Abstract
Stroke has emerged as the second leading cause of mortality. Insomnia after stroke is a highly prevalent complication of stroke with a complex mechanism, impacting daily activities and hindering neurological function rehabilitation while also increasing the risk of stroke recurrence. With the development of molecular biology, intestinal flora has garnered considerable interest in the past few years because of its significant implications for human physiology and pathology. Numerous studies have emphasized the crucial function of intestinal flora in the pathological changes associated with insomnia after stroke. It can influence sleep patterns following a stroke by modulating various pathways, including the hypothalamic-pituitary-adrenal (HPA) axis, immune responses, and neural mechanisms. Disruption of intestinal flora can adversely affect post-stroke sleep quality, while sleep after stroke can also lead to intestinal flora imbalance. Based on the intestinal flora, this paper explores the involvement of hypothalamic-pituitary-adrenal axis (HPA axis), immune pathway and neural pathway in insomnia after stroke, aiming to offer insights for the prevention, treatment, and research of post-stroke insomnia.
Keywords: intestinal flora, stroke, insomnia, insomnia after stroke, mechanism
Introduction
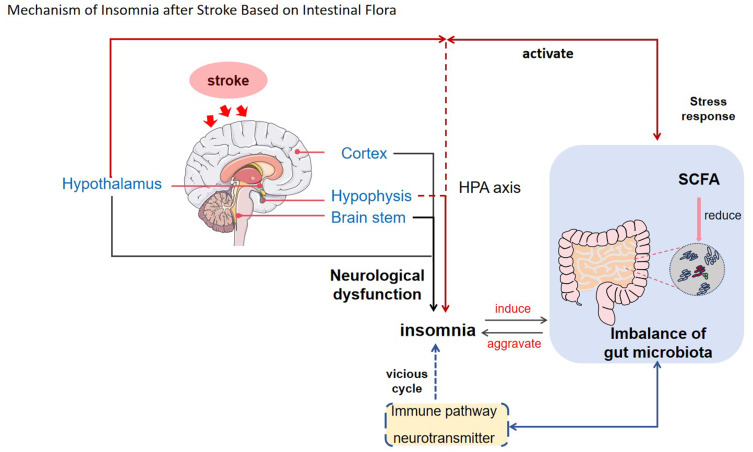
Stroke, also known as a cerebrovascular accident, is a prevalent cerebrovascular disease. It has emerged as a significant global health concern due to the increasing incidence of stroke resulting from the aging population. Currently, stroke ranks as the second leading cause of mortality and the third most common cause of impairment.1 Stroke patients often experience a range of sequelae, such as physical activity disorders, psychological disorders and so on. These conditions significantly impact the well-being and daily life of patients, placing immense pressure on both the individuals affected, their families, and society as a whole. Insomnia following a stroke is characterized by a decline in the quality of sleep or the presence of sleep disorders after experiencing a stroke. It is a common complication, with studies indicating that the prevalence of insomnia following a stroke ranges from 38.2% to 40.7%.2 This not only impacts the neurological rehabilitation process of patients but also increases the risk of recurrent strokes,3 leading to lasting negative effects on future quality of life. Research has shown that approximately 12–18% of stroke survivors experience disruptions in their daily activities due to insomnia.4 The etiology of post-stroke insomnia remains unclear and is believed to be associated with local brain damage, neurological dysfunction, psychological factors, gender, and other related factors. Recent studies have indicated that the majority of anatomical components involved in regulating sleep mechanisms are situated in the hypothalamus and brain stem. Damage to these areas may disrupt the sleep-wake cycle system, potentially leading to post-stroke insomnia. Additionally, a decrease in the sleep cycle among stroke patients may also be linked to damage in the medial part of the pons tectum. The brain stem has been reported as having the highest incidence of insomnia following brain injury, while the cerebellum has shown the lowest.5 Furthermore, it has been observed that individuals who have suffered a right hemisphere stroke experience more instances of insomnia compared to those with left hemisphere strokes.6 Neurological dysfunction leads to abnormal release and metabolism of neurotransmitters, which significantly contributes to post-stroke insomnia. Damage to brain tissue disrupts the secretion system of neurotransmitters related to sleep, such as glutamate (Glu), 5-hydroxytryptamine (5-HT), gamma-aminobutyric acid (GABA), acetylcholine (Ach), norepinephrine (NE) and dopamine (DA). This imbalance in the sleep-wake system results in insomnia.7 After a stroke, patients experience a decrease in self-care ability and changes in social status, which can easily lead to anxiety, depression, and other adverse psychological emotions that also contribute to insomnia.8 Studies indicate that women have a higher incidence of insomnia than men among stroke patients.9 Additionally, physical activity disorders, personality changes, speech disorders, and environmental factors are independent risk factors for insomnia in stroke patients.7 With the continuous advancement of molecular biology, the study of intestinal flora has emerged as a prominent research focus in recent years. It has become a new target for the prevention and treatment of numerous diseases. Intestinal flora, a complex and vast microbiota symbiotic with the human body, Plays an essential part in maintaining overall health. Disruption of the intestinal flora not only triggers related gastrointestinal reactions but also leads to pathological changes within the body.10 Furthermore, it is worth noting that intestinal flora significantly influences the development of insomnia following a stroke, while insomnia after stroke can also contribute to disturbances in intestinal flora (Figure 1).11 This article provides an overview of the mechanisms underlying post-stroke insomnia based on intestinal flora, aiming to establish a foundation for disease prevention and treatment strategies.
