Abstract
Objective
This study aims to investigate the relationship between serum albumin levels and neonatal acute respiratory distress syndrome (NARDS) in patients with newborn pneumonia, providing new insights for clinical interventions targeting NARDS.
Methods
A retrospective analysis of medical records of neonatal pneumonia patients admitted to the neonatal intensive care unit (NICU) at a tertiary medical institution from January 2021 to December 2023 was conducted. Patients were stratified based on hypoalbuminemia (defined as serum albumin levels < 35 g/L), clinical thresholds, and albumin level quartiles. To eliminate the impact of potential confounding factors on the results, multivariable logistic regression and propensity score matching (PSM) analyses were performed to calculate the adjusted odds ratio (OR) and 95% confidence interval (95% CI) for the occurrence of NARDS in these patients. Additionally, subgroup analyses were conducted to explore interaction effects.
Results
In this retrospective cohort study, a total of 342 patients with neonatal pneumonia admitted to the NICU were included. The multivariable logistic regression analysis revealed that the incidence of NARDS in patients with hypoalbuminemia was significantly higher than in those with normal albumin levels (OR = 2.16, 95% CI 1.47–4.06, p = 0.017). Compared to patients in quartile Q1 (≥39 g/L), those in quartile Q4 (≤33 g/L) exhibited a significantly increased risk of NARDS (OR = 4.40, 95% CI 1.53–12.63, p = 0.006). After conducting PSM, these associations remained significant. Furthermore, treating serum albumin levels as a continuous variable revealed that each 1 g/L increase was associated with a 17% reduction in NARDS risk (95% CI, 1.08–1.15).
Conclusion
Low serum albumin levels in patients with neonatal pneumonia are closely associated with NARDS, indicating a significant dose-response relationship between the two.
Keywords: neonatal, newborn pneumoniae, neonatal acute respiratory distress syndrome, albumin, hypoalbuminemia
Introduction
Due to the immaturity of the innate immune system in neonates, their ability to defend against pulmonary infections is limited,1,2 making them more susceptible to infectious pulmonary diseases during the neonatal period. Neonatal pneumonia is a leading cause of morbidity and mortality in infants worldwide.3,4 It is estimated that between 152,000 and 490,000 infants under one year of age die from pneumonia each year.5 Among pediatric populations, pulmonary diseases, including pneumonia and lower respiratory tract infections, remain the most common risk factors for NARDS.6 Evidence suggests that pulmonary infections can decrease the biosynthesis of surfactant or impair its secretion, ultimately leading to the formation of hyaline membranes and alveolar collapse.7–9
NARDS, also called hyaline membrane disease of the newborn10, results from a deficiency of pulmonary surfactant (PS) in neonates, leading to impaired effective ventilation and gas exchange, or from immature lung development,11,12 affecting up to 7% of neonates.13,14 Previous studies have indicated that low serum albumin levels are a risk factor for early-onset sepsis-related NARDS.15 Research by Ying Q et al highlighted a close correlation between the decline in serum albumin on the first day of life and the incidence of late preterm respiratory distress syndrome.16 Additionally, Moison RM et al confirmed that albumin levels in RDS patients were lower than those in preterm infants.17 Finally, a study by Torer B et al found that the prevalence and mortality rates of NARDS and sepsis were significantly higher in groups with average serum albumin levels below 25.5 ± 3.8 g/L.18 Although several studies have established a relationship between serum albumin levels and respiratory distress in neonates, the relatively simplified conclusions limit the specificity of clinical practice and hinder the promotion of albumin management in NICU neonatal patients.
This study employs a retrospective cohort design to meticulously categorize serum albumin levels in neonates and investigate the associations between different levels of serum albumin and NARDS. The aim is to provide pediatricians with precise and quantitative predictive associations and to determine beneficial serum albumin threshold levels for patients. This study seeks to address several key issues through its retrospective cohort design: 1) to emphasize modifiable and clinically manageable risk factors, providing new insights for interventions targeting NARDS in neonates with pneumonia; 2) to quantify and visually represent the intensity of NARDS risk associated with varying serum albumin levels and to establish a dose-response relationship between serum albumin levels and NARDS while examining potential interactions with other risk factors.
Methods
Data Sources and Patient
This study is a retrospective cohort investigation that collected clinical data from neonates with pneumonia and those who developed NARDS secondary to neonatal pneumonia, hospitalized in the NICU of our institution between January 2021 and December 2023. Researchers conducted an anonymous analysis of the data extracted from electronic medical records to ensure the protection of personal identity information. The study adhered to the ethical principles outlined in the Declaration of Helsinki of 1964 and received approval from the Institutional Review Board (IRB) for all aspects of the research, waiving the requirement for written informed consent.
The diagnostic criteria for NARDS were established based on the 2017 Montreux criteria.19 The diagnosis of neonatal pneumonia relied on clinical and laboratory findings, requiring fulfillment of the following three criteria: persistent consolidation, imaging evidence of cavitation, atelectasis, infiltration, or pleural effusion; evidence of gas exchange deterioration; and at least three additional clinical and/or laboratory findings.3,20
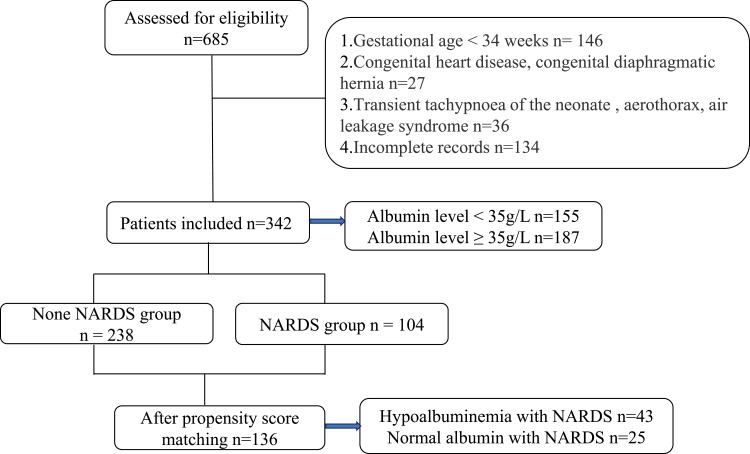
Inclusion criteria were: (a) gestational age (GA) ≥ 34 weeks; (b) neonatal age < 1 month; and (c) clinical cases diagnosed as neonatal pneumonia. Exclusion criteria included: (a) GA < 34 weeks or neonatal age ≥ 1 month; (b) congenital heart disease, congenital diaphragmatic hernia, transient tachypnea of the newborn, pneumothorax, or leak syndrome; and (c) incomplete records. The flowchart is presented in Figure 1.
