Abstract
Human herpesvirus-6B (HHV-6B) reactivation has been associated with non-relapse mortality (NRM) and overall mortality (OM) following allogeneic hematopoietic stem cell transplant (HCT). We performed a systematic review and meta-analysis to better quantify the association. Studies were included if they systematically tested a cohort of HCT recipients for HHV-6 infection or reactivation and described mortality for patients with and without HHV-6B. Random effects models were used to assess the pooled effect of HHV-6B positivity on each outcome of interest. Bayesian aggregation was additionally performed if models included 10 or fewer studies. Eight studies were included in the NRM analysis, which demonstrated a significant association between HHV-6 detection and NRM (pooled effect: 1.84; 95% CI: 1.29–2.62) without significant heterogeneity (I2 = 0.0%, p = 0.55). A Bayesian aggregation of the raw data used to construct the NRM random effects model supported these findings (95% credible interval: 0.15–1.13). Twenty-five studies were included in OM analysis, which showed a significant positive association (pooled effect: 1.37; 95% CI: 1.07–1.76), though considerable heterogeneity was observed (I2 = 36.7%, p < 0.05). HHV-6 detection is associated with NRM and OM following HCT. Randomized trials are warranted to evaluate if preventing or treating HHV-6B reactivation improves outcomes.
Subject terms: Haematological cancer, Haematological diseases
Introduction
HHV-6 is a member of the Roseolovirus genus of the beta-herpesvirus subfamily of human herpesviruses [1]. Human herpesvirus 6 (HHV-6) is the collective name of two HHV-6 species, HHV-6A and HHV-6B. The epidemiology of HHV-6A has not been defined. In contrast, HHV-6B infects most individuals in early childhood and the virus reactivates in 30-70% of HCT recipients [2–6]. Because HHV-6B is the pathogenic species known to reactivate in HCT, the current work focuses on evaluating HHV-6B and mortality [7]. Risk factors associated with HHV-6B reactivation include receiving allogeneic (versus autologous) HCT, myeloablative conditioning regimen, transplants from unrelated or human leukocyte antigen (HLA)-mismatched donors, and umbilical cord blood transplants [3, 4, 8–13]. HHV-6B reactivation has been associated with subsequent encephalitis, central nervous system (CNS) dysfunction, bone marrow suppression, acute graft-versus-host-disease (aGVHD), cytomegalovirus (CMV) reactivation, lower respiratory tract disease, and mortality [2, 4, 5, 8–10, 13–29].
While some studies have reported an association between HHV-6B and mortality [20, 22, 30–36], results have been conflicting [37]. To date, no prior work has systematically aggregated data from studies of HHV-6B and mortality. The goal of the current study is to fill this gap and characterize mortality outcomes in HCT recipients documented to have HHV-6B reactivation compared to those without HHV-6B reactivation.
Methods
A search of the PubMed database was performed using the terms:
(hematopoietic OR stem cell OR cord blood OR bone marrow OR transplant*)
AND
(HHV-6 OR MeSH term: herpesvirus 6 OR HHV6 OR human herpesvirus-6 OR HHV-6B OR HHV6B OR human herpesvirus-6B OR HHV-6A OR HHV6A OR human herpesvirus-6A)
This search was performed according to PRISMA guidelines [38] on 2/1/24 and returned 1,319 results. Manuscripts (or studies) were screened for the following entry criteria (1) inclusion of a cohort of HCT recipients systematically tested for HHV-6 (2) report of the number of patients with HHV-6 infection or reactivation, and (3) report of the number of patients with at least one outcome of interest. Outcomes of interest included non-relapse mortality (NRM), relapse-related mortality (RM), overall survival (OS), treatment-related mortality (TRM), and overall mortality/all-cause mortality (OM/ACM). In instances where raw data describing an outcome of interest by HHV-6B positivity were unclear or not described, the study’s authors were contacted to request these data.
Definitions
NRM was defined as any mortality not attributable to relapse. ACM and OM were defined as mortality due to any reason. OS was defined as survival during the follow up period. RM was defined as mortality due to relapse of disease. TRM was defined as mortality attributed to any treatment-related cause. Studies that assessed OS or ACM were converted to OM by subtracting the number of patients who survived from the total number of patients whose outcomes were described.
Statistical analysis
Study effect sizes were calculated as odds ratios using the raw data for the number of HHV-6B positive and negative patients who did and did not experience each outcome of interest. Studies that did not include at least 10 patients with and 10 patients without HHV-6B detection were excluded from statistical models. Studies were divided into OM, RM, NRM, and TRM. Random effects models were used to pool the effect sizes using the Mantel-Haenszel method [39], with a Paule-Mandel estimator for τ2 [40] and Hartung-Knapp adjustments [41]. Models were assessed for heterogeneity using an I2 test and Cochrane’s Q [42]. Subgroup analyses by stem cell source (CBT, non-CBT, CBT and non-CBT, or source unclear), age of cohort (adult, pediatric, or both), and follow up period (less than the median follow-up period of all studies, or greater than or equal to the median follow up period of all studies) were performed. These analyses were included in the main results if all subgroups contained at least 3 studies and were otherwise detailed in the supplementary materials (pages 4-6, and 8-10) due to the limited utility of subgroup analyses with a small number of studies.
In models that contained 10 or more studies, publication bias was assessed with a linear regression of funnel plot asymmetry using the algorithm described by Peters et al. [43] for binary outcome data. In models with 10 or fewer studies, raw data were evaluated with Bayesian aggregation of the treatment effect on the logarithm of the odds ratio (logOR). The rationale for this additional analysis is that Bayesian modeling provides additional context to the results of random effects models that include a small number of studies due to the minimal impact of small datasets on Bayesian inferences. Rubin models with partial pooling and weakly informative priors were used. These models were constructed using the Markov Chain Monte Carlo method using 10 chains set to 20,000 iterations each and were not interpreted if r-hat exceeded 1.05.
Results
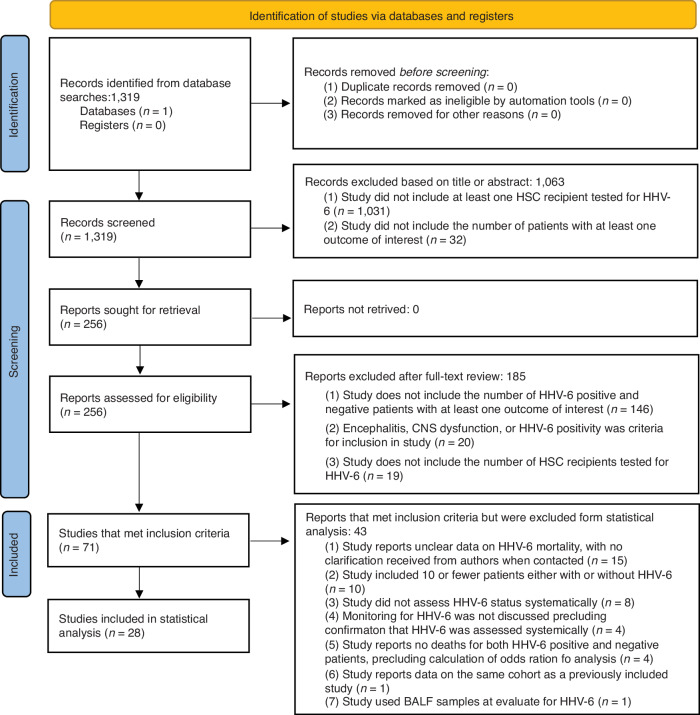
Of the 1319 results, 1063 were excluded during screening, leaving 256 articles for full-text review. During full-text review, 185 articles were excluded and 71 articles met inclusion criteria. Among studies that met inclusion criteria, 28 were included in statistical analyses (based on the minimum number of patients required) with a total of 4241 patients included across all studies [2, 20–22, 30, 32–35, 44–62]. A PRISMA flowchart detailing reasons for exclusion is provided in Fig. 1. Characteristics of patients included in analyses are given in Table 1. A full dataset with all included and excluded studies, along with all collected data and all reasons for exclusion, is provided in Supplementary Table 1. Mortality data stratified by HHV-6 status is provided in Supplementary Table 2.