Figure 1 .
This Figure illustrates the role of intestinal flora in insomnia following a stroke. Intestinal flora can influence the HPA axis, immune pathways, and neural pathways, among other mechanisms, thereby affecting the development of insomnia post-stroke. Moreover, dysbiosis of intestinal flora interacts reciprocally with both stroke and insomnia, potentially leading to a vicious cycle. The arrows indicate the direction of influence or causality.
The Correlation Between Gut Microbiota and the Incidence of Stroke and Insomnia
Intestinal flora is a broad term referring to a diverse array of symbiotic microbial communities within the human intestinal tract, which is both dynamic and complex. The human intestinal tract is colonized by over 1000 different types of bacteria, amounting to a total of 100 trillion.12 Intestinal microorganisms outnumber human cells by a significant margin, containing approximately 100 times the number of genes found in the human genome. As such, intestinal microorganisms are often referred to as the “second genome” or “second brain” of the human body. The main constituents of intestinal flora include Firmicutes, Proteobacteria, Bacteroidetes, Verrucomicrobiales and Actinobacteria. Among these, Bacteroidetes and Firmicutes are predominant in the human body, collectively accounting for more than 90% of intestinal bacteria in healthy individuals.13 According to the role of intestinal flora, it can be categorized into three groups: harmful bacteria (such as Proteus and Staphylococcus), beneficial bacteria (including Lactobacillus, Bifidobacterium, and Bacteroides), and opportunistic pathogens (such as Escherichia coli, Staphylococcus, and Streptococcus). The balance between pathogenic and non-pathogenic microorganisms in the gut is crucial for maintaining human health. Intestinal flora produces a range of metabolites while participating in the regulation of various bodily functions, collectively Reestablishing the balance of the entire gut microbiota to maintain homeostasis. When a specific flora is imbalanced, it not only leads to related intestinal reactions but also causes pathological changes in the body.10 After a stroke, there are dynamic changes in both the diversity and abundance of gut microbiota as well as in the concentrations of metabolic compounds.14 Studies have identified that an imbalance in intestinal flora independently contributes to the risk of having a stroke,15 with this imbalance exacerbating stroke outcomes.16 Furthermore, a large number of animal experiments and clinical studies have shown that intestinal flora and its metabolites—particularly short-chain fatty acids (SCFA)—can regulate GABA, Brain-derived neurotrophic factor (BDNF), microglia, and adaptive immune cells. This regulation enhances neural network plasticity while reducing nerve inflammation; promoting nerve function repair; ultimately improving patient prognosis.17,18 Insomnia is closely linked to the composition of intestinal flora. Liu et al found that patients with insomnia experienced a notable reduction in the variety and amount of gut bacteria. Additionally, it has been observed that the richness and abundance of intestinal flora are linked to improved sleep efficiency and longer total sleep duration, as well as a reduction in sleep disruption (WASO). The structure of Firmicutes and Bacteroidetes in individuals with insomnia has been found to undergo significant changes, with the abundance of Bacteroidetes and Firmicutes is positively associated with the effectiveness of sleep. These findings provide a theoretical basis for targeting intestinal flora as a potential treatment for insomnia.19 Furthermore, Zhao et al discovered that long-term circadian rhythm disorders led to damage in the intestinal epithelial barrier of rats, resulting in increased intestinal permeability and subsequent alterations in the structure and composition of their intestinal flora.20 Through 16S rDNA gene sequencing, Liu et al also revealed that compared to the normal group, individuals within the insomnia group exhibited lower diversity within their intestinal flora. Moreover, both α and β diversity within this group underwent significant changes, along with statistically significant differences in relative abundance.21 Intestinal flora is closely associated with circadian rhythm, sleep disorders, and mental fatigue. Restoring intestinal flora homeostasis can lead to improved sleep quality and prolonged sleep duration.22 Fecal Microbiota Transplantation (FMT) has been found to increase the number of Bacteroidetes and butyrate-producing bacteria, while decreasing the number of Firmicutes. This significantly enhances the sleep quality of patients.23 Studies have demonstrated that by influencing histone deacetylase activity, intestinal flora can further regulate the expression of genes related to metabolism and impact food intake, thus stabilizing and adjusting the circadian rhythm.24 Continuous broad-spectrum antibiotic treatment in inflammatory mouse models not only reduced their intestinal flora but also significantly decreased