up. Median (min–max) follow-up time for the surviving patients was 76 (1–168) months. Patient characteristics were similar between RD and Control groups (Table 1). Ninety-seven patients underwent RICT at a median of 10 (3–33) weeks post inclusion while 27/124 (22%) patients did not reach transplant due to early relapse (n = 18), medical infirmity (n = 4), withdrawn consent (n = 2) or death (n = 3).
Table 1.
Characteristics of patients in the RD vs Control primary endpoint analysis.
| Controls (n = 77) | RICT/RD (n = 124) | p-value | |
|---|---|---|---|
| Gender F/M – n (%) | 36 (47)/41 (53) | 55 (44)/69 (56) | NS |
| Median age at Inclusion, years (min–max) | 63 (51–70) | 63 (51–71) | NS |
| Risk group IR/HR – n (%) | 50 (65)/27 (35) | 68 (55)/56 (45) | NS |
| Median time from diagnosis to inclusion, days (min–max) | 64 (32–256) | 64 (29–319) | NS |
| Given therapy | |||
| Chemotherapy only | 69 | 27 | |
| Transplanted | 8a | 97b | |
| Median time from inclusion to transplant, days (min–max) | 73 (23–236) | ||
| Causes of death – n (%) | 0.003 | ||
| GvHD or related infection | – | 9 (7) | |
| Infection | 1 (1) | 11 (9) | |
| Other | 3 (4) | 4 (3) | |
| Relapse | 50 (65) | 54 (44) | |
| Secondary malignancy | 3 (4) | 3 (2) | |
a6 URD, 1 cord blood, 1 haploidentical HCT.
bAll RICT/RD.
Overall survival
81/124 (65%) and 57/77 (74%) patients died in the RD and Control groups, respectively. Most deaths were due to relapse; 67% in the RD group and 88% in the Control group. Other causes are shown in Table 1. OS is shown in Fig. 2. At three years it was 0.41 (95% CI; 0.32–0.50) and 0.49 ((95% CI; 0.37–0.59); p = 0.50, logrank test) in the RD and control group, respectively. The data are not consistent with a constant hazard ratio over time: HR = 2.31 (95% CI: 1.34–4.01) before one year and HR = 0.57 (0.34–0.95) after one year (p = 0.0003 for difference) suggesting relatively higher mortality in the RD group the first year after inclusion and lower thereafter.
Fig. 2. Overall survival from study inclusion in RD vs Controls.
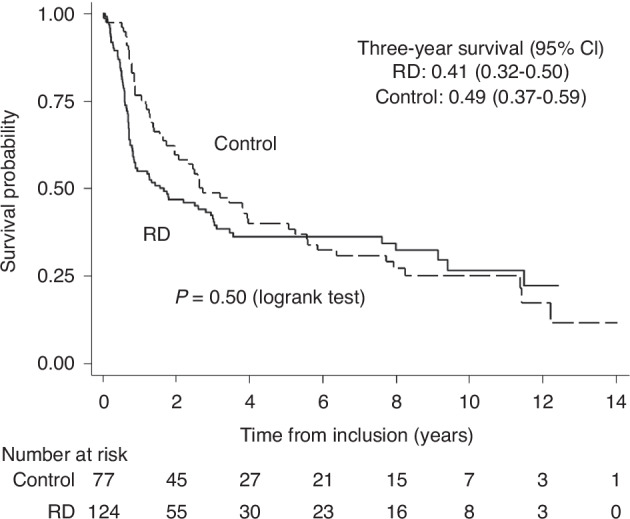
Kaplan-Meier estimates of 3-year survival in RD and control groups (study parts 1 and 2).
In an analysis performed with RD transplant as a time-dependent covariate, the analysis reflects the given treatment: A patient always starts as a Control patient and shifts to an RD patient at transplantation. The HR was 0.82 (0.56–1.18, p = 0.28) between the hazard for patients who received a transplant to the those who did not. Follow-up was censored at transplantation for the eight Controls transplanted off-protocol with alternative donors.