Fig. 1.
PRISMA Flowchart.
Table 1.
Characteristics of Included Studies.
| Characteristics | Included in Statistcal Analysis | Included in OM Meta-Analysis (Fig. 3) | Included in NRM Meta-Analysis (Fig. 2) | Included in RM Meta-Analysis | |
|---|---|---|---|---|---|
| Total Studies | N | 28 | 25 | 8 | 7 |
| Average number of patients per study | N (range) | 151 (25–738) | 162 (26–738) | 191 (25–738) | 229 (26–738) |
| Adult vs. pediatric studies % (age range) | Adult | 9 (32.1%) | 7 (28.0%) | 3 (37.5%) | 3 (42.9%) |
| Pediatric | 8 (28.6%) | 7 (28.0%) | 2 (25.0%) | 1 (14.3%) | |
| Both | 11 (39.3%) | 11 (44.0%) | 3 (37.5%) | 3 (42.9%) | |
| Study Design | Prospective | 16 (57.1%) | 15 (60.0%) | 5 (62.5%) | 4 (57.1%) |
| Retrospective | 12 (42.9%) | 10 (40.0%) | 3 (37.5%) | 3 (42.9%) | |
| Underlying diseases (N patients across all studies) | ALL | 306 (6.2%) | 221 (4.4%) | 160 (12.5%) | 207 (15.5%) |
| AML | 355 (7.3%) | 342 (6.8%) | 206 (16.1%) | 193 (14.5%) | |
| CML | 85 (1.7%) | 78 (1.5%) | 9 (0.7%) | 12 (0.9%) | |
| NHL | 15 (0.3%) | 12 (0.2%) | 7 (0.5%) | 4 (0.3%) | |
| AML or MDS | 146 (2.9%) | 137 (2.7%) | 12 (1%) | 17 (1%) | |
| Myeloma | 157 (3.1%) | 141 (2.8%) | 0 (0.0%) | 16 (1.2%) | |
| Unspecified lymphoma | 286 (5.8%) | 275 (5.4%) | 0 (0.0%) | 11 (0.8%) | |
| Unspecified leukemia | 1490 (40.0%) | 1490 (29.5%) | 0 (0.0%) | 0 (0.0%) | |
| Neuroblastoma | 7 (0.1%) | 7 (0.1%) | 0 (0.0%) | 0 (0.0%) | |
| Unspecified malignancy | 1274 (32.3%) | 1263 (25.0%) | 668 (52.2%) | 657 (49.3%) | |
| Non-malignancy | 509 (10.8%) | 508 (10.0%) | 146 (11.4%) | 145 (10.9%) | |
| Unspecified | 583 (12.6%) | 583 (11.5%) | 72 (5.6%) | 72 (5.4%) | |
| GVHD prophylaxis | CsA | 219 (14.1%) | 219 (13.4%) | 58 (27.6%) | 58 (19.5%) |
| TAC | 89 (5.3%) | 89 (5.4%) | 0 (0.0%) | 0 (0.0%) | |
| MTX | 22 (1.3%) | 3 (0.2%) | 19 (9.0%) | 0 (0.0%) | |
| TAC + MTX | 9 (0.5%) | 9 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| Sirolimus | 161 (10.0%) | 161 (9.8%) | 0 (0.0%) | 0 (0.0%) | |
| Cyclophosphamide | 101 (6.0%) | 101 (6.2%) | 0 (0.0%) | 0 (0.0%) | |
| ATG | 136 (8.3%) | 136 (8.3%) | 0 (0.0%) | 0 (0.0%) | |
| alemtuzumab | 34 (2.0%) | 34 (2.1%) | 0 (0.0%) | 0 (0.0%) | |
| CsA + prednisolone | 172 (10.7%) | 172 (10.5%) | 0 (0.0%) | 0 (0.0%) | |
| CsA + unspecified steroids | 26 (1.5%) | 26 (1.6%) | 26 (12.4%) | 26 (8.7%) | |
| CsA + MTX | 436 (32.5%) | 431 (26.3%) | 5 (2.4%) | 0 (0.0%) | |
| CsA + MMF | 82 (4.8%) | 82 (5.0%) | 0 (0.0%) | 0 (0.0%) | |
| CsA + MMF + TAC | 53 (3.1%) | 0 (0.0%) | 0 (0.0%) | 53 (17.8%) | |
| MMF + MTX | 3 (0.2%) | 3 (0.2%) | 0 (0.0%) | 0 (0.0%) | |
| CsA MMF + MTX | 174 (10.9%) | 174 (10.6%) | 102 (48.6%) | 102 (34.2%) | |
| TAC/MTX/MMF | 59 (3.4%) | 0 (0.0%) | 0 (0.0%) | 59 (19.8%) | |
| Conditioning regimen | RIC | 1179 (34.7%) | 1170 (34.6%) | 400 (27.1%) | 391 (27.0%) |
| MAC | 2223 (65.3%) | 2207 (65.4%) | 1074 (72.9%) | 1058 (73.0%) | |
| Studies that administered antiviral prophylaxis (N) | ACY | 12 (42.9%) | 10 (40.0%) | 2 (25.0%) | 2 (28.6%) |
| GAN | 2 (7.1%) | 2 (8.0%) | 0 (0.0%) | 0 (0.0%) | |
| ACY and GAN | 4 (14.3%) | 4 (16.0%) | 1 (12.5%) | 1 (14.3%) | |
| IVIg | 1 (3.6%) | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | |
| Unclear or ND | 9 (32.1%) | 9 (36.0%) | 4 (50.0%) | 4 (57.1%) | |
| Outcomes reported | (Range of mortality %) | 4–65% | 7–65% | 4–42% | 6–30% |
| Mean follow-up period | (Days and range) | 99.9 (28–365) | 99.9 (28–365) | 87.6 (28–120) | 87.6 (28–120) |
| HHV-6 Monitoring frequency | 2x per week | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) |
| 1x per week | 15 (53.6%) | 12 (48.0%) | 4 (50.0%) | 3 (42.9%) | |
| 1x per 2 weeks | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | |
| Other | 5 (17.9%) | 5 (20.0%) | 2 (25.0%) | 2 (28.6%) | |
| Unclear or ND | 6 (21.4%) | 6 (24.0%) | 2 (25.0%) | 2 (28.6%) | |
| Samples used for testing (N) | Whole blood | 5 (17.9%) | 5 (20.0%) | 0 (0.0%) | 0 (0.0%) |
| Plasma | 17 (60.7%) | 16 (64.0%) | 7 (87.5%) | 6 (85.7%) | |
| PBMC | 3 (10.7%) | 2 (8.0%) | 1 (12.5%) | 0 (0.0%) | |
| Unclear or ND | 3 (10.7%) | 2 (8.0%) | 0 (0.0%) | 1 (14.3%) | |
| Threshold for HHV-6 detection, copies/mL (N) | 1000 | 4 (14.3%) | 4 (16.0%) | 1 (12.5%) | 1 (14.3%) |
| 500 | 3 (10.7%) | 3 (12.0%) | 1 (12.5%) | 1 (14.3%) | |
| 200 | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | |
| 125 | 1 (3.6%) | 1 (4.0%) | 1 (12.5%) | 1 (14.3%) | |
| 120 | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | |
| 100 | 2 (7.1%) | 2 (8.0%) | 1 (12.5%) | 1 (14.3%) | |
| 50 | 3 (10.7%) | 3 (12.0%) | 0 (0.0%) | 0 (0.0%) | |
| 25 | 3 (10.7%) | 3 (12.0%) | 1 (12.5%) | 1 (14.3%) | |
| 20 | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | |
| 10 | 1 (3.6%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | |
| Unclear or ND | 8 (28.6%) | 5 (20.0%) | 3 (37.5%) | 2 (28.6%) | |
| Type of HCT | BMT | 921 (16.5%) | 850 (15.7%) | 154 (11.8%) | 914 (76.0%) |
| CBT | 753 (13.5%) | 750 (13.8%) | 864 (66.1%) | 289 (24.0%) | |
| PBSC | 1382 (24.7%) | 1292 (23.8%) | 289 (22.1%) | 0 (0.0%) | |
| Unspecified | 2538 (45.4%) | 2538 (46.7%) | 0 (0.0%) | 0 (0.0%) |
ALL Acute lymphoblastic leukemia, AML acute myeloid leukemia, CML chronic myeloid leukemia, NHL Non-Hodgkin lymphoma, MDS Myelodysplastic syndrome, RIC Reduced-intensity conditioning, MAC myeloablative conditioning, ACY Acyclovir, GAN Ganciclovir, ND Not discussed, OM Overall mortality, RM Relapse mortality, NRM Non-relapse mortality.
Of the 28 studies included in statistical analyses, 25 provided OM data, 8 provided NRM data, 7 provided RM data, and 1 provided TRM data. Models were not built to assess TRM because only 1 study was identified. Some studies described multiple outcomes of interest and were thus included in multiple analyses. Subgroup analyses for studies assessing OM were included in main results. An insufficient number of studies included the data required for subgroup analyses of RM and NRM; these results are available in the supplementary materials on pages 4-6, and 8-10 for completeness but are not described in the main results.
HHV-6B detection and non-relapse mortality
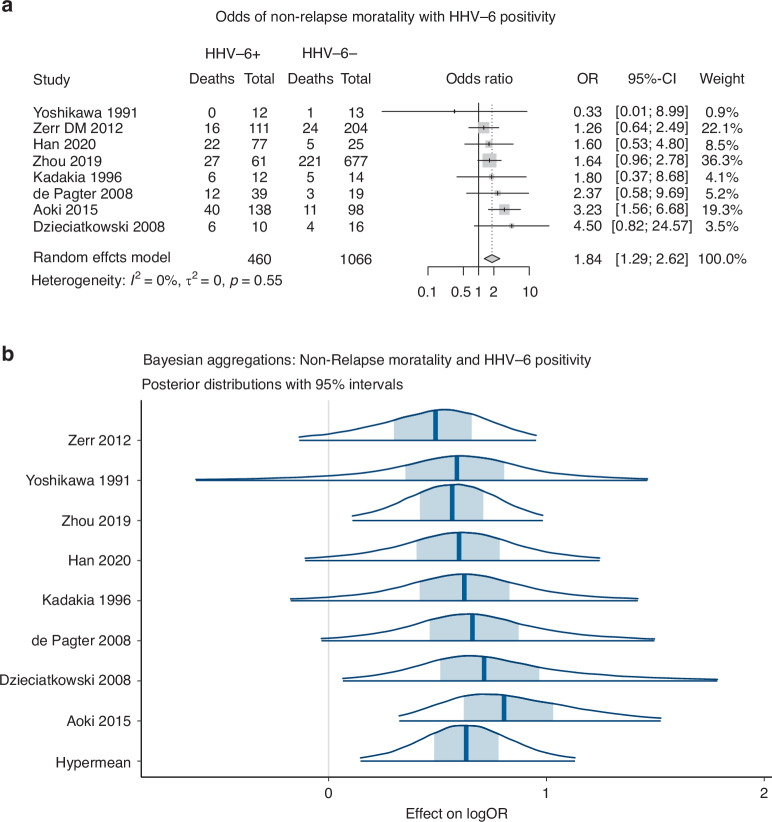
Eight studies were included in analysis of non-relapse mortality. A random effects model demonstrated a pooled effect size of 1.84 (95% CI: 1.29–2.62, p < 0.01), indicating that patients with HHV-6B detection had significantly increased odds of NRM (Fig. 2a). The model did not exhibit significant heterogeneity, with 0% of the observed effects attributed to variation between studies (I2 = 0.0%, Q = 5.94, p = 0.55).
Fig. 2.
a Non relapse mortality associated with HHV-6 positivity: Random effects model. b Non relapse mortality associated with HHV-6 positivity: Bayesian Aggregation.
Due to the small number of studies assessed, a Bayesian aggregation was performed to understand if the observed association may be influenced by the limited number of studies. The hypermean of the aggregate treatment effect on logOR was 0.63, with a 95% credible interval of 0.15 to 1.13 (Fig. 2b). This model can be interpreted as indicating that there is a 95% probability that patients diagnosed with HHV-6B infection or reactivation were between 15% and 113% more likely to have an outcome of NRM. These results provide reasonable confidence that HHV-6B positivity predicts non-relapse mortality, in support of the random effects model.