non-REM sleep time while increasing REM sleep time.25 There is a strong correlation between intestinal flora imbalance and post-stroke insomnia. Studies have found that patients with post-stroke insomnia exhibit obvious intestinal flora imbalance and a higher number of harmful flora compared to healthy adults.14 Grosicki et al found that Bacteroidetes was the predominant group in the insomnia group, whereas Firmicutes and Proteobacteria were more abundant in the normal group. The ratio of F/B was negatively correlated with the Pittsburgh Sleep Quality Index (PSQI) score, indicating that lower F/B ratios were associated with higher PSQI scores. In other words, poorer sleep quality may be influenced by the flora under Bacteroidetes and Firmicutes through their regulation of food intake and circadian rhythm.26 Short-chain fatty acids, particularly butyrate which are metabolites of intestinal microorganisms, play a significant role in sleep. Increased butyrate concentration in mice led to longer sleep duration and decreased sleep latency.27 Intestinal microbial disorders in post-stroke patients, such as significant reductions in butyrate-producing bacteria like Clostridium, Ackermann, and Staphylococcus, result in a notable increase in the incidence of sleep problems.14 Yu et al found that warm needling can improve levels of intestinal microflora and serum metabolites while significantly increasing butyrate content in feces, thereby improving post-stroke insomnia.28
The Intestinal Microflora Plays a Role in the Mechanism of Post-Stroke Insomnia
Hypothalamic-Pituitary-Adrenal Axis (HPA Axis)
The HPA axis is a critical component of the neuroendocrine system and plays a significant role in the gut-brain axis. It regulates sleep, mood, and the immune system, serving as the core mechanism for stress response. Dysfunction of this system can lead to insomnia due to its inhibition during sleep. Increased secretion of ACTH and cortisol from the HPA axis has been associated with insomnia. In a study on foreign subjects, it was found that under stress, the HPA axis becomes activated and stimulates amygdala tissue in the brain. This stimulation promotes the release of Corticotropin Releasing Hormone (CRH) from the hypothalamus, which then triggers the anterior pituitary to release ACTH. Subsequently, ACTH stimulates the adrenal gland to release cortisol, leading to increased sympathetic excitability and arousal that can cause insomnia.29 Ehichioya et al conducted research on offspring mice from pregnant rats at different stages of pregnancy to study how sleep deprivation affects their HPA axis function. The results showed that maternal sleep deprivation impacted offspring mice’s HPA axis function and altered corticosterone levels in these mice while inhibiting glucocorticoid receptor expression in their hippocampus.30 The HPA axis is controlled by a mechanism of negative feedback, where cortisol hinders the release of ACTH and additionally reduces CRH secretion, resulting in overall inhibition of the HPA axis system.31 After experiencing a stroke, there is an excessive activation of the HPA axis, leading to impaired negative feedback mechanisms and an inability to maintain balance. This results in excessive production and accumulation of cortisol.32 Cortisol is a common metabolite in the HPA axis and plays an essential part in the regulation of internal balance. Prolonged high levels of cortisol not only promote wakefulness but also lead to neurotoxicity and an increased risk of complications following a stroke.33 A study has shown that serum cortisol levels in patients with chronic insomnia are positively correlated with spatial working memory (SWM) scores and Pittsburgh Sleep Quality Index (PSQI) scores (P < 0.05).34 The interaction between intestinal flora and the HPA axis is significant. Changes in intestinal microorganisms can impact the HPA axis by modifying neurotransmitter-related neuro-endocrine signaling pathways or inflammatory response. Conversely, activation of the HPA axis can affect gastrointestinal function, leading to alterations to the composition of intestinal flora.35 Wu et al found that specific intestinal microorganisms can inhibit the activation of the HPA axis and reduce corticosterone levels.36 Short Chain Fatty Acids (SCFA), a metabolite of intestinal flora, have been shown to enhance the physiological function of the blood-brain barrier in germ-free mice, suppress HPA axis reactions, and promote sleep. Tan et al observed a notable decrease in SCFA-producing bacteria in stroke patients’ intestines and significantly lower SCFA levels in their blood compared to healthy controls.37 This suggests that disturbances in SCFA-producing flora may be a crucial factor contributing to sleep disorders after a stroke.38 In conclusion, insomnia is associated with hyperfunctioning of the HPA axis, with intestinal flora playing a central role in its regulation. The disruption of intestinal flora following a stroke leads to HPA axis activation and insomnia; conversely, insomnia can also disrupt intestinal flora, creating a vicious cycle.