Other outcomes
RFS: 85/124 (69%) and 60/77 (78%) events occurred in the RD and Control groups, respectively (HR = 0.97 (0.69–1.37); p = 0.87). The RFS at 3 years was 0.38 (95% CI 0.29–0.46) in RD patients and 0.35 (95% CI 0.24–0.45) in Controls.
NRM: 27/124 (22%) and 7/77 (9%) non-relapse deaths occurred in the RD groups and Control groups, respectively. With relapse as a competing event, the cumulative NRM incidence at 3 years was 0.17 (0.11–0.25) in the RD group, and 0.039 (0.01–0.10) in Controls (p = 0.033, Gray’s test). RI: 58/124 (47%) and 53/77 (69%) patients relapsed in the RD and Control groups, respectively; With NRM as a competing risk, the cumulative relapse incidence at 3 years was 0.45 (95% CI 0.36–0.54) in the RD group, and 0.61 (95% CI 0.50–0.71) in Controls (p = 0.097, Gray’s test).
Unrelated Donor (URD) versus No donor (Part 2)
Results. At study closure, 65/116 (56%) patients were alive after a follow-up of median 39 (3–70) months. 16/96 (17%) of the patients in the URD group did not undergo transplantation.
39/96 (41%) patients in the URD-group and 12/20 Controls (60%) died. Patient characteristics and causes of death are shown in Table 2.
Table 2.
Characteristics of patients in the URD vs Control analysis.
| Controls (n = 20) | RICT/URD (n = 96) | p-value | |
|---|---|---|---|
| Gender F/M – n (%) | 10 (50)/10 (50) | 36 (38)/60 (63) | NS |
| Median age at Inclusion, years (min–max) | 63 (55–69) | 64 (52–71) | NS |
| Risk group IR/HR – n (%) | 10 (50)/10 (50) | 46 (48)/50 (52) | NS |
| Median time from diagnosis to inclusion, days (min–max) | 57 (32–160) | 64 (28–244) | NS |
| Given therapy | |||
| Chemotherapy only | 14 | 16 | |
| Transplanted | 6a | 80b | |
| Median time from inclusion to transplant, days (min–max) | 93 (23–302) | ||
| Cause of death – n (%) | NS | ||
| GvHD or rel inf | – | 1 (1) | |
| Infection | 1 (5) | 5 (5) | |
| Other | 0 | 3 (3) | |
| Relapse | 10 (50) | 30 (31) | |
| Secondary malignancy | 1 (5) | 0 | |
a1 cord blood, 5 haploidentical HCT.
bAll RICT/URD.
The three-year OS was 0.60 (95% CI 0.50–0.70) in the URD-HCT group and 0.37 (95% CI 0.13–0.62) in the Control group (p = 0.10; Fig. 3). The HR comparing URD-HCT and Controls was 0.59 (95% CI 0.31–1.12; p = 0.11). There was neither any significant difference in RFS between URD-HCT and Controls (three-year values with 95% CI 0.52 (0.41,0.61) and 0.39 (0.19–0.60) respectively, p = 0.21), nor in NRM (0.10 (0.05–0.17) and 0.10 (0.02–0.27), p = 0.85), or RI (0.39 (0.29–0.48) and 0.51 (0.27–0.70), p = 0.32.
Fig. 3. Overall survival from study inclusion in patients without potential sibling donor vs. controls.
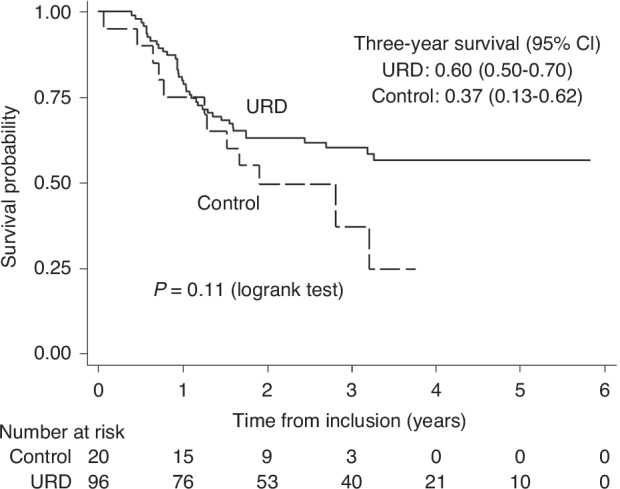
Kaplan-Meier estimated 3-year survival in patients without potential sibling donor (only study part 2).