HHV-6B detection and overall mortality
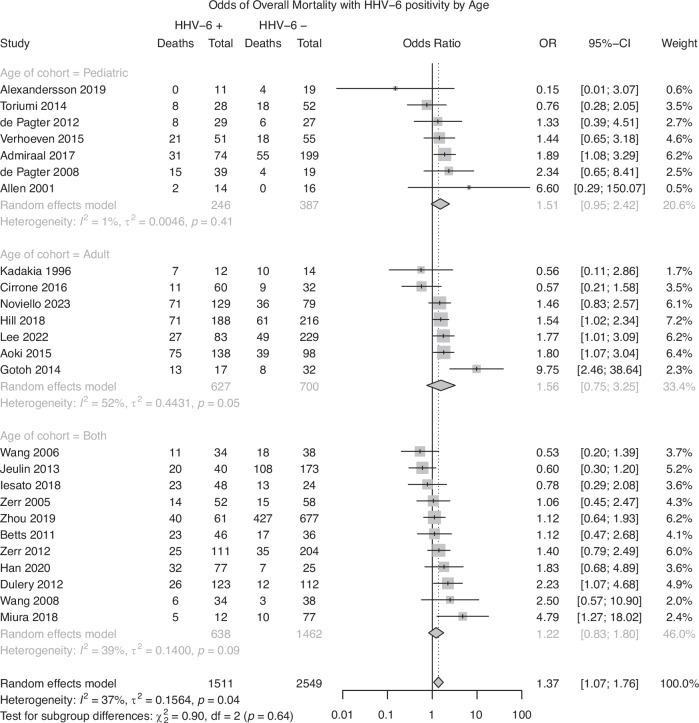
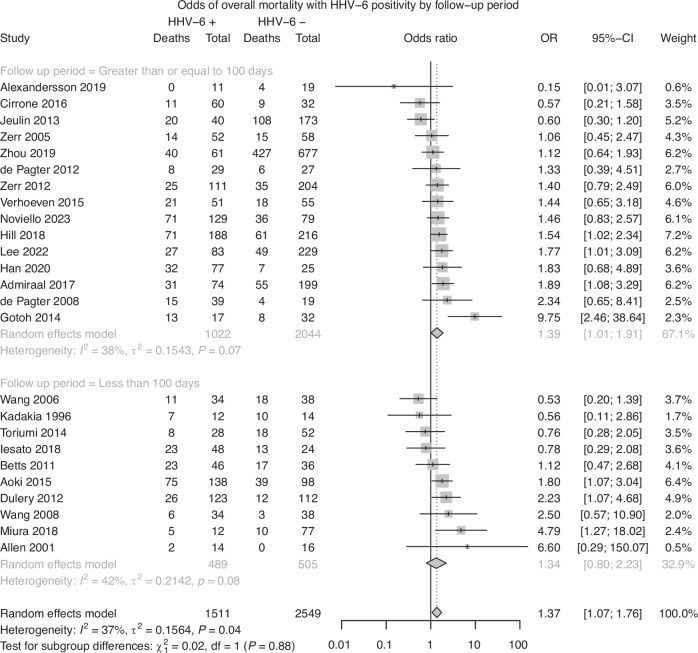
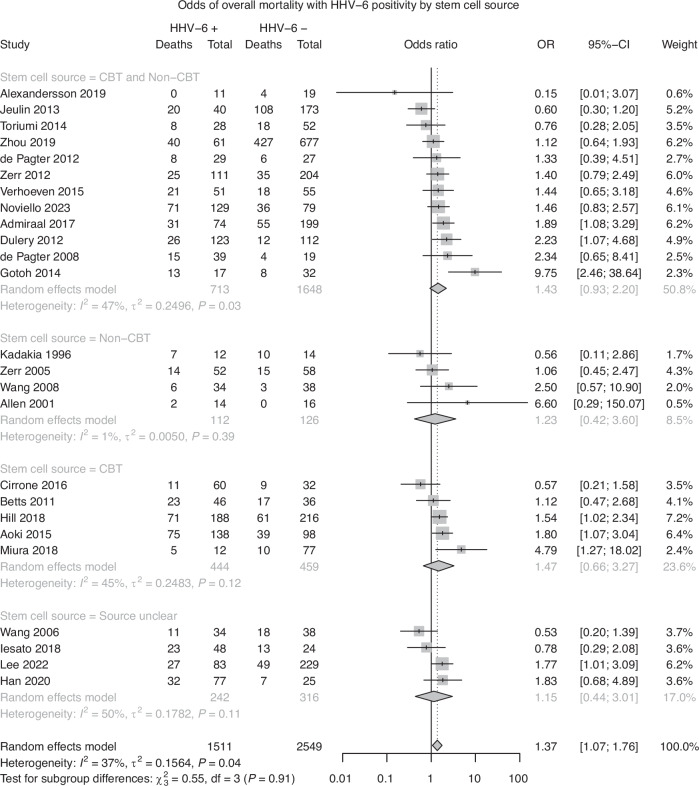
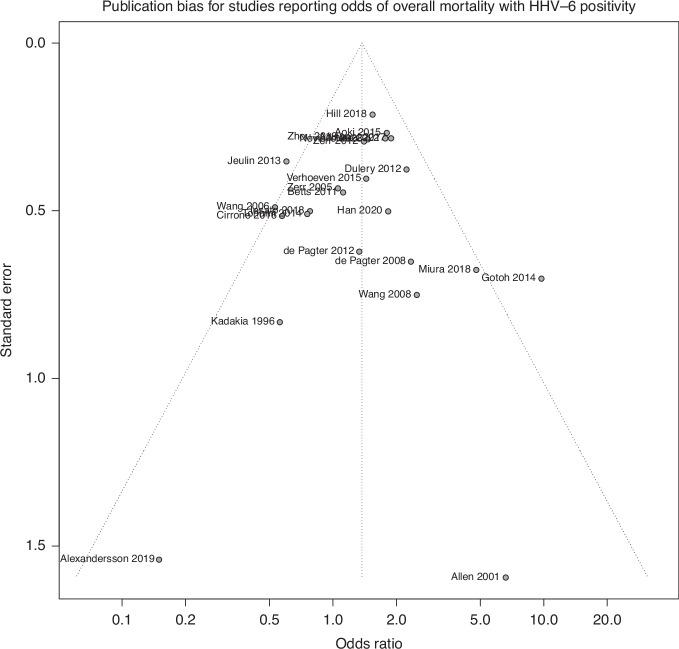
Twenty-five studies were included in analysis of overall mortality. A random effects model demonstrated a pooled effect size of 1.37 (95% CI: 1.07–1.76, p < 0.05), indicating that patients with HHV-6B detection had significantly increased odds of death due to any cause. Significant heterogeneity was observed in the model, with 37% of the pooled effect attributable to between-study heterogeneity (I2 = 36.7%, Q = 37.9, p < 0.05). The heterogeneity observed in the model suggests that the observed effect may be due to variation between studies, rather than a true pooled effect. Subgroup analyses did not reveal a significant difference between groups based on stem cell source (Q = 0.55, p = 0.91; Fig. 3a), age (Q = 0.90, p = 0.64; Fig. 3b), or follow-up period based on a median follow-up period of 100 days (Q = 0.02, p = 0.88; Fig. 3c). A test of funnel plot asymmetry did not indicate a significant influence of publication bias on the current results (t = −0.18, p = 0.85; Fig. 4).
Fig. 3.
a Risk of Overall Mortality with HHV-6 Positivity, Subgroup analysis by Stem Cell Source. b Risk of Overall Mortality with HHV-6 Positivity, Subgroup analysis by Age. c Risk of Overall Mortality with HHV-6 Positivity, Subgroup analysis by Follow-up Period.
Fig. 4.
Funnel Plot of Studies evaluating Overall Mortality.
HHV-6B detection and relapse mortality
Seven studies were included in analysis of relapse mortality and HHV-6B positivity. A random effects model demonstrated a pooled effect size of 0.74 (95% CI: 0.28–1.96) without significant heterogeneity observed (I2 = 40.1%, Q = 10.0, p = 0.12), indicating that HHV-6B detection did not increase the odds of relapse mortality. A Bayesian aggregation was performed due to the low number of studies included, which supported a lack of association (Credible interval: −1.18 to 0.54).