Immune Pathway
The gastrointestinal tract is the most densely populated gathering place of immune cells in the human body and serves as the largest immune organ. The diversity of intestinal flora directly impacts the stability of immune function, with the establishment and maintenance of intestinal flora homeostasis playing a crucial role in shaping, developing, and regulating the immune system.39 Intestinal flora has the ability to stimulate immune cells to release relevant immune factors, thereby exerting specific effects on the body. As an organ with the largest contact surface between the human body and external environment, it is imperative for the intestinal tract to maintain a complete barrier in order to uphold intestinal homeostasis and prevent diseases. The biological mechanical barrier of the intestinal tract is formed by both intestinal flora and mucosal cells, effectively preventing invasion by bacteria and viruses. Following a stroke or disruption of circadian rhythm, there may be a disturbance in intestinal flora leading to decreased relative abundance of normal microbiota, resulting in impaired intestinal barrier function40 and increased permeability of epithelial cell membranes within the intestine. To further reduce the clearance rate of intestinal flora and bacteria in the bloodstream, intestinal pathogens may exit the intestinal tract and enter the bloodstream, triggering an immune response. This process can impact levels of inflammatory factors such as interferon-γ(IFN-γ), interleukin-6 (IL-6), C-reactive protein (CRP), interleukin-1(IL-1), nuclear factor-κB (NF-κB) and tumor necrosis factor-α (TNF-α), and disrupt the normal sleep-wake cycle.41 It may also be associated with inhibition of the vagus nerve caused by these inflammatory factors.42 Wu et al found that levels of IL-6, IL-1β and TNF-α in the hypothalamus were lower in an insomnia model compared to a control group. After electroacupuncture treatment at various frequencies, IL-6, IL-1β and TNF-α content in the hypothalamus significantly increased compared to those in the model group.43 Huang et al studied changes in sleep time, IL-25 and TNF-α contents in serum and hippocampus of insomnia rats as well as gastric mucosa injury rats through acupuncture. The results showed that sleep time, IL-25 and TNF-α contents increased in serum and hippocampus of insomnia rats. Sleep time was shortened for insomnia rats with gastric mucosal injury while inflammatory cytokine content decreased indirectly indicating a relationship between gastrointestinal health, inflammation cytokines, and insomnia. Furthermore, these three cytokines are closely linked to activation of gastrointestinal immune cells as well as stimulation from metabolites produced by intestinal flora. Sleep can influence immune system stability leading to enhanced expression of pro-inflammatory cytokines. Therefore, it is evident that there is an interaction between insomnia, intestinal flora homeostasis, and pro-inflammatory cytokine expression.44 Studies have indicated that an increase in the proliferation of Aeromonas can trigger an inflammatory response, a reduction in the level of butyric acid (a metabolite of flora), and a decrease in melatonin, leading to abnormal sleep patterns.45 In the brain and spinal cord, which make up the central nervous system, there are immune cells called microglia that are spread throughout. These cells have the ability to promptly react to various stimuli by continuously monitoring and detecting changes in the surrounding environment.46 After experiencing a stroke, gut microbiota plays a crucial role in protecting the brain primarily through endogenous aromatic hydrocarbon receptor (AHR) ligands and short-chain fatty acids (SCFAs), which contribute to activating microglia.47 Intestinal flora can also influence the activity of microglia through the blood-brain barrier, resulting in disruptions to the rhythmicity of sleep patterns.48 Disorders in circadian rhythms can lead to decreased levels of lactobacilli, increased microglial activity, and activated immune responses.49 Hence, it is clear that a reciprocal connection can be observed between the gut microbiota, the immune system of the host, and the occurrence of insomnia. Intestinal flora can impact sleep through immune and inflammatory pathways. Conversely, insomnia can affect intestinal flora through immune responses and inflammatory pathways caused by microglia. This may result in a vicious cycle effect.