Figure 1.
Flow diagram for the present study.
Based on our hospital’s antibiotic usage policy for the NICU and recommendations from the World Health Organization21, all enrolled cases were treated with ampicillin and third-generation cephalosporins to cover special bacterial infections such as Group B Streptococcus (GBS) and Escherichia coli. Furthermore, supportive treatment for neonatal pneumonia has been associated with improved outcomes and reduced mortality rates22, which includes the use of oxygen therapy, temperature regulation, and either parenteral nutrition or nasogastric feeding.
Data Collection
Patient data were extracted from the hospital’s health information system. In this study, we collected a range of indicators, including demographic information, maternal perinatal factors, and laboratory test results. The demographic data included gender, age, weight, and the 5-minute Apgar score. Maternal perinatal factors encompassed gestational age, mode of delivery, pregnancy-related comorbidities such as hypertension and diabetes, parity status, history of multiple births, history of premature rupture of membranes, and fetal distress. Laboratory test results included glucose (Glu), C-reactive protein (CRP), white blood cell count (WBC), absolute neutrophil count (ANC), platelet count (PLT), lactate dehydrogenase (LDH), total protein (TP), and albumin (ALB). All laboratory data were collected within 24 hours of patient admission and were obtained, processed, and analyzed according to standard international biochemical laboratory protocols.
Exposure
The primary exposure of interest is hypoalbuminemia at admission, defined as serum albumin levels < 35 g/L. To examine the dose-response relationship, albumin levels were categorized into four groups based on clinical thresholds: normal albumin (Group C1): ≥ 35 g/L, mild hypoalbuminemia (Group C2): 34.9–30 g/L, moderate hypoalbuminemia (Group C3): 29.9–25 g/L, and severe hypoalbuminemia (Group C4): ≤ 24.9 g/L. Additionally, patients were further stratified into quartiles based on their admission albumin levels: Group Q1 (≥ 39 g/L), Group Q2 (36–38 g/L), Group Q3 (34–35 g/L), and Group Q4 (≤ 33 g/L).
Outcome
The primary outcome of this study is neonatal pneumonia complicated by NARDS. The diagnostic criteria for NARDS were established according to the 2017 Montreux definition.19 The diagnosis of neonatal ARDS should meet the following requirements at the same time: ① the onset of the disease, and the acute attack (within 1 week) after identifying or suspicious inducements (asphyxia, choking, meconium aspiration, infection, etc).; ②transient tachypnea of newborn (TTN) or dyspnea caused by congenital malformation were excluded; ③pulmonary imaging showed bilateral diffuse and irregular decreased light transmittance, exudation or white lung, which could not be explained by other reasons, such as local effusion, atelectasis, NRDS, TTN or congenital malformation; ④ The cause of pulmonary edema, which can not be explained by congenital heart disease, can be confirmed by cardiac ultrasound; ⑤ According to oxygen index, OI = FiO2 × Mean airway pressure (Paw) × 100/PaO2] Assessment of oxygenation disorder and disease severity: mild ARDS 4≤OI<8, moderate ARDS 8≤OI<16, severe ARDS ≥ 16.
Statistical Analysis
As the continuous data did not meet the normality assumption determined by the Kolmogorov–Smirnov test, baseline characteristics of the patients were expressed as medians (interquartile ranges) or numbers (percentages). Chi-square tests were used to compare categorical data, while the Kruskal–Wallis test was employed to assess continuous data. Statistically significant covariates (P < 0.05) from univariate logistic regression analysis were included in a multivariate logistic regression model to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI). To reduce bias, we conducted a propensity score-matched study (PSM) for all included covariates. In the 1:1 PSM approach, we utilized a nearest neighbor matching algorithm to match the normal albumin group with the hypoalbuminemia group, with a caliper width of 0.02 standard deviations. Subsequently, logistic regression analysis was performed on the PSM-matched data to obtain adjusted ORs and 95% CI. Additionally, we conducted subgroup analyses to explore potential interactions between variables. Patients were stratified into subgroups based on covariates, and separate logistic regression analyses were performed for each subgroup. By comparing the differences in ORs across various subgroups, we assessed the presence of interactions.
All statistical procedures were performed using SPSS Statistics 25.0 for Windows (IBM Corp., Armonk, NY) and R software version 4.4.0 for Windows (R Foundation for Statistical Computing, Boston, MA, United States).
Results
Study Population and Baseline Characteristics
In this retrospective study, a total of 342 patients were enrolled (Figure 1). Among them, 155 patients (45.3%) had serum albumin levels below 35 g/L, and 65 patients (19.0%) developed NARDS. The selection process is illustrated in Figure 1.
Table 1 presents the baseline characteristics of the patients, categorized into four groups based on serum albumin quartiles: Group Q1 (≥ 39 g/L), Group Q2 (36–38 g/L), Group Q3 (34–35 g/L), and Group Q4 (≤ 33 g/L). The distribution of patients in these groups was as follows: 63 (18.4%), 89 (26.0%), 85 (24.9%), and 105 (30.7%), respectively. The average age of all included patients was 0.15 hours, with males comprising 63.2% of the cohort. The Kruskal–Wallis test indicated statistically significant trends across various variables as serum albumin levels decreased. These variables included age, weight, 5-minute Apgar score, gestational age, primiparity, multiple births, pregnancy-related hypertension, white blood cell count, neutrophil count, lactate dehydrogenase, and total protein levels.
Table 1.
Baseline Characteristics of the Patients by Admission Albumin Levels (Quartile-Based Four-Category)
| Characteristics | Total patients n = 342 |
Admission Albumin Levels(g/L) | P for Trend | |||
|---|---|---|---|---|---|---|
| Q1(≥39) n=63 |
Q2(36–38) n=89 |
Q3(34–35) n=85 |
Q4(≤33) n=105 |
|||
| Demographics | ||||||
| Male(n,%) | 216(63.2) | 33(52.4) | 54(60.7) | 60(70.6) | 69(65.7) | 0.128 |
| Weight, g (M, IQR) |
2600.0(2147.5,3252.5) | 3050.0(2400.0,3650.0) | 2900.0(2300.0,3525.0) | 2450.0(2110.0,3047.5) | 2400.0(2000.0,2880.0) | 0.001 |
| Age, Hour (M, IQR) |
0.15(0.12,0.25) | 0.17(0.12,0.42) | 0.17(0.12,2.21) | 0.15(0.11,0.23) | 0.15(0.11,0.20) | 0.030 |
| Apgar5(M, IQR) | 9(8,9) | 9(9,10) | 9(8,10) | 9(9,9) | 9(8,9) | 0.023 |
| perinatal factors | ||||||
| GA, week (M, IQR) |
36(34.4,37.6) | 38.1(35.3,40.1) | 37.0(35.3,39.1) | 35.3(34.3,37.0) | 35.2(34.1,37.0) | 0.001 |
| Cesarean section(n,%) | 220(64.3) | 34(54.0) | 52(58.4) | 59(69.4) | 75(71.4) | 0.056 |
| Gestational diabetes(n,%) | 107(31.3) | 15(23.8) | 31(34.8) | 28(32.9) | 33(31.4) | 0.519 |
| primipara(n,%) | 258(75.4) | 56(88.9) | 72(80.9) | 59(69.4) | 71(67.6) | 0.005 |
| Twins(n,%) | 41(12.0) | 8(12.7) | 4(4.5) | 8(9.4) | 21(20.0) | 0.009 |
| HBP(n,%) | 78(22.8) | 7(11.1) | 15(16.9) | 24(28.2) | 32(30.5) | 0.009 |
| PROM(n,%) | 124(36.3) | 22(34.9) | 35(39.3) | 36(42.4) | 31(29.5) | 0.280 |
| FD(n,%) | 34(9.9) | 9(14.3) | 11(12.4) | 9(4.8) | 5(4.8) | 0.165 |
| laboratory tests | ||||||
| Glu,mmol/L (M, IQR) |
4.4(3.4,5.5) | 4.4(4.0,5.9) | 4.4(3.2,5.4) | 4.1(3.5,5.6) | 4.2(3.1,5.2) | 0.159 |
| CRP,mg/L (M, IQR) |
0.2(0.2,0.5) | 0.2(0.2,1.2) | 0.2(0.2,0.9) | 0.2(0.2,0.3) | 0.2(0.2,0.5) | 0.573 |
| WBC,×10^9/L (M, IQR) |
12.2(8.9,16.6) | 15.0(10.6,19.8) | 13.7(9.8,19.5) | 10.7(7.9,14.3) | 10.3(8.3,14.2) | 0.001 |
| ANC,×10^9/L (M, IQR) |
6.8(4.4,10.9) | 9.7(5.7,14.4) | 9.1(5.1,13.1) | 5.6(3.9,8.2) | 5.2(3.6,8.5) | 0.001 |
| PLT,×10^9/L (M, IQR) |
236(203,278) | 240(203,290) | 238(200,290) | 238(195,279) | 233(211,266) | 0.836 |
| LDH,U/L (M, IQR) |
516(437,650) | 622(492,828) | 568(446,712) | 519(435,620) | 470(412,547) | 0.001 |
| TP,g/L(M, IQR) | 51(46,55) | 60(56,62) | 54(52,56) | 49(4752) | 45(42,47) | 0.001 |
Abbreviations: GA, gestational age; HBP, hypertensive disorders of pregnancy; PROM, premature rupture of membrane; FD, fetal distress; Glu blood glucose; WBC, white blood cells; ANC, neutrophil count; PLT, platelet; CRP, c-reactive protein; LDH, L-lactate dehydrogenase; TP, total protein; M, median; IQR, Interquartile Range.
Association Between Serum Albumin Levels and NARDS Risk
Covariates that were statistically significant in the univariate logistic regression analysis were included in a multivariate logistic regression model, with results presented in Table 2. We found that neonatal pneumonia complicated by NARDS was significantly associated with factors including male sex, 5-minute Apgar score, gestational age, mode of delivery, blood glucose levels, neutrophil counts, platelet counts, and serum albumin levels (p < 0.05). Adjusted results based on covariates are shown in Table 3: when considering serum albumin levels as a continuous variable, the correlation between albumin levels and NARDS risk was indicated by an odds ratio (OR) of 0.83 (95% CI: 0.75–0.91, p < 0.001). In other words, for every 1 g/L increase in albumin level, the risk of NARDS decreased by 17%. When serum albumin levels were treated as a dichotomous variable, the risk of NARDS was significantly higher in the hypoalbuminemia group compared to the normal albumin group (OR = 2.74, 95% CI: 1.70–4.41, p = 0.001). After adjusting for potential confounding factors in the multivariate logistic regression model, this association remained significant (Table 3), with hypoalbuminemic patients having a 2.16-fold increased risk of NARDS (adjusted OR = 2.16, 95% CI: 1.47–4.06, p = 0.017). Even after propensity score matching (PSM), the results were statistically significant (PSM-adjusted OR: 2.31, 95% CI: 1.16–4.61, p = 0.017).
Table 2.
Multivariate Analysis for NARDS
| Characteristics | Univariate | P | Multivariate | P | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Demographics | ||||||
| Male(n,%) | 1.67 | 1.01–2.74 | 0.044 | 1.89 | 1.01–3.52 | 0.045 |
| Age(M, IQR) | 0.95 | 0.89–1.02 | 0.164 | <NA> | <NA> | <NA> |
| Weight(M, IQR) | 0.99 | 0.99–1.00 | 0.001 | 1.00 | 1.00–1.00 | 0.197 |
| Apgar5(M, IQR) | 0.61 | 0.47–0.81 | 0.001 | 0.60 | 0.44–0.81 | 0.001 |
| perinatal factors | ||||||
| GA,week(M, IQR) | 0.62 | 0.53–0.72 | 0.001 | 0.45 | 0.33–0.62 | 0.001 |
| Cesarean section(n,%) | 1.66 | 1.01–2.74 | 0.048 | 1.96 | 1.02–3.75 | 0.043 |
| Gestational diabetes(n,%) | 0.91 | 0.55–1.50 | 0.697 | <NA> | <NA> | <NA> |
| primipara(n,%) | 0.72 | 0.43–1.22 | 0.225 | <NA> | <NA> | <NA> |
| Twins(n,%) | 1.55 | 0.79–3.04 | 0.204 | <NA> | <NA> | <NA> |
| HBP(n,%) | 1.19 | 0.69–2.05 | 0.523 | <NA> | <NA> | <NA> |
| PROM(n,%) | 0.80 | 0.49–1.30 | 0.365 | <NA> | <NA> | <NA> |
| FD(n,%) | 1.70 | 0.82–3.50 | 0.154 | <NA> | <NA> | <NA> |
| laboratory tests | ||||||
| Glu,mmol/L(M, IQR) | 0.61 | 0.50–0.75 | 0.001 | 0.68 | 0.54–0.86 | 0.001 |
| CRP,mg/L(M, IQR) | 0.93 | 0.85–1.02 | 0.120 | <NA> | <NA> | <NA> |
| WBC,×10^9/L(M, IQR) | 0.95 | 0.91–0.99 | 0.017 | 0.88 | 0.75–1.05 | 0.152 |
| ANC,×10^9/L(M, IQR) | 0.93 | 0.88–0.98 | 0.006 | 1.37 | 1.10–1.72 | 0.005 |
| PLT,×10^9/L(M, IQR) | 0.99 | 0.98–0.99 | 0.001 | 0.99 | 0.98–0.99 | 0.012 |
| LDH,U/L(M, IQR) | 1.00 | 0.99–1.00 | 0.576 | <NA> | <NA> | <NA> |
| TP,g/L(M, IQR) | 0.95 | 0.92–0.98 | 0.001 | 1.01 | 0.96–1.07 | 0.600 |
| ALB(M, IQR) | 0.82 | 0.76–0.88 | 0.001 | 0.83 | 0.75–0.91 | 0.001 |
Abbreviations: GA, gestational age; HBP, hypertensive disorders of pregnancy; PROM, premature rupture of membrane; FD, fetal distress; Glu blood glucose; WBC, white blood cells; ANC, neutrophil count; PLT, platelet; CRP, c-reactive protein; LDH, L-lactate dehydrogenase; TP, total protein; ALB, albumin levels; M, median; IQR, Interquartile Range.
Table 3.
Unadjusted and Adjusted Association Between Admission Albumin Levels and NARDS
| Albumin (g/L) | Events n (%) | Unadjusted OR (95% CI) | P | Multivariable regression adjusted OR (95% CI) | P | PSM adjusted OR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Continuous | Per 1 | NA | 0.82(0.76–0.88) | 0.001 | 0.83(0.75–0.91) | 0.001 | NA | NA |
| Dichotomy | Normal, ≥ 35 | 39(37.5%) | 1 [Reference] | 0.001 | 1 [Reference] | 0.017 | 1 [Reference] | 0.017 |
| HypoAlb, < 35 | 65(62.5%) | 2.74(1.70–4.41) | 2.16(1.47–4.06) | 2.31(1.16–4.61) | ||||
| Quartile | ||||||||
| Q1(≥39) | 8(12.7%) | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | |
| Q2(36–38) | 26(29.2%) | 2.84(1.18–6.78) | 0.019 | 2.31(0.83–6.43) | 0.108 | 1.37(0.49–3.86) | 0.547 | |
| Q3(34–35) | 20(23.5%) | 2.12(0.86–5.18) | 0.101 | 1.30(0.44–3.79) | 0.634 | 1.77(0.61–5.17) | 0.296 | |
| Q4(≤33) | 50(47.6%) | 6.25(2.71–14.40) | 0.001 | 4.40(1.53–12.63) | 0.006 | 4.07(1.44–11.53) | 0.008 | |
| Clinical threshold | ||||||||
| C1(≥ 35) | 39(20.9%) | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | |
| C2(34.9–30) | 49(35.8%) | 2.11(1.28–3.47) | 0.003 | 1.51(0.79–2.90) | 0.218 | 1.78(0.87–3.64) | 0.112 | |
| C3(29.9–25) | 15(88.2%) | 28.46(6.24–129.74) | 0.001 | 26.45(4.48–156.05) | 0.000 | 15.6(1.88–129.46) | 0.008 | |
| C4(≤ 24.9) | 1(100.0%) | NA# | NA# | NA# | NA# | NA# | NA# | |
Notes: The factors of the multivariable regression: Male, Weight, Apgar5, GA, Cesarean section, Glu, WBC, ANC,PLT,TP. NA# The number of individuals in the C4 group is too small to conduct logistic regression and propensity score matching.
Abbreviations: HypoAlb, Hypoalbuminemia; NA, Not Applicable; CI, Confidence Interval; OR, Odds Ratio; PSM, Propensity Scores Matching.
After adjusting for confounding factors in the multivariate regression model, patients in Group Q4 (serum albumin ≤ 33 g/L) exhibited a significantly higher risk compared to those in Group Q1 (serum albumin ≥ 39 g/L) (OR 4.40, 95% CI: 1.53–12.63, p = 0.006). This correlation persisted even after PSM (Q4: PSM-adjusted OR 4.07, 95% CI: 1.44–11.53, p = 0.008). Furthermore, similar results were observed when comparing patients in Group C3 (serum albumin 29.9–25 g/L) with those in Group C1 (serum albumin ≥ 35 g/L) (p = 0.008). Collectively, these findings suggest that lower serum albumin levels at admission are associated with a higher risk of NARDS in this patient cohort.
Dose–Response Relationship
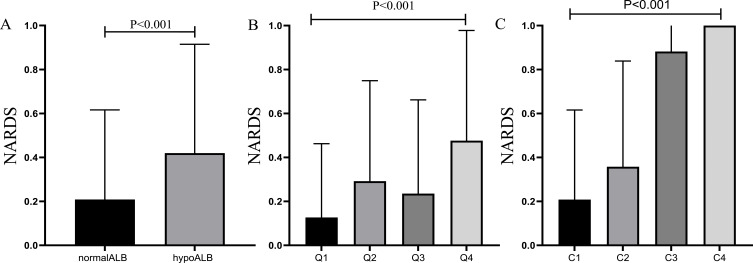
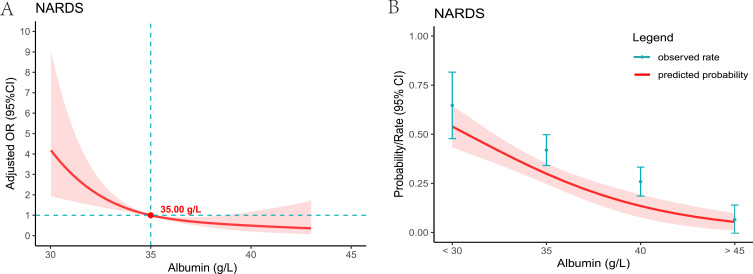
Compared to the normal albumin level group, the incidence of NARDS was higher in the hypoalbuminemia group, with the occurrence of NARDS increasing as the severity of hypoalbuminemia intensified (Figure 2, p < 0.001). Figure 3A presents the restricted cubic spline plot illustrating the relationship between albumin levels and NARDS. This model was adjusted for the clinical severity of albumin and all covariates included in this study. The results indicated that as albumin levels decreased, the risk of NARDS increased. When albumin levels fell below the critical threshold of 35 g/L (odds ratio of 1), the risk of NARDS significantly increased. Furthermore, Figure 3B illustrates the association between albumin levels and the probability and incidence of NARDS, emphasizing that lower albumin levels are associated with an increased incidence of NARDS.
Figure 2.
Relationship between different albumin level groups and NARDS rates in patients with neonatal pneumoniae. (A) The incidence of NARDS was 20.9% in the group with normal preoperative serum albumin levels, and 41.9% in the hypoalbuminemia group. (B) Patients were divided into 4 quartiles, comparing NARDS rates among the 4 quartiles. Q1:12.7%; Q2:29.2%; Q3:23.5%; Q4:47.6%. (C) Patients were categorized into 4 groups using clinical thresholds, comparing NARDS rates among the 4 groups. C1: 20.9%; C2: 35.8%; C3: 88.2%; C4: 100%.
Figure 3.
For a line plot depicting the relationship between serum albumin levels in NARDS patients. (A) The restricted cubic spline plot was used to demonstrate the association between serum albumin levels and NARDS. The Y-axis represents the adjusted odds ratio, while the X-axis indicates serum albumin levels. The model has been adjusted for all included covariates. The shaded red area signifies the 95% confidence intervals. The critical value of OR = 1 is denoted by the point. (B) The plot illustrates the relationship between serum albumin levels and the predicted probability of NARDS. The Y-axis represents the predicted probability, while the X-axis indicates serum albumin levels. The shaded red area signifies the 95% confidence intervals.
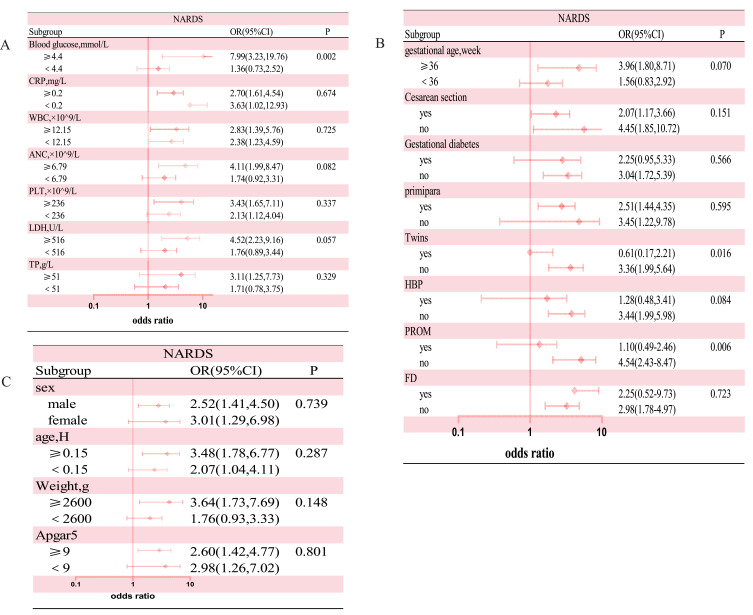
Subgroup analysis depicted the interaction between albumin levels (normal versus hypoalbuminemia) and NARDS(Figure 4), along with other covariates. The results revealed that neonatal blood glucose levels, maternal twin pregnancies, and premature rupture of membranes had interactive effects on the association between neonatal hypoalbuminemia and NARDS (p< 0.01), suggesting that the impact of hypoalbuminemia on NARDS risk may vary according to neonatal blood glucose levels, maternal twin pregnancies, and premature rupture of membranes. However, other variables did not have a significant effect on the association between admission hypoalbuminemia and NARDS.