Discussion
The main objective of the present work was to determine if there was a significant association between HHV-6B reactivation and mortality following HCT. We found that HHV-6B detection is associated with NRM as supported by a significant pooled effect, a lack of heterogeneity, and Bayesian aggregation, while OM is associated with HHV-6B as supported by a pooled effect limited by heterogeneity.
HHV-6B can cause encephalitis [63] and has been associated with pneumonitis [29]. However, these complications do not occur frequently enough to explain the degree of increased NRM we observed. A conjecture that more fully explains our findings is that HHV-6B may be involved in immune dysregulation after HCT, which may explain more frequent adverse events such as acute graft-vs-host disease (aGVHD). HHV-6B reactivation has been associated with delayed reconstitution in NK cells [64], as well as impaired neutrophil [65] and platelet [35] engraftment. Most importantly, HHV-6B can efficiently infect CD4 + T cells [66] and has been shown to decrease T cell reconstitution after HCT [67]. CD4 + T-cell reconstitution after HCT has been associated with improved outcomes [68–71] whereas delayed CD4 + T-cell reconstitution has been associated with increased risk of aGVHD [30] as well as increased mortality [68–71]. Furthermore, some have postulated that HHV-6 may also deplete regulatory CD4 + T cells and increase the likelihood of dysregulated immune responses, like aGVHD [66]. Studies using mouse models potentially support the theory that HHV-6-mediated immune dysregulation causes aGVHD by demonstrating that pretreatment of allografts with inhibition against OX40, the entry receptor for HHV-6B, results in decreased severity of GVHD [72]. aGVHD is a significant source of morbidity and mortality after alloHCT [73] and a 2018 meta-analysis reported that HHV-6 reactivation is independently associated with nearly a 3-fold increased risk of developing grade II to IV acute GVHD [23]. Further investigation of the role of HHV-6 may play in aGVHD is needed.
Approximately 0.3–2.9% of individuals are known to have HHV-6B or HHV-6A integrated into the chromosome of every nucleated cell, which can be passed down to offspring in a mendelian fashion (iciHHV-6). Consequently, these patients have strikingly and continuously high HHV-6 DNA viral levels in whole blood ( > 5.5 log10 copies/mL) without necessarily having demonstrated HHV-6 reactivation. It should be noted that antiviral therapy for iciHHV-6 patients in the absence of reactivation is ineffective and would only expose patients to the risk of drug side effects. Thus, it is important to distinguish between active replication and iciHHV-6, which is inconsistently reported in the context of the papers reviewed.
Treatments for HHV-6B disease are limited and there are currently no FDA-approved therapies for HHV-6B disease. Both ganciclovir and foscarnet are used off-label as first line agents for HHV-6 encephalitis [74]. Unfortunately, use of these antivirals is limited by side effects of myelosuppression (ganciclovir) and nephrotoxicity (foscarnet); for this reason, prophylactic use is not recommended [74]. Other agents, such as cidofovir have only anecdotal evidence for use [7, 74]. Clinical trials exploring the efficacy of viral specific T-cell therapies have been previously attempted but were discontinued due to a low probability of meeting the primary endpoints [73, 75]. Artesunate has also recently been studied for its in vitro effect on HHV-6 [76], but it has not been studied in the context of HSCT. Considering our findings of higher mortality associated with HHV-6B reactivation, there is an urgent need for improved antiviral agents specifically against HHV-6.
Our findings are limited by the following considerations: (1) Despite an available HHV-6 PCR international standard [7, 77], this is not widely used to date, and this limits standardization of viral load assessments across studies. (2) Only one database was accessed to perform this systematic review so there may have been literature that was missed including papers with no English translation, poster presentations, abstracts that did not undergo peer review, or other forms of grey literature. (3) Differing treatment methodologies between different hospitals further complicates the analysis due to potential confounding variables not considered such as protocols for the use of foscarnet or ganciclovir for preemptive treatment of HHV-6 or prophylaxis for CMV. (4) The median follow-up period was 100 days, which indicates that many studies have a relatively short follow-up period and highlights the need for studies assessing the effect of HHV-6B on mortality to include longer follow-up periods. Studies with relatively short follow-up periods were included in the interest of gathering comprehensive results. (5) This analysis did not account for viral load, and higher viral loads may be more strongly associated with our outcomes of interest. (6) The analysis did not adjust for confounders that could contribute to HHV-6 detection and/or mortality due to a lack of consistency in covariables across studies. For the purposes of standardization and future meta-analyses, we recommend several covariables be considered when studying outcomes associated with HHV-6B reactivation in this setting, including transplant source, preconditioning regimen, occurrence of GVHD, steroid usage, and CMV reactivation characteristics. Like HHV-6B, CMV is immunomodulatory and independently associated with increased mortality; this could be affecting our analyses, but we are unable to account for the independent contribution of CMV with the available data in the included studies. However, prior work that adjusted for CMV has shown an independent association between HHV-6 and mortality [52]. 7) Specific contributions to NRM were not consistently available for analysis, so we were unable to provide this data. However, beyond GVHD [23], there may be other factors contributing to NRM associated with HHV-6B reactivation worth investigating such as encephalitis, hepatitis, pneumonitis, myelosuppression, neutropenic fever, nephrotoxicity, and rash. 8) Publication bias was evaluated using a regression of funnel plot asymmetry, and it is possible that this method does not account for all sources of publication bias.
In conclusion, we demonstrate that HHV-6B reactivation was associated with increased NRM and weakly associated with OM in a meta-analysis of 28 studies. Due to the biological consequences of HHV-6B, HHV-6B activity might contribute to a higher NRM. These results provide quantitative context for prior work establishing a link between HHV-6B and NRM and suggest the possible need for improved therapeutic strategies to manage HHV-6B reactivation after HCT. It is critical to note that the current results suggest a correlative, but not causative, relationship between HHV-6B and mortality. Future studies must carefully account for variables that could contribute to mortality, such as the reactivation of other herpesviruses.
Supplementary information
Sup Table 1 - Full Dataset - Mortality after HCT and HHV-6.xlsx
Sup Table 2 - Mortality by HHV-6 Status.xlsx
Acknowledgements
The authors would like to thank the HHV-6 Foundation for their support of this work.
Author contributions
CS and HZ designed the study, performed data collection, wrote the manuscript, and prepared submission materials. KC designed and reviewed statistical analyses. TP and DT reviewed the data, performed data analysis, and assisted with manuscript revisions. JAH and DMZ provided expert review of all materials and assisted in project design.
Data availability
All scripts used to perform analyses and generate figures and all raw data used in analyses are publicly available at https://github.com/dannytoomey/hhv6-mortality-ma.
Code availability
Analyses were performed using the R programming language, version 4.3.2. All scripts and raw data used in analysis are publicly available on GitHub [78].
Competing interests
CS, HZ, KC, TP, and DT have no conflicts to disclose. JAH provides consulting for Allovir, Gilead, Karius, Symbio, and receives research support from Allovir, Gilead, Karius, and Merck. DMZ received research funding from Merck and served as a consultant for AlloVir by serving on clinical endpoint adjudication committees for two studies.