Neural Pathways
Neurotransmitters
The intestinal flora plays an essential role as the primary producer of numerous neurotransmitters, with over 90% of brain neurotransmitters being primarily synthesized in the human intestine.50 The gut microbiota interacts with the nervous system by influencing various neurotransmitters and plays a role in the regulation of sleep. For example, dopamine (DA) is produced by Bacillus, Serratia, and Escherichia coli; gamma-aminobutyric acid (GABA) is synthesized by lactic acid bacteria, Escherichia coli, Bifidobacterium, and Pseudomonas; Norepinephrine (NE) is produced by yeasts and Bacillus; Candida, Escherichia coli, Bifidobacterium infantis, Streptococcus, Enterococcus, and Lactobacillus bulgaricus are involved in the synthesis of 5-hydroxytryptamine (5-HT).16 Insomnia is associated with the levels of 5-HT, GABA, DA, NE, melatonin (MT), and other neurotransmitters. Studies have found that plasma levels of 5-HT, GABA, DA, ACH and NE in patients with insomnia after craniocerebral injury are significantly lower than those in patients with primary insomnia, and the difference is statistically significant (P < 0.05).51 The vagus nerve plays an essential role in the bidirectional regulation between the gut and the brain, with 90% of its extension from the gut to the brain.52 It has also been found that intestinal flora or its metabolites can directly impact the functional synapses of primary afferent neurons in the enteric nervous system. This action then sends signals to vagal afferents to either excite or inhibit corresponding neurons in the central nervous system for sleep regulation.53 Studies have shown that transcutaneous vagus nerve stimulation can effectively treat primary insomnia by modulating neurotransmitter concentrations such as GABA, NE, and 5-HT, thereby participating in sleep regulation.54 Furthermore, after 4 weeks of percutaneous vagal nerve stimulation treatment, patients with insomnia showed significantly decreased PSQI scores, with statistically significant differences. Polysomnographic data also indicated reduced time to fall asleep and increased duration of slow-wave sleep, demonstrating that transcutaneous vagal stimulation can effectively treat insomnia.54 5-HT plays a crucial role in the regulation of sleep-wake cycles. It is released from nerve axon terminals and subsequently influences the synthesis of substances related to sleep within the brain. Approximately 90% of 5-HT is produced in the gut, particularly in enterochromaffin cells,55 and it is influenced by intestinal flora and its metabolites such as short-chain fatty acids and spore-producing bacteria.56 Imbalance of intestinal flora after a stroke can lead to impaired intestinal endothelial cell function which affects normal production of 5-HT resulting in reduced sleep quality. Research conducted by Hou et al has demonstrated that acupuncture can regulate intestinal flora, impact serum and central 5-HT content, and improve sleep.57 Additionally, 5-HT serves as a precursor to melatonin which can be converted into melatonin at night to help reduce sleep latency. There exists a negative feedback regulation system involving “5-HT-melatonin” for gastrointestinal motility regulation. Studies have shown that intraperitoneal injection of physiological doses of melatonin in rats can stimulate the central nervous system to secrete 5-HT. Conversely, injection of physiological doses of 5-HT can increase melatonin levels in the central and gastrointestinal tract. It has been observed that these two substances antagonize each other at the level of melatonin receptor and 5-HT receptor.58 Melatonin (MT) is a hormone released by the pineal gland that acts as a neurotransmitter, with various properties such as regulating circadian rhythm and possessing anti-inflammatory effects.59 Its secretion increases at night, inhibits the HPA axis, reduces cortisol concentration during human activities and stress to improve sleep quality, and maintains wakefulness by reducing secretion during the day. This plays a essential role in regulating the body’s sleep-wake mechanism.60 Additionally, MT has been found to decrease the size of infarcts, relieve brain swelling, enhance neurological function, and maintain the integrity of the blood-brain barrier in mice with stroke.61 Research suggests that MT is closely linked to intestinal microorganisms and their metabolism; indeed, a significant amount of MT can be synthesized and secreted by the gastrointestinal tract. Disruptions in intestinal flora may lead to changes in MT levels due to their interaction with one another.62 ENKHMURUN C discovered that melatonin (MT) has the potential to reverse the expression of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) induced by lipopolysaccharide (LPS). This is achieved by inhibiting neuroinflammation mediated by BV-2 microglia activation. These findings indicate that MT may hold potential as an effective treatment for post-stroke insomnia.63 Gamma-Aminobutyric acid (GABA) is predominantly present in the brain and arousal-related systems, serving as the predominant inhibitory neurotransmitter in the nervous system. By binding to corresponding ion channels, GABA can inhibit the release of abnormal brain waves and prevent neurons from becoming overexcited, thereby aiding in promoting sleep.27 GABA produced by the intestinal flora may have direct effects on the central nervous system (CNS) through vagal nerve transmission, thereby influencing sleep.64 Additionally, GABA plays a role in maintaining gastrointestinal tract homeostasis by regulating gut microbiota, indicating a close relationship between intestinal microorganisms and circulating GABA levels.65 Furthermore, chronic antibiotic treatment has been shown to increase the content of inhibitory neurotransmitters glycine and GABA in the intestinal tract of inflamed mice, leading to significant changes in their sleep/wake cycle.66 Research has demonstrated that GABA can inhibit glutamate-mediated excitatory neurotoxicity and promote functional recovery after stroke by regulating the mechanism of long-term potentiation of damaged neurons.67 DA is primarily derived from the nigrostriatal and mesolimbic systems. The nigrostriatal pathway releases DA to facilitate sleep, whereas the limbic system releases DA to promote wakefulness. DA acts on neurons expressing dopamine receptor D2 in the basolateral amygdala, thereby inducing the transition from non-rapid eye movement (NREM) sleep to rapid eye movement (REM) sleep.68 Liu et al discovered that mice lacking dopamine receptors exhibit varying degrees of differences in their intestinal flora composition at various taxonomic levels compared to normal control mice. This suggests that dopamine receptor deficiency can lead to dysbiosis in mouse gut microbiota.69
Brain-Derived Neurotrophic Factor
BDNF is the neurotrophic factor that is most commonly found in the human brain, promoting and sustaining the growth and development of various neurons, particularly 5-HT and dopaminergic neurons. It can enhance the morphology of neurons in the brain and plays an essential role in sleep homeostasis.70 In patients with insomnia, BDNF levels in peripheral blood may be reduced to varying degrees, showing a negative correlation with the severity of insomnia.71 Following a stroke, BDNF can facilitate neural function repair by modulating intestinal flora.72 Changes in intestinal flora can impact BDNF expression; an imbalance or increase of harmful bacteria may lead to decreased BDNF levels, affecting neuronal activity, especially hippocampal neurons. Probiotics within the flora, such as Bifidobacterium, have been shown to increase BDNF expression,73 leading to significant improvement in serum BDNF levels following probiotic supplementation.74 Research has shown that Jiawei Jiaotai Pill can effectively mitigate abnormal activation of the BDNF/TrkB pathway, improve intestinal flora structure and composition in rats, promote beneficial bacteria production, and enhance sleep quality.75
Conclusion
The extensive and complex microbiota of the intestines, which symbiotically interacts with the human body, is essential for maintaining overall health. Disruption of the intestinal flora not only leads to related gastrointestinal issues but also triggers various pathological changes throughout the body. The relationship between intestinal flora and post-stroke insomnia is closely intertwined. Intestinal flora can impact the onset and progression of post-stroke insomnia by influencing the HPA axis, immune pathways, neural pathways, and other mechanisms. The imbalance of intestinal flora, stroke, and insomnia interact in a manner that easily perpetuates a vicious cycle. Therefore, an in-depth exploration of intestinal flora holds promise for identifying new targets for preventing and treating post-stroke insomnia. In the past few years, there has been notable advancement in comprehending the various pathways through which gut flora influences the onset of insomnia following a stroke. However, there remains a lack of direct research into the mechanisms linking post-stroke insomnia with imbalances in intestinal flora; thus further clarification is needed on their relationship. Currently, numerous studies on the relationship between flora and disease rely on animal models, which may not accurately reflect human flora due to its diverse and complex nature. The interaction between flora is common, thus necessitating more clinical observations for verification. In modern society, psychological pressure often leads to mental and emotional problems such as insomnia after stroke. Nevertheless, there is a shortage of clinical research on the influence of intestinal flora on post-stroke insomnia with psychological issues. At present, significant progress has been made in establishing diagnostic criteria for insomnia and models for assessing and predicting sleep quality based on the composition and diversity of gut microbiota. These advancements aim to prevent and enhance the treatment of post-stroke insomnia. Probiotics, fecal microbial transplantation, and other therapeutic approaches have demonstrated efficacy; however, they have primarily been tested in small sample trials and are not yet widely implemented. With the increasing sophistication of metagenomic sequencing techniques, including 16S rRNA amplicon sequencing, more opportunities arise for investigating the relationship between intestinal flora and post-stroke insomnia. We believe that future laboratory and clinical studies should be conducted to validate existing evidence regarding this relationship. It is essential to fully explore the role of gut microbiota in the prevention, diagnosis, and treatment of post-stroke insomnia. We hope that these findings will provide valuable insights for researchers pursuing similar avenues of inquiry.