Figure 4.
Subgroup analysis was performed to evaluate potential interaction between hypoalbuminemia and each covariate. A value of p less than 0.01 was considered statistically significant. (A) Subgroup analysis of variables related to laboratory tests. (B)Subgroup analysis of variables related to perinatal factors (C) Subgroup analysis of variables related to demographics.
Discussion
Neonatal Acute Respiratory Distress Syndrome (NARDS) is a common, life-threatening respiratory condition observed in the NICU, with a mortality rate ranging from 17% to 33%.23–25 However, the significance of albumin in NARDS has been evaluated in relatively few studies, and the somewhat simplified conclusions have limited the specificity of clinical practice. Therefore, it is crucial to comprehensively understand the varying risk levels of NARDS associated with different albumin levels. This understanding may provide valuable insights for clinicians to identify high-risk NARDS patients early and to formulate targeted interventions.
In our study, we precisely estimated the strength of the association between different albumin levels and NARDS using stratified analysis of serum albumin levels and multivariable logistic regression analysis. We found that hypoalbuminemia is an independent risk factor for the occurrence of NARDS in neonates with pneumonia. A linear dose-response relationship exists between serum albumin levels and NARDS among neonates with pneumonia: lower albumin levels correlate with higher incidence rates of NARDS. This association persists even after adjusting for potential confounding factors. Moreover, when albumin levels fall below 35.00 g/L, the risk of NARDS significantly increases. This finding is consistent with the observations of Ying Q et al,16 who reported a higher correlation between albumin levels below 34 g/L and the incidence of neonatal respiratory distress syndrome, indicating a sensitivity of 83.9% and a specificity of 62.5% at the 34 g/L cutoff. However, considering factors such as sample size, caution is warranted in interpreting the interactions between blood glucose levels and premature rupture of membranes. Furthermore, the association between blood glucose levels and hypoalbuminemia warrants further investigation and validation in future studies.
Acute Respiratory Distress Syndrome is characterized by diffuse alveolar damage and increased capillary permeability.26 Damage to the alveolar-capillary membrane can lead to the accumulation of protein-rich fluid in the lung parenchyma and interstitium, inactivation of surfactant, atelectasis, and impaired gas exchange.26–28 Research shows that pulmonary infectious diseases remain a common risk factor for NARDS (Neonatal Acute Respiratory Distress Syndrome).23,29,30 Pulmonary infections can damage type II alveolar epithelial cells, leading to variable secondary impairments in surfactant function or surfactant quantity.31 Additionally, inflammatory cells release various reactive oxygen species (ROS), including hydrogen peroxide, superoxide, and nitric oxide, through enzymatic reactions, which subsequently damage the structure and function of lipids and proteins, ultimately resulting in the degradation of pulmonary surfactant.32
In this study, low serum albumin levels were independently associated with the development of NARDS in neonates with pneumonia. Several factors may explain the occurrence of NARDS in this context. Firstly, serum albumin plays a crucial role in maintaining vascular volume and fluid balance.33,34 Low serum albumin levels can lead to reduced pulmonary oncotic pressure, facilitating the leakage of protein-rich fluid (including albumin, serum, lysolipids, etc.) into the pulmonary interstitial tissue, which subsequently decreases lung compliance and contributes to surfactant inactivation.35 Mechanistically, albumin and other plasma proteins impair surfactant activity primarily through competitive adsorption, thereby reducing the availability of surfactant at the air-liquid interface within the alveoli.36–39 Secondly, albumin is considered the most significant extracellular antioxidant, accounting for nearly three-quarters of plasma antioxidant capacity.40 Research indicates that the antioxidant and free radical scavenging properties of albumin depend on its strong ligand-binding characteristics, which enable it to bind metals such as copper and iron, as well as free fatty acids, preventing their peroxidation and the formation of reactive oxygen species.41,42 Moreover, the thiol groups present on its surface can act as free radical scavengers, further enhancing its antioxidant capabilities.34,43 Consequently, hypoalbuminemia diminishes the capacity to counter oxidative stress, thus increasing the risk of pulmonary oxidative damage and ultimately leading to the development of respiratory distress. Lastly, during disease states, inflammatory mediators can negatively affect the synthesis capacity of albumin. Studies have shown that these mediators can directly inhibit the transcription of genes responsible for albumin synthesis, while the synthesis of albumin is also diminished due to a redirection of protein synthesis priorities towards acute phase proteins.16,18 In summary, albumin plays a critical role in the context of pneumonia complicated by respiratory failure in neonates.
Hypoalbuminemia is typically caused by inflammation, but it can also result from hepatocyte injury and decreased albumin synthesis, insufficient dietary amino acids, or increased albumin excretion. Regardless of the underlying cause, hypoalbuminemia has a strong predictive value for both mortality and morbidity.44 A study examining nutritional indicators in NICU revealed that for every increase of 1 g/dL in serum albumin levels, the need for respiratory support on the first day after birth decreased by a factor of 0.001.45 A large cohort study conducted by Watchko et al found that neonates with serum albumin levels below 2.5 g/dL had a higher risk of mortality compared to those with levels above this threshold.46 Furthermore, research by Park JH et al emphasizes that the lowest serum albumin level is the most effective predictor of mortality in very low birth weight infants beyond the first week of life.47 In patients with sepsis, low serum albumin levels are independently associated with an increased risk of mortality. During the acute phase, lower serum albumin levels correlate closely with heightened risks of severe sepsis and organ failure.48 Additionally, research by Mansbach et al indicated that low serum albumin levels were associated with an increased risk of apnea in infants suffering from severe bronchiolitis.49 Collectively, these studies suggest that serum albumin levels can serve as a reliable indicator for assessing respiratory diseases and outcomes in neonates, providing crucial reference points for clinical practice.
It is important to acknowledge some limitations inherent to our study. Firstly, it should be noted that our study design is retrospective. While our findings indicate a strong association between exposure factors and outcomes, causal relationships cannot be inferred. Secondly, despite adjustments made, residual confounding factors may still affect the interpretation of results. Given the multifactorial origins and complex mechanisms underlying NARDS, addressing its occurrence solely through the lens of serum albumin levels presents significant challenges. Therefore, we recommend a comprehensive, multifactorial management approach to effectively mitigate the incidence of NARDS. Thirdly, during the initial data collection phase, we did not include relevant information on patient outcomes, which resulted in a lack of investigation into the impact of albumin levels on the prognosis of neonatal pneumonia with concurrent NARDS in this study. This limitation highlights the need for future research to address this aspect for a more comprehensive understanding of the condition. Finally, given that our study was conducted at a single center and constrained by sample size, generalizing our findings to all medical centers may pose challenges.
Conclusions
We found a linear dose-response relationship between serum albumin levels and the occurrence of respiratory distress in neonates with pneumonia. Our study identified a risk threshold for serum albumin levels at 35 g/L, below which adverse outcomes may arise for infants suffering from pneumonia. Therefore, routine assessment of serum albumin levels and the implementation of personalized management strategies are of paramount importance for this patient population.
Acknowledgments
The authors wish to thank patients and participating investigators associated with the clinical studies.
Funding Statement
Not applicable.
Data Sharing Statement
All the data used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted according to the Declaration of 1964 helsinki and approved by the Ethics Committee of the Dandong Central Hospital (Approval No. DDZX-20240808). Waiving the requirement for written informed consent due to the retrospective study design and anonymized patient data.
Consent for Publication
Not Applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors state that they have no competing interests.
References
- 1.Kwak DJ, Augustine NH, Borges WG, et al. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun. 2000;68(1):320–327. doi: 10.1128/IAI.68.1.320-327.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Pine TR, Joyner JL, Augustine NH, et al. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B Streptococci. Pediatr Res. 2003;54(2):276–281. doi: 10.1203/01.PDR.0000072515.10652.87 [DOI] [PubMed] [Google Scholar]
- 3.Hooven TA, Polin RA. Pneumonia. Semin Fetal Neonatal Med. 2017;22(4):206–213. doi: 10.1016/j.siny.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nissen MD. Congenital and neonatal pneumonia. Paediatr Respir Rev. 2007;8(3):195–203. doi: 10.1016/j.prrv.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltramo F, Khemani RG. Definition and global epidemiology of pediatric acute respiratory distress syndrome. Ann translat Med. 2019;7(19):502. doi: 10.21037/atm.2019.09.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasser JR, Mallampalli RK. Surfactant and its role in the pathobiology of pulmonary infection. Microbes Infect. 2012;14(1):17–25. doi: 10.1016/j.micinf.2011.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang Z, Hao Y, Hwang S, et al. The Pseudomonas aeruginosa flagellum confers resistance to pulmonary surfactant protein-A by impacting the production of exoproteases through quorum-sensing. Mol Microbiol. 2011;79(5):1220–1235. doi: 10.1111/j.1365-2958.2010.07516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissinger R, Carlson C, Hulsey T, Eicher D. Secondary surfactant deficiency in neonates. J Perinatol. 2004;24(10):663–666. doi: 10.1038/sj.jp.7211154 [DOI] [PubMed] [Google Scholar]
- 10.Chernick V. Hyaline-membrane disease: therapy with constant lung-distending pressure. N Engl J Med. 1973;289(6):302–304. doi: 10.1056/NEJM197308092890606 [DOI] [PubMed] [Google Scholar]
- 11.Wirbelauer J, Speer CP. The role of surfactant treatment in preterm infants and term newborns with acute respiratory distress syndrome. J Perinatol. 2009;29(Suppl 2):S18–22. doi: 10.1038/jp.2009.30 [DOI] [PubMed] [Google Scholar]
- 12.Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019;115(4):432–450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14(1):29–36. doi: 10.1016/j.prrv.2012.02.002 quiz 36–37. [DOI] [PubMed] [Google Scholar]
- 14.Sweet LR, Keech C, Klein NP, et al. Respiratory distress in the neonate: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48):6506–6517. doi: 10.1016/j.vaccine.2017.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You T, Zhou Y-R, Liu X-C, Li L-Q. Risk factors and clinical characteristics of neonatal acute respiratory distress syndrome caused by early onset sepsis. Front Pediatr. 2022;10:847827. doi: 10.3389/fped.2022.847827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ying Q, You X-Q, Luo F, Wang J-M. Maternal-neonatal serum albumin level and neonatal respiratory distress syndrome in late-preterm infants. Front Pediatr. 2021;9:666934. doi: 10.3389/fped.2021.666934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moison RM, Haasnoot AA, Van Zoeren-Grobben D, Berger HM. Plasma proteins in acute and chronic lung disease of the newborn. Free Radic Biol Med. 1998;25(3):321–328. doi: 10.1016/s0891-5849(98)00070-7 [DOI] [PubMed] [Google Scholar]
- 18.Torer B, Hanta D, Yapakci E, et al. Association of serum albumin level and mortality in premature infants. J Clin Lab Anal. 2016;30(6):867–872. doi: 10.1002/jcla.21949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid ERA, Ali WH, Azmy A, et al. Oxidative stress and anti-oxidant markers in premature infants with respiratory distress syndrome. Open Access Maced J Med Sci. 2019;7(17):2858–2863. doi: 10.3889/oamjms.2019.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Liu F, Liu Y, et al. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 2014;146(2):383–388. doi: 10.1378/chest.13-2852 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Management of the child with a serious infection or severe malnutrition: guidelines for care at the first-referral level in developing countries. World Health Organization. 2000. [Google Scholar]
- 22.Duke T. Neonatal pneumonia in developing countries. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F211–219. doi: 10.1136/adc.2003.048108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Luca D, Tingay DG, Van Kaam AH, et al. Epidemiology of neonatal acute respiratory distress syndrome: prospective, multicenter, international cohort study. Pediatr Crit Care Me. 2022;23(7):524–534. doi: 10.1097/PCC.0000000000002961 [DOI] [PubMed] [Google Scholar]
- 24.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7(2):115–128. doi: 10.1016/S2213-2600(18)30344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong JJ-M, Jit M, Sultana R, et al. Mortality in pediatric acute respiratory distress syndrome: a systematic review and meta-analysis. J Intensive Care Med. 2019;34(7):563–571. doi: 10.1177/0885066617705109 [DOI] [PubMed] [Google Scholar]
- 26.Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Internal Med. 2004;141(6):460–470. doi: 10.7326/0003-4819-141-6-200409210-00012 [DOI] [PubMed] [Google Scholar]
- 27.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Ann Rev Pathol. 2011;6(1):147–163. doi: 10.1146/annurev-pathol-011110-130158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlrab P, Kraft F, Tretter V, et al. Recent advances in understanding acute respiratory distress syndrome. F1000Res 7. 2018:7. F1000 Faculty Rev-263. doi: 10.12688/f1000research.11148.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Li J, Shi Y. Clinical characteristics and outcomes in neonates with perinatal acute respiratory distress syndrome in China: a national, multicentre, cross-sectional study. eClinicalMedicine. 2022;55:101739. doi: 10.1016/j.eclinm.2022.101739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Luca D, van Kaam AH, Tingay DG, et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5(8):657–666. doi: 10.1016/S2213-2600(17)30214-X [DOI] [PubMed] [Google Scholar]
- 31.Hallman M, Glumoff V, Rämet M. Surfactant in respiratory distress syndrome and lung injury. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2001;129(1):287–294. doi: 10.1016/s1095-6433(01)00324-5 [DOI] [PubMed] [Google Scholar]
- 32.Dushianthan A, Cusack R, Goss V, et al. Clinical review: exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome--where do we go from here? Critical Care. 2012;16(6):238. doi: 10.1186/cc11512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev. 2010;24(1):53–63. doi: 10.1016/j.tmrv.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836–1846. doi: 10.1002/hep.26338 [DOI] [PubMed] [Google Scholar]
- 35.Taverna M, Marie A-L, Mira J-P, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intens Care. 2013;3(1):4. doi: 10.1186/2110-5820-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zasadzinski JA, Alig TF, Alonso C, et al. Inhibition of pulmonary surfactant adsorption by serum and the mechanisms of reversal by hydrophilic polymers: theory. Biophys J. 2005;89(3):1621–1629. doi: 10.1529/biophysj.105.062646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernsler JG, Zasadzinski JA. Competitive adsorption: a physical model for lung surfactant inactivation. Langmuir. 2009;25(14):8131–8143. doi: 10.1021/la8039434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taeusch HW, Bernardino de la Serna J, Perez-Gil J, et al. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys J. 2005;89(3):1769–1779. doi: 10.1529/biophysj.105.062620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holm BA, Wang Z, Notter RH. Multiple mechanisms of lung surfactant inhibition. Pediatr Res. 1999;46(1):85–93. doi: 10.1203/00006450-199907000-00015 [DOI] [PubMed] [Google Scholar]
- 40.Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. 2001;3(2):293–311. doi: 10.1089/152308601300185241 [DOI] [PubMed] [Google Scholar]
- 41.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057 [DOI] [PubMed] [Google Scholar]
- 43.Saleh MA, De Miguel C, Stevens DI, et al. Free radical scavenging decreases endothelin-1 excretion and glomerular albumin permeability during type 1 diabetes. Physiol Rep. 2016;4(24):e13055. doi: 10.14814/phy2.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(Suppl S3):S193–199. doi: 10.1007/s11739-012-0802-0 [DOI] [PubMed] [Google Scholar]
- 45.Jang HM, Choi SJ, Park S-H, et al. Association between the nutritional status at birth and need for respiratory support on the first day of life. Neonatal Med. 2019;26(1):24–33. doi: 10.5385/nm.2019.26.1.24 [DOI] [Google Scholar]
- 46.Watchko JF, Spitzer AR, Clark RH. Prevalence of hypoalbuminemia and elevated bilirubin/albumin ratios in a large cohort of infants in the neonatal intensive care unit. J Pediatr. 2017;188:280–286.e4. doi: 10.1016/j.jpeds.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Chang YS, Ahn SY, et al. Predicting mortality in extremely low birth weight infants: comparison between gestational age, birth weight, Apgar score, CRIB II score, initial and lowest serum albumin levels. PLoS One. 2018;13(2):e0192232. doi: 10.1371/journal.pone.0192232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiedermann CJ, Wiedermann CJ. Hypoalbuminemia as Surrogate and Culprit of Infections. Int J Mol Sci. 2021;22(9):4496. doi: 10.3390/ijms22094496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansbach JM, Geller RJ, Hasegawa K, et al. Association of serum albumin with apnea in infants with bronchiolitis: a secondary analysis of data from the MARC-35 study. JAMA Network Open. 2019;2(7):e197100. doi: 10.1001/jamanetworkopen.2019.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used and analyzed during the current study are available from the corresponding author upon reasonable request.