Ethics approval and consent to participate
Our study is exempt from IRB approval as all data is publicly available.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christopher J. Stathis, Harrison Zhu.
These authors jointly supervised this work: Joshua A. Hill, Danielle M. Zerr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-024-02398-w.
References
- 1.Pantry SN, Medveczky PG. Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses. 2017;9:194 10.3390/v9070194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerr DM, Corey L, Kim HW, Huang M-L, Nguy L, Boeckh M. Clinical Outcomes of Human Herpesvirus 6 Reactivation after Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2005;40:932–40. 10.1086/428060 [DOI] [PubMed] [Google Scholar]
- 3.Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, et al. Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis After Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clin Infect Dis. 2013;57:671–81. 10.1093/cid/cit358 [DOI] [PubMed] [Google Scholar]
- 4.Hentrich M, Oruzio D, Jäger G, Schlemmer M, Schleuning M, Schiel X, et al. Impact of human herpesvirus-6 after haematopoietic stem cell transplantation. Br J Haematol. 2005;128:66–72. 10.1111/j.1365-2141.2004.05254.x [DOI] [PubMed] [Google Scholar]
- 5.Ljungman P, Wang F-Z, Clark DA, Emery VC, Remberger M, Ringdén O, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774–81. 10.1111/j.1365-2141.2000.02422.x [PubMed] [Google Scholar]
- 6.Yoshikawa T, Asano Y, Ihira M, Suzuki K, Ohashi M, Suga S, et al. Human Herpesvirus 6 Viremia in Bone Marrow Transplant Recipients: Clinical Features and Risk Factors. The. J Infect Dis. 2002;185:847–53. 10.1086/339411 [DOI] [PubMed] [Google Scholar]
- 7.Ward KN, Hill JA, Hubacek P, Camara R, de la, Crocchiolo R, Einsele H, et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019;104:2155–63. 10.3324/haematol.2019.223073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human Herpesvirus 6 DNA in Plasma after Allogeneic Stem Cell Transplantation: Incidence and Clinical Significance. J Infect Dis. 2006;193:68–79. 10.1086/498531 [DOI] [PubMed] [Google Scholar]
- 9.Yamane A, Mori T, Suzuki S, Mihara A, Yamazaki R, Aisa Y, et al. Risk Factors for Developing Human Herpesvirus 6 (HHV-6) Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation and Its Association with Central Nervous System Disorders. Biol Blood Marrow Transplant. 2007;13:100–6. 10.1016/j.bbmt.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 10.Zerr DM, Fann JR, Breiger D, Boeckh M, Adler AL, Xie H, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117:5243–9. 10.1182/blood-2010-10-316083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sashihara J, Tanaka-Taya K, Tanaka S, Amo K, Miyagawa H, Hosoi G, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. 2002;100:2005–11. [PubMed] [Google Scholar]
- 12.Chevallier P, Hebia-Fellah I, Planche L, Guillaume T, Bressolette-Bodin C, Coste-Burel M, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transpl. 2010;45:1204–11. 10.1038/bmt.2009.326 [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Ali A, Woan KV, Tam E, Yaghmour G, Flores A, et al. Unique Challenges to Diagnosing Human Herpesvirus-6 (HHV-6) Encephalitis Following Post-Hematopoietic Stem Cell Transplant: A Case and Brief Review. Cell Transpl. 2022;31. 10.1177/09636897221119734 [DOI] [PMC free article] [PubMed]
- 14.Ogata M, Satou T, Kawano R, Takakura S, Goto K, Ikewaki J, et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transpl. 2010;45:129–36. 10.1038/bmt.2009.116 [DOI] [PubMed] [Google Scholar]
- 15.Drobyski WR, Dunne WM, Burd EM, Knox KK, Ash RC, Horowitz MM, et al. Human Herpesvirus-6 (HHV-6) Infection in Allogeneic Bone Marrow Transplant Recipients: Evidence of a Marrow-Suppressive Role for HHV-6 In Vivo. J Infect Dis. 1993;167:735–9. 10.1093/infdis/167.3.735 [DOI] [PubMed] [Google Scholar]
- 16.Wang FZ, Dahl H, Linde A, Brytting M, Ehrnst A, Ljungman P. Lymphotropic herpesviruses in allogeneic bone marrow transplantation. Blood. 1996;88:3615–20. [PubMed] [Google Scholar]
- 17.Maeda Y, Teshima T, Yamada M, Shinagawa K, Nakao S, Ohno Y, et al. Monitoring of human herpesviruses after allogeneic peripheral blood stem cell transplantation and bone marrow transplantation. Br J Haematol. 1999;105:295–302. [PubMed] [Google Scholar]
- 18.Imbert-Marcille BM, Tang XW, Lepelletier D, Besse B, Moreau P, Billaudel S, et al. Human Herpesvirus 6 Infection after Autologous or Allogeneic Stem Cell Transplantation: A Single-Center Prospective Longitudinal Study of 92 Patients. Clin Infect Dis. 2000;31:881–6. 10.1086/318142 [DOI] [PubMed] [Google Scholar]
- 19.Savolainen H, Lautenschlager I, Piiparinen H, Saarinen‐Pihkala U, Hovi L, Vettenranta K. Human herpesvirus‐6 and‐7 in pediatric stem cell transplantation. Pediatr Blood Cancer. 2005;45:820–5. [DOI] [PubMed] [Google Scholar]
- 20.Pagter PJA, de, Schuurman R, Visscher H, Vos M, de, Bierings M, Loon AMvan, et al. Human Herpes Virus 6 Plasma DNA Positivity after Hematopoietic Stem Cell Transplantation in Children: an Important Risk Factor for Clinical Outcome. Biol Blood Marrow Transplant. 2008;14:831–9. 10.1016/j.bbmt.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 21.Wang L-R, Dong L-J, Zhang M-J, Lu D-P. Correlations of human herpesvirus 6B and CMV infection with acute GVHD in recipients of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transpl. 2008;42:673–7. 10.1038/bmt.2008.238 [DOI] [PubMed] [Google Scholar]
- 22.Zerr DM, Boeckh M, Delaney C, Martin PJ, Xie H, Adler AL, et al. HHV-6 Reactivation and Associated Sequelae after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2012;18:1700–8. 10.1016/j.bbmt.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan TL, Carlin K, Ljungman P, Politikos I, Boussiotis V, Boeckh M, et al. Human Herpesvirus-6B Reactivation Is a Risk Factor for Grades II to IV Acute Graft-versus-Host Disease after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transplant. 2018;24:2324–36. 10.1016/j.bbmt.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tormo N, Solano C, Cámara R, de la, Garcia-Noblejas A, Cardeñoso L, Clari MÁ, et al. An Assessment of the Effect of Human Herpesvirus-6 Replication on Active Cytomegalovirus Infection after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:653–61. 10.1016/j.bbmt.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 25.Wang F-Z, Larsson K, Linde A, Ljungman P. Human herpesvirus 6 infection and cytomegalovirus-specific lymphoproliferative responses in allogeneic stem cell transplant recipients. Bone Marrow Transpl. 2002;30:521–6. 10.1038/sj.bmt.1703657 [DOI] [PubMed] [Google Scholar]