Funding Statement
Heilongjiang Province TCM Research Project (ZHY2024-150); Heilongjiang Province Key R&D Program (GZ21C001); and Heilongjiang Province TCM Research Project (ZYW2022-128).
Abbreviations
DA, dopamine; NE, norepinephrine; BDNF, brain-derived neurotrophic factor; HPA axis, hypothalamic-pituitary-adrenal axis; Glu, glutamate; 5-HT, 5-hydroxy tryptamine; GABA, gamma-aminobutyric acid; Ach, acetylcholine; SCFA, short-chain fatty acids.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, execution, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
There are no conflicts of interest.
References
- 1.Parr E, Ferdinand P, Roffe C. Management of acute stroke in the older person. Geriatrics. 2017;2(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. [DOI] [PubMed] [Google Scholar]
- 3.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurotherapeutics. 2018;18(7):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyung-Lim J, Won-Hyoung K, Ha-Yoon C, et al. The effect of sleep disturbances on the functional recovery of rehabilitation inpatients following mild and moderate stroke. Am J Phys Med Rehabil. 2017;96(10):734–740. [DOI] [PubMed] [Google Scholar]
- 5.Na-Ying T, Shuo-Lin W, Chun-Xue W, Li-Jun L, Ning Z, Zi-Xuan W. The clinical characteristics of patients with insomnia after stroke and risk factor analysis. Chin Med J. 2018;53(9):979–982. [Google Scholar]
- 6.Da Rocha P, Barroso M, Dantas A, Melo L, Campos TF. Predictive factors of subjective sleep quality and insomnia complaint in patients with stroke: implications for clinical practice. Anais da Academia Brasileira de Ciências. 2013;85(3):1197–1206. [DOI] [PubMed] [Google Scholar]
- 7.Tsai HJ, Wong YS, Ong CT. Clinical course and risk factors for sleep disturbance in patients with ischemic stroke. PLoS One. 2022;17(11):e0277309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li-Jun L, Yang Y, Bo-Yuan G, et al. Insomnia is associated with increased mortality in patients with first-ever stroke: a 6-year follow-up in a Chinese cohort study. Stroke Vasc Neurol. 2018;3(4):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim W-H, Jung H-Y, Choi H-Y, et al. The associations between insomnia and health-related quality of life in rehabilitation units at 1 month after stroke. J Psychosom Res. 2017;96:10–14. [DOI] [PubMed] [Google Scholar]
- 10.Bolun Z, Yutong Y, Shanshan Z, et al. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front Immunol. 2020;11:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangkyu K, Michal JS. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junjie Q, Ruiqiang L, Jeroen R, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer KC, Rees T, Finlay BB. The gut microbiota-brain axis expands neurologic function: a nervous rapport. BioEssays. 2019;41(10):e1800268. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Wang X, Sun C, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiuli Z, Xuxuan G, Yu P, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front Cell Infect Microbiol. 2019;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pluta R, Januszewski S, Czuczwar SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. 2021;22(2):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuxia Z, Meiqi J, Jiahui R, et al. New insight into gut microbiota and their metabolites in ischemic stroke: a promising therapeutic target. Biomed Pharmacother. 2023;162:114559. [DOI] [PubMed] [Google Scholar]
- 18.Paul C, Nicolas B, Chloé D, Sophie J, Hubert V, Laura M. Gut microbiota and stroke: new avenues to improve prevention and outcome. Eur J Neurol. 2023;30(11):3595–3604. [DOI] [PubMed] [Google Scholar]
- 19.Bingdong L, Weifeng L, Shujie C, et al. Corrigendum: gut microbiota as an objective measurement for auxiliary diagnosis of insomnia disorder. Front Microbiol. 2020;11:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo-Jie Z, Yue L, Bo C, et al. Circadian rhythm disorders of rat intestinal flora and the influence of the intestinal barrier. Mod Preventive Med. 2022;49(08):1495–1500. [Google Scholar]
- 21.Liu B, Lin W, Chen S, et al. Gut microbiota as an objective measurement for auxiliary diagnosis of insomnia disorder. Front Microbiol. 2019;10:1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds AC, Paterson JL, Ferguson SA, Stanley D, Wright KP, Dawson D. The shift work and health research agenda: considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev. 2016;34:3–9. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506–512. [DOI] [PubMed] [Google Scholar]