RD versus URD versus Controls, according to given treatment (Part 2)
OS from study inclusion was analyzed with one time-varying covariates for each type of transplant. The HR´s were 0.70 (0.39–1.26) for RD-HCT versus Controls, and 0.27 (0.15–0.47) for URD-HCT versus Controls. A direct comparison yields that HR for URD-HCT was significantly lower than for RD-HCT (p = 0.0041) indicating that survival after URD was superior to RD.
RD versus URD from transplantation (Part 2)
Patient characteristics are shown in Table 3. At study closure, 73 of 123 (59%) patients were alive after median 38 (1–66) months of follow-up from transplants. 24/43 (56%) patients died in the RD and 26/80 (33%) in the URD group. The OS was higher (p = 0.005) for patients undergoing URD (Fig. 4). Three year survival was 0.42 (0.26–0.57) in the RD-group and 0.67 (0.55–0.76) in the URD group. Furthermore, RFS was higher in the URD group (3-year RFS 0.61 [0.49,0.71] vs. 0.36 [0.21,0.51]; p = 0.01), NRM was lower (0.11 [0.05,0.19] vs. 0.30 [0.16,0.44]; p = 0.01), while there was no significant difference in RI (0.29 [0.19,0.39] vs 0.34 [0.20,0.49]; p = 0.46).
Table 3.
Characteristics of RD vs URD patients in study period 2.
| RICT/RD (n = 43) | RICT/URD (n = 80) | p-value | |
|---|---|---|---|
| Gender F/M – n (%) | 18 (42)/25 (58) | 30 (38)/50 (63) | NS |
| Median age at Inclusion, years (min–max) | 63 (53–71) | 65 (53–71) | NS |
| Time from CR to inclusion, days (min–max) | 14 (0–173) | 14 (0–138) | NS |
| Time from Inclusion to HCT days (min–max) | 79 (23–236) | 93 (23–302) | |
| Risk group IR/HR – n (%) | 16 (37)/27 (63) | 35 (44)/45 (56) | NS |
| Flu/Bu p.o./Not Flu/Bu p.o. – n (%) | 16 (37)/27 (63) | 50 (63)/30 (38) | 0.008 |
| No ATG/ATG – n (%) | 33 (77)/10 (23) | 5 (6)/75 (94) | <0.0001 |
| Immunosuppression treatment – n (%) | 0.008 | ||
| CyA+MTX | 22 | 61 | |
| Other | 21 | 19 | |
| Donor age, median, yr (range) | 61 (49–73) | 25 (18–68) | <0.0001 |
| Female donor to male recipient – n (%) | 0.052 | ||
| Yes | 10 (23) | 7 (9) | |
| No | 33 (77) | 73 (92) |
Fig. 4. Overall survival in patients transplanted from unrelated or sibling donors.
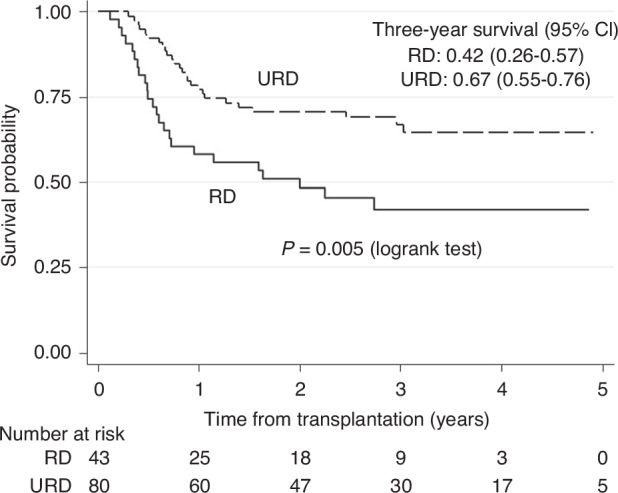
Kaplan-Meier estimates of 3-year survival in patients transplanted from URD or RD (only study period 2).
Acute and chronic GVHD in RD (Parts 1 + 2) and URD (Part 2)
There was a lower risk for acute GVHD grades III–IV in patients undergoing URD HCT (6% URD vs 19% RD; p = 0.01) and a lower risk for extensive chronic GVHD at 12 months (URD 12.7% vs. RD 40.3%; p < 0.001), 24 months (9.6% URD vs. 38.3% RD; p < 0.001), and 36 months (8.3% URD vs. 40% RD; p = 0.003) after transplantation compared to RD HCT patients (Supplementary Fig. 1).
Discussion
Whether RICT allogeneic HCT in elderly patients with AML results in improved long-term survival has been discussed for many years. It has been exceedingly difficult to perform proper randomized prospective studies in allogeneic HCT in general due to the donor selection process including the willingness of patients and donors to be randomized to non-HCT therapy. Studies using other approaches have reported that RICT-HCT can result in long-term leukemia-free survival [1, 12–16]. However, a recent large prospective cohort study has challenged this concept in elderly patients and those with co-morbidities [17].
The aim of this study was to use pseudo-randomization based on the availability of at least one sibling willing to be typed and with the starting date either the day of a sample obtained for HLA-typing of a potential sibling donor or the day of CR if typing was performed before the patient entered CR. Our study, designed now several years ago, shows now with extended follow-up no advantage for a reduced intensity allogeneic-HCT with a sibling donor compared to Controls. As expected, the NRM was higher in the transplant group compared to Controls and although there was a slightly higher incidence of relapse in the Controls, this did not compensate for the higher risk for NRM. Similar results were seen when an URD was used, but the low number of Controls makes it difficult to draw any firm conclusion from this comparison. The main cause of death in all study groups was leukemia relapse and not NRM. Many relapses occurred in the “transplant groups” before the patient could get to transplant.
Several important advances in supportive care especially the introduction of new drugs against infections have occurred since the study was initiated improving the outcome of allogeneic HCT. Furthermore, the selected conditioning regimen has been shown to be inferior to more intensive conditioning regimens[10, 18], and it is possible that a more intensive but still reduced intensity regimen could have yielded better results. Interestingly, a large prospective study including patients during the latter part of our study period (2013–2017) also failed to show a survival benefit in elderly patients and in these who were medically infirm [17]. Moreover, new alternatives for non-transplant therapy of AML in the elderly have been introduced changing the therapeutic landscape [19]. The study included patients with a median age of 62–64 years in the different groups so not real elderly according to what is the clinical practice today. This, however, ought to have reduced rather than increased the risk for NRM if we translate the results to today’s situation.
The results of URD transplants were better than the results of HLA-identical sibling transplants with higher OS and RFS. Moreover, the NRM and the risks for especially severe acute and chronic GVHD were lower in the URD group suggesting that the best donor for an elderly patient might not be an HLA-identical sibling. This might be due to the positive impact of younger donor age on outcome of allo-HCT as shown in other studies [20–22]. Indeed, the median unrelated donor age was 25 years compared to 61 years in the RD group. It is important, however, that 94% of URD patients received ATG compared to only 11% of the patients receiving HLA-identical sibling donor transplants, which may explain the lower frequency of severe GVHD in the URD-group.
The strengths of this study are that it is large and multinational with analyses based on biological randomization enabling unbiased comparisons between HCT and Control. There are, however, also several weaknesses most importantly that many developments have occurred since the design of the study. Furthermore, the controls for the URD group were few making it difficult to draw conclusions from this comparison. Moreover, molecular risk stratification has not been evenly applied to study patients and Hematopoietic cell transplant comorbidity score data was not collected.
In summary, this study doesn’t support the use of elderly HLA-identical siblings as donors for older AML patients undergoing a RICT allogeneic HCT in first CR. It moreover indicates that a younger URD might be superior to an elderly RD.
Supplementary information
Acknowledgements
Inger Andersson and Joanne Blais for their work for the study. Economic support was granted by the Swedish Research Council (2012-38102-91941-45), the Swedish Cancer Foundation, CA 2017/364, 2017/369, the Swedish State under the ALF agreement, Lion´s Cancer Research Fund of Western Sweden, and NordForsk Project No. 71306. The study was supported by Cell Therapy Transplant Canada (formerly named Canadian Blood and Marrow Transplant Group), and Otsuka pharmaceuticals (Canada).
Author contributions
MB, PL, TK designed the study. MB, TK obtained funding. HA performed the statistical analyses, MN, EW worked on the data collection and data management. PL, MB, MN, EW, HA wrote the paper. All other authors participated as investigators, collected data, and reviewed the manuscript.
Funding
Open access funding provided by Karolinska Institute.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to patient and donor confidentiality but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mats Brune, Thomas Kiss.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-024-02408-x.
References
- 1.Feinstein LC, Sandmaier BM, Hegenbart U, McSweeney PA, Maloney DG, Gooley TA, et al. Non-myeloablative allografting from human leucocyte antigen-identical sibling donors for treatment of acute myeloid leukaemia in first complete remission. Br J Haematol. 2003;120:281–8. [DOI] [PubMed] [Google Scholar]
- 2.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. [DOI] [PubMed] [Google Scholar]
- 3.Ustun C, Le-Rademacher J, Wang HL, Othus M, Sun Z, Major B, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33:2599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farag SS, Maharry K, Zhang MJ, Perez WS, George SL, Mrozek K, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transpl. 2011;17:1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungman P, de WT, Verdonck L, Gahrton G, Freycon F, Gravett P, et al. Bone marrow transplantation for acute myeloblastic leukaemia: an EBMT Leukaemia Working Party prospective analysis from HLA-typing. Br J Haematol. 1993;84:61–6. [DOI] [PubMed] [Google Scholar]
- 7.Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups [see comments]. N Engl J Med. 1995;332:217–23. [DOI] [PubMed] [Google Scholar]
- 8.Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission [see comments]. N Engl J Med. 1998;339:1649–56. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–66. [DOI] [PubMed] [Google Scholar]
- 10.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. [DOI] [PubMed] [Google Scholar]
- 12.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–72. [DOI] [PubMed] [Google Scholar]
- 14.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–93. [DOI] [PubMed] [Google Scholar]
- 15.Blaise D, Farnault L, Faucher C, Marchetti N, Furst S, El Cheikh J, et al. Reduced-intensity conditioning with Fludarabin, oral Busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol. 2010;38:1241–50. [DOI] [PubMed] [Google Scholar]
- 16.Devillier R, Forcade E, Garnier A, Guenounou S, Thepot S, Guillerm G, et al. In-depth time-dependent analysis of the benefit of allo-HSCT for elderly patients with CR1 AML: a FILO study. Blood Adv. 2022;6:1804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Gooley TA, Storer BE, Gerds AT, Sekeres MA, Medeiros BC, et al. An 8-year pragmatic observation evaluation of the benefits of allogeneic HCT in older and medically infirm patients with AML. Blood. 2023;141:295–308. [DOI] [PubMed] [Google Scholar]
- 18.Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–e39. [DOI] [PubMed] [Google Scholar]
- 19.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 20.Bastida JM, Cabrero M, Lopez-Godino O, Lopez-Parra M, Sanchez-Guijo F, Lopez-Corral L, et al. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leuk Res. 2015;39:828–34. [DOI] [PubMed] [Google Scholar]
- 21.Ayuk F, Beelen DW, Bornhauser M, Stelljes M, Zabelina T, Finke J, et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transpl. 2018;24:2558–67. [DOI] [PubMed] [Google Scholar]
- 22.Seo S, Usui Y, Matsuo K, Atsuta Y, Igarashi A, Fukuda T, et al. Impact of the combination of donor age and HLA disparity on the outcomes of unrelated bone marrow transplantation. Bone Marrow Transpl. 2021;56:2410–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to patient and donor confidentiality but are available from the corresponding author on reasonable request.