- 26.Cone RW, Hackman RC, Huang M-LW, Bowden RA, Meyers JD, Metcalf M, et al. Human Herpesvirus 6 in Lung Tissue from Patients with Pneumonitis after Bone Marrow Transplantation. N. Engl J Med. 1993;329:156–61. 10.1056/NEJM199307153290302 [DOI] [PubMed] [Google Scholar]
- 27.Carrigan DR, Tapper MA, Knox KK, Drobyski WR, Ash RC, Russler SK. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991;338:147–9. 10.1016/0140-6736(91)90137-E [DOI] [PubMed] [Google Scholar]
- 28.Cone RW, Huang M-LW, Hackman RC. Human Herpesvirus 6 and Pneumonia. Leuk Lymphoma. 1994;15:235–41. 10.3109/10428199409049719 [DOI] [PubMed] [Google Scholar]
- 29.Hill JA, Vande Vusse LK, Xie H, Chung EL, Yeung CCS, Seo S, et al. Human Herpesvirus 6B and Lower Respiratory Tract Disease After Hematopoietic Cell Transplantation. JCO. 2019;37:2670–81. 10.1200/JCO.19.00908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Admiraal R, Koning CCH, de, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140:1643–50.e9. 10.1016/j.jaci.2016.12.992 [DOI] [PubMed] [Google Scholar]
- 31.de Pagter PJA, Schuurman R, Keukens L, Schutten M, Cornelissen JJ, van Baarle D, et al. Human herpes virus 6 reactivation: important predictor for poor outcome after myeloablative, but not non-myeloablative allo-SCT. Bone Marrow Transpl. 2013;48:1460–4. 10.1038/bmt.2013.78 [DOI] [PubMed] [Google Scholar]
- 32.Han T-T, Zhang Y-N, Sun Y-Q, Kong J, Wang F-R, Wang Z-D, et al. Human herpesvirus 6 reactivation in unmanipulated haploidentical hematopoietic stem cell transplantation predicts the occurrence of grade II to IV acute graft-versus-host disease. Transpl Infect Dis. 2021;23:e13544 10.1111/tid.13544 [DOI] [PubMed] [Google Scholar]
- 33.Aoki J, Numata A, Yamamoto E, Fujii E, Tanaka M, Kanamori H. Impact of Human Herpesvirus-6 Reactivation on Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:2017–22. 10.1016/j.bbmt.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, O’Dwyer DN, Xia M, Miller HK, Chan PR, Trulik K, et al. First-Onset Herpesviral Infection and Lung Injury in Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med. 2019;200:63–74. 10.1164/rccm.201809-1635OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dulery R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, et al. Early Human Herpesvirus Type 6 Reactivation after Allogeneic Stem Cell Transplantation: A Large-Scale Clinical Study. Biol Blood Marrow Transplant. 2012;18:1080–9. 10.1016/j.bbmt.2011.12.579 [DOI] [PubMed] [Google Scholar]
- 36.Hill JA, Lee YJ, Vande Vusse LK, Xie H, Chung EL, Waghmare A, et al. HHV-6B detection and host gene expression implicate HHV-6B as pulmonary pathogen after hematopoietic cell transplant. Nat Commun. 2024;15:542 10.1038/s41467-024-44828-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashidi A, Ebadi M, Said B, Cao Q, Shanley R, Curtsinger J, et al. Absence of early HHV-6 reactivation after cord blood allograft predicts powerful graft-versus-tumor effect. Am J Hematol. 2018;93:1014–9. 10.1002/ajh.25141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 40.Paule RC, Mandel J. Consensus Values and Weighting Factors. J Res Natl Bur Stand (1977). 1982;87:377–85. 10.6028/jres.087.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 42.Cochran WG. Some Methods for Strengthening the Common χ2 Tests. Biometrics. 1954;10:417–51. 10.2307/3001616 [Google Scholar]
- 43.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 44.Alexandersson A, Koskenvuo M, Tiderman A, Lääperi M, Huttunen P, Saarinen-Pihkala U, et al. Viral infections and immune reconstitution interaction after pediatric allogenic hematopoietic stem cell transplantation. Infect Dis (Lond). 2019;51:772–8. 10.1080/23744235.2019.1650198 [DOI] [PubMed] [Google Scholar]
- 45.Allen UD, Tellier R, Doyle J, Petric M, Wasfy S, Kumar P, et al. The utility of plasma polymerase chain reaction for human herpes virus-6 among pediatric bone marrow transplant recipients: results of a pilot study. Bone Marrow Transpl. 2001;28:473–7. 10.1038/sj.bmt.1703153 [DOI] [PubMed] [Google Scholar]
- 46.Baker M, Wang H, Rowley SD, Cai L, Pecora AL, Skarbnik A, et al. Comparative Outcomes after Haploidentical or Unrelated Donor Bone Marrow or Blood Stem Cell Transplantation in Adult Patients with Hematological Malignancies. Biol Blood Marrow Transpl. 2016;22:2047–55. 10.1016/j.bbmt.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 47.Betts BC, Young J-AH, Ustun C, Cao Q, Weisdorf DJ. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biol Blood Marrow Transpl. 2011;17:1562–8. 10.1016/j.bbmt.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cirrone F, Ippoliti C, Wang H, Zhou XK, Gergis U, Mayer S, et al. Early human herpes virus type 6 reactivation in umbilical cord blood allogeneic stem cell transplantation. Leuk Lymphoma. 2016;57:2555–9. 10.3109/10428194.2016.1157873 [DOI] [PubMed] [Google Scholar]
- 49.de Pagter APJ, Boelens JJ, Scherrenburg J, Vroom-de Blank T, Tesselaar K, Nanlohy N, et al. First analysis of human herpesvirus 6T-cell responses: specific boosting after HHV6 reactivation in stem cell transplantation recipients. Clin Immunol. 2012;144:179–89. 10.1016/j.clim.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 50.Dzieciątkowski T, Przybylski M, Torosian T, Tomaszewska A, Łuczak M. Prevalence of human herpesvirus 6 antibodies and DNA in allogeneic stem cell transplant patients: two-year single centre experience. Arch Immunol Ther Exp. 2008;56:201–6. 10.1007/s00005-008-0021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotoh M, Yoshizawa S, Katagiri S, Suguro T, Asano M, Kitahara T, et al. Human herpesvirus 6 reactivation on the 30th day after allogeneic hematopoietic stem cell transplantation can predict grade 2-4 acute graft-versus-host disease. Transpl Infect Dis. 2014;16:440–9. 10.1111/tid.12229 [DOI] [PubMed] [Google Scholar]
- 52.Hill JA, Mayer BT, Xie H, Leisenring WM, Huang M-L, Stevens-Ayers T, et al. Kinetics of Double-Stranded DNA Viremia After Allogeneic Hematopoietic Cell Transplantation. Clin Infect Dis. 2018;66:368–75. 10.1093/cid/cix804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iesato K, Hori T, Yoto Y, Yamamoto M, Inazawa N, Kamo K, et al. Long-term prognosis of human herpesvirus 6 reactivation following allogeneic hematopoietic stem cell transplantation. Pediatr Int. 2018;60:547–52. 10.1111/ped.13551 [DOI] [PubMed] [Google Scholar]
- 54.Jeulin H, Agrinier N, Guery M, Salmon A, Clément L, Bordigoni P, et al. Human herpesvirus 6 infection after allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation. 2013;95:1292–8. 10.1097/TP.0b013e318289958b [DOI] [PubMed] [Google Scholar]
- 55.Kadakia MP, Rybka WB, Stewart JA, Patton JL, Stamey FR, Elsawy M, et al. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation. Blood. 1996;87:5341–54. [PubMed] [Google Scholar]
- 56.Lee YJ, Su Y, Cho C, Tamari R, Perales M-A, Jakubowski AA, et al. Human Herpesvirus 6 DNAemia Is Associated With Worse Survival After Ex Vivo T-Cell-Depleted Hematopoietic Cell Transplant. J Infect Dis. 2022;225:453–64. 10.1093/infdis/jiab412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miura H, Kawamura Y, Hattori F, Tanaka M, Kudo K, Ihira M, et al. Late-phase human herpesvirus 6B reactivation in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2018;20:e12916 10.1111/tid.12916 [DOI] [PubMed] [Google Scholar]
- 58.Noviello M, Lorentino F, Xue E, Racca S, Furnari G, Valtolina V, et al. Human herpesvirus 6-specific T-cell immunity in allogeneic hematopoietic stem cell transplant recipients. Blood Adv. 2023;7:5446–57. 10.1182/bloodadvances.2022009274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toriumi N, Kobayashi R, Yoshida M, Iguchi A, Sarashina T, Okubo H, et al. Risk factors for human herpesvirus 6 reactivation and its relationship with syndrome of inappropriate antidiuretic hormone secretion after stem cell transplantation in pediatric patients. J Pediatr Hematol Oncol. 2014;36:379–83. 10.1097/MPH.0b013e3182a11676 [DOI] [PubMed] [Google Scholar]
- 60.Verhoeven DHJ, Claas ECJ, Jol-van der Zijde CM, Thijssen JCP, Lankester AC, Bredius RGM, et al. Reactivation of Human Herpes Virus-6 After Pediatric Stem Cell Transplantation: Risk Factors, Onset, Clinical Symptoms and Association With Severity of Acute Graft-Versus-Host Disease. Pediatr Infect Dis J. 2015;34:1118–27. 10.1097/INF.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 61.Wang L-R, Dong L-J, Zhang M-J, Lu D-P. The Impact of Human Herpesvirus 6B Reactivation on Early Complications following Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2006;12:1031–7. 10.1016/j.bbmt.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 62.Yoshikawa T, Suga S, Asano Y, Nakashima T, Yazaki T, Sobue R, et al. Human herpesvirus-6 infection in bone marrow transplantation. Blood. 1991;78:1381–4. [PubMed] [Google Scholar]
- 63.Toomey D, Phan TL, Phan T, Hill JA, Zerr DM. Viral Encephalitis after Hematopoietic Cell Transplantation: A Systematic Review. Transpl Cell Ther. 2023;29:636.e1–636.e9. 10.1016/j.jtct.2023.06.022 [DOI] [PubMed] [Google Scholar]
- 64.Eliassen E, Di Luca D, Rizzo R, Barao I. The Interplay between Natural Killer Cells and Human Herpesvirus-6. Viruses. 2017;9:367 10.3390/v9120367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miura H, Kawamura Y, Ozeki E, Ihira M, Ohashi M, Yoshikawa T. Pathogenesis of Severe Neutropenia in Patients With Primary Human Herpesvirus 6B Infection. Pediatr Infect Dis J. 2015;34:1003 10.1097/INF.0000000000000777 [DOI] [PubMed] [Google Scholar]
- 66.Phan TL, Pritchett JC, Leifer C, Zerr DM, Koelle DM, Di Luca D, et al. HHV-6B infection, T-cell reconstitution, and graft-vs-host disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2018;53:1508–17. 10.1038/s41409-018-0225-2 [DOI] [PubMed] [Google Scholar]
- 67.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2018;2:428–32. 10.1182/bloodadvances.2017012724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartelink IH, Belitser SV, Knibbe CAJ, Danhof M, de Pagter AJ, Egberts TCG, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transpl. 2013;19:305–13. 10.1016/j.bbmt.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 69.Berger M, Figari O, Bruno B, Raiola A, Dominietto A, Fiorone M, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transpl. 2008;41:55–62. 10.1038/sj.bmt.1705870 [DOI] [PubMed] [Google Scholar]
- 70.Fedele R, Martino M, Garreffa C, Messina G, Console G, Princi D, et al. The impact of early CD4+ lymphocyte recovery on the outcome of patients who undergo allogeneic bone marrow or peripheral blood stem cell transplantation. Blood Transfus. 2012;10:174–80. 10.2450/2012.0034-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 ×10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transpl. 2006;37:1119–28. 10.1038/sj.bmt.1705381 [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Feng S, Tang R, Du B, Xu K, Pan X. Efficacy of pretreatment of allografts with methoxypolyethylene glycol-succinimidyl-propionic acid ester in combination with an anti-OX40L monoclonal antibody in relieving graft-versus-host disease in mice. Int J Hematol. 2010;92:609–16. 10.1007/s12185-010-0701-y [DOI] [PubMed] [Google Scholar]
- 73.Holtan SG, Yu J, Choe HK, Paranagama D, Tang J, Naim A, et al. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: a multicenter chart review. Bone Marrow Transpl. 2022;57:1581–5. 10.1038/s41409-022-01764-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogata M, Uchida N, Fukuda T, Ikegame K, Kamimura T, Onizuka M, et al. Clinical practice recommendations for the diagnosis and management of human herpesvirus-6B encephalitis after allogeneic hematopoietic stem cell transplantation: the Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2020;55:1004–13. 10.1038/s41409-019-0752-5 [DOI] [PubMed] [Google Scholar]
- 75.AlloVir. AlloVir Provides Updates on Phase 3 Clinical Development Program for Posoleucel, an Allogeneic Virus-Specific T Cell Therapy | AlloVir, Inc. n.d. https://ir.allovir.com/news-releases/news-release-details/allovir-provides-updates-phase-3-clinical-development-program/ (accessed January 15, 2024).
- 76.Milbradt J, Auerochs S, Korn K, Marschall M. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J Clin Virol. 2009;46:24–8. 10.1016/j.jcv.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 77.WHO. 1st WHO International Standard for HHV-6B virus DNA 2017. https://nibsc.org/documents/ifu/15-266.pdf
- 78.Stathis et al. Supplementary Material. GitHub n.d github.com/dannytoomey/hhv6-mortality-ma/ (accessed November 21, 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sup Table 1 - Full Dataset - Mortality after HCT and HHV-6.xlsx
Sup Table 2 - Mortality by HHV-6 Status.xlsx
Data Availability Statement
All scripts used to perform analyses and generate figures and all raw data used in analyses are publicly available at https://github.com/dannytoomey/hhv6-mortality-ma.
Analyses were performed using the R programming language, version 4.3.2. All scripts and raw data used in analysis are publicly available on GitHub [78].