- 24.Vivienne W, Theresa A. Epigenetic regulation by gut microbiota. Gut Microbes. 2022;14(1):2022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yukino O, Chika M, Nozomu O, et al. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci Rep. 2020;10(1):19554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosicki GJ, Riemann BL, Flatt AA, Valentino T, Lustgarten MS. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: a pilot study. Sleep Med. 2020;73:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Han X, Cen S, et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol Res. 2020;233:126409. [DOI] [PubMed] [Google Scholar]
- 28.Hong Y, Hui Y, Lengge S, et al. Influence of warm acupuncture on gut microbiota and metabolites in rats with insomnia induced by PCPA. PLoS One. 2022;17(4):e0267843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisa S, Roee A. From childhood adversity to latent stress vulnerability in adulthood: the mediating roles of sleep disturbances and HPA axis dysfunction. Neuropsychopharmacology. 2023;48(10):1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehichioya DE, Taufique ST, Anigbogu CN, Jaja SI. Effect of rapid eye movement sleep deprivation during pregnancy on glucocorticoid receptor regulation of HPA axis function in female offspring. Brain Res. 2022;1781:147823. [DOI] [PubMed] [Google Scholar]
- 31.Asarnow LD. Depression and sleep: what has the treatment research revealed and could the HPA axis be a potential mechanism? Curr Opin Psychol. 2020;34:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barca ML, Eldholm RS, Persson K, et al. Cortisol levels among older people with and without depression and dementia. Int Psychogeriatr. 2018;31(4):597–601. [DOI] [PubMed] [Google Scholar]
- 33.Zhili C, Poornima V, Don S, Michael C, Tao Y, Jieli C. Brain-heart interaction: cardiac complications after stroke. Circ Res. 2017;121(4):451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SuJie L, YuanYan G, Zheng W, XiaoXi X. Chronic insomnia patients serum cortisol, BNDF, level of CD4 ~ + and the correlation of sleep quality and cognitive function. J Clin Exp Med. 2022;21(03):262–266. [Google Scholar]
- 35.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. [DOI] [PubMed] [Google Scholar]
- 36.Wu W, Adame M, Liou C, et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 2021;595(7867):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuhong T, Qiheng W, Huidi W, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr. 2020;45(3):518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Błażej M, Igor Ł, Wojciech M, et al. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog Neuro Psychopharmacol Biol Psychiatry. 2020;102:109951. [DOI] [PubMed] [Google Scholar]
- 39.Kuziel GA, Rakoff-Nahoum S. The gut microbiome. Curr Biol. 2022;32(6):R257–R264. [DOI] [PubMed] [Google Scholar]
- 40.Zakaria GE. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek. 2020;113(12):2019–2040. [DOI] [PubMed] [Google Scholar]
- 41.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong TS, Mayer E. Advances in brain-gut-microbiome interactions: a comprehensive update on signaling mechanisms, disorders, and therapeutic implications. Cell Mol Gastroenterol Hepatol. 2024;18(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jian-li W, Dong-Mei D, Wen-Qiang S, et al. Experimental study on the effect of electrical term on hypothalamic immune cytokines in insomnia rats. J Hunan Univ Chin Medi. 2017;37(06):679–683. [Google Scholar]
- 44.Ying-Hua H, Qian L, Ping Y, Ya-Nan Y, Hui-Fang M. Effects of acupuncture stimulation of different acupoint groups on sleeping latency, serum and hippocampal TNF-α and IL-25 contents in rats with gastric mucosal injury. Zhen Ci Yan Jiu = Acupuncture Research. 2015;40(2):131–135. [PubMed] [Google Scholar]
- 45.Xintong W, Zixu W, Jing C, Yulan D, Yaoxing C. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soares NL, Vieira HL. Microglia at the centre of brain research: accomplishments and challenges for the future. Neurochem Res. 2021;47(2):1–16. [DOI] [PubMed] [Google Scholar]
- 47.Han H, Safe S, Jayaraman A, Chapkin RS. Diet-host-microbiota interactions shape aryl hydrocarbon receptor ligand production to modulate intestinal homeostasis. Annu Rev Nutr. 2021;41:455–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choudhury ME, Mikami K, Nakanishi Y, et al. Insomnia and depressive behavior of MyD88-deficient mice: relationships with altered microglial functions. J Neuroimmunol. 2021;363:577794. [DOI] [PubMed] [Google Scholar]
- 49.Zhi-Jun S, Qan-Yi Z, Yi-Peng X, Zheng-Yu Z. Advances in signaling pathways related to microglia activation after sleep disorders. Acta Physiologica sinica. 2023;75(04):569–574. [PubMed] [Google Scholar]
- 50.Ting-Ting H, Jian-Bo L, Yan-Li D, Yi X, Lie-Min R, Shao-Hua H. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genetics. 2019;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ming-Jun H, Gang W, JinNing S, