Abstract
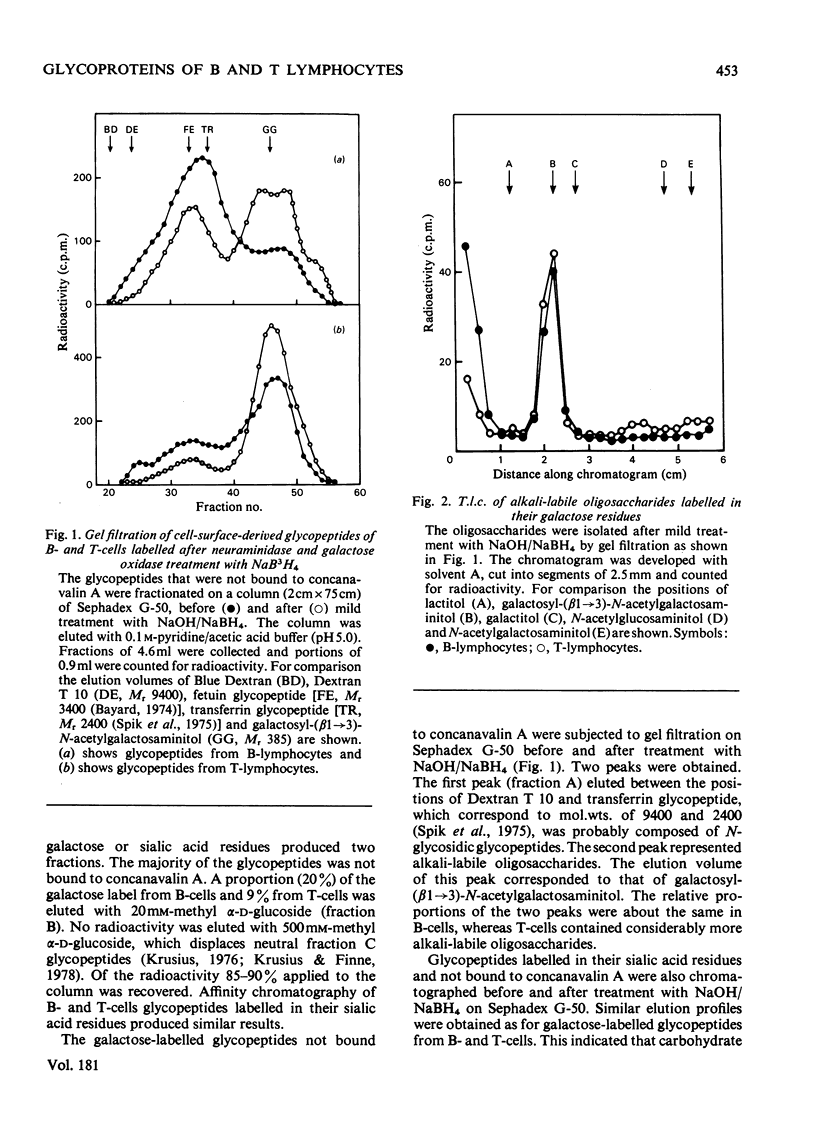
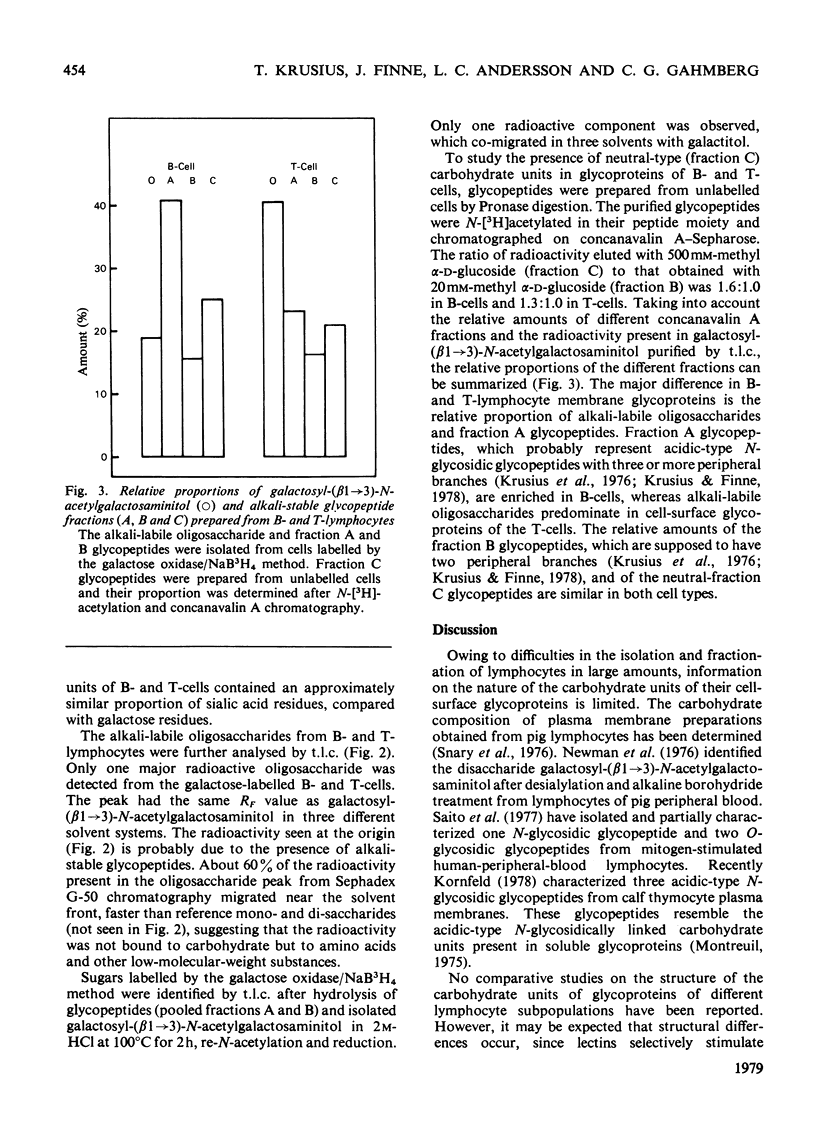
Carbohydrate units of cell-surface glycoproteins of mouse B- and T-lymphocytes, labelled in their sialic acid residues by the periodate/NaB3H4 method and in their galactose residues by the galactose oxidase/NaB3H4 method after neuraminidase treatment, have been studied. Glycopeptides were prepared from the labelled cells by Pronase digestion and fractionated by concanavalin A affinity chromatography into two fractions (A and B). Alkali-labile oligosaccharides were isolated after mild NaOH/NaBH4 treatment by gel filtration. The alkali-labile oligosaccharides were further analysed by t.l.c. To study the relative proportion of neutral mannose-rich carbohydrate units (fraction C) in lymphocyte glycoproteins, glycopeptides were also prepared from unlabelled cells and subjected to concanavalin A affinity chromatography after N-[3H]acetylation of their peptide moiety. The major alkali-labile oligosaccharide component of both cell types was identified as galactosyl-(beta 1 leads to 3)-N-acetylgalactosaminitol. T-Lymphocytes were characterized by a high proportion of this oligosaccharide and a lower proportion of alkali-stable fraction A glycopeptides, whereas the opposite was observed for B-lymphocytes. The relative proportions of the concanavalin A-binding fractions B and C were similar in both cell types. The differences observed may correlate with the different surface properties of B- and T-lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Nordling S., Häyry P. Fractionation of mouse T and B lymphocytes by preparative cell electrophoresis. Efficiency of the method. Cell Immunol. 1973 Aug;8(2):235–248. doi: 10.1016/0008-8749(73)90113-5. [DOI] [PubMed] [Google Scholar]
- Arima T., Spiro R. G. Studies on the carbohydrate units of thyroglobulin. Structure of the mannose-N-acetylglucosamine unit (unit A) of the human and calf proteins. J Biol Chem. 1972 Mar 25;247(6):1836–1848. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Finne J., Krusius T. O-glycosidic carbohydrate units from glycoproteins of different tissues: demonstration of a brain-specific disaccharide, alpha-galactosyl-(1 leads to 3)-N-acetylgalactosamine. FEBS Lett. 1976 Jul 1;66(1):94–97. doi: 10.1016/0014-5793(76)80593-5. [DOI] [PubMed] [Google Scholar]
- Finne J., Mononen I., Kärkkäinen J. Analysis of hexosaminitol-containing disaccharide alditols from rat brain glycoproteins and gangliosides as O-trimethylsilyl derivatives by gas chromatography mass spectrometry. Biomed Mass Spectrom. 1977 Oct;4(5):281–283. doi: 10.1002/bms.1200040502. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Leukocyte surface origin of human alpha1-acid glycoprotein (orosomucoid). J Exp Med. 1978 Aug 1;148(2):507–521. doi: 10.1084/jem.148.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gahmberg C. G., Häyry P., Andersson L. C. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976 Mar;68(3):642–653. doi: 10.1083/jcb.68.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. Structure of the oligosaccharides of three glycopeptides from calf thymocyte plasma membranes. Biochemistry. 1978 Apr 18;17(8):1415–1423. doi: 10.1021/bi00601a009. [DOI] [PubMed] [Google Scholar]
- Krusius T. A simple method for the isolation of neutral glycopeptides by affinity chromatography. FEBS Lett. 1976 Jul 1;66(1):86–89. doi: 10.1016/0014-5793(76)80591-1. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J. Structural features of tissue glycoproteins. Fractionation and methylation analysis of glycopeptides derived from rat brain, kidney and liver. Eur J Biochem. 1977 Sep;78(2):369–379. doi: 10.1111/j.1432-1033.1977.tb11749.x. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Glöckner W. M., Uhlenbruck G. G. Immunochemical detection of the Thomsen-Friedenreich antigen (T-antigen) on the pig lymphocyte plasma membrane. Eur J Biochem. 1976 May 1;64(2):373–380. doi: 10.1111/j.1432-1033.1976.tb10311.x. [DOI] [PubMed] [Google Scholar]
- Saito M., Toyoshima S., Osawa T. Isolation of glycopeptides from the lectin-stimulated human peripheral lymphocyte cell surface. J Biochem. 1977 May;81(5):1203–1208. [PubMed] [Google Scholar]
- Snary D., Allen A. K., Faulkes R. A., Crumpton M. J. Carbohydrate composition of lymphocyte plasma membrane from pig mesenteric lymph node. Biochem J. 1976 Jan 1;153(1):75–78. doi: 10.1042/bj1530075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Bayard B., Fournet B., Strecker G., Bouquelet S., Montreuil J. Studies on glycoconjugates. LXIV. Complete structure of two carbohydrate units of human serotransferrin. FEBS Lett. 1975 Feb 15;50(3):296–299. doi: 10.1016/0014-5793(75)80513-8. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Ralph P., Bevan M. J. Differences in the surface proteins of mouse B and T cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):157–161. doi: 10.1073/pnas.72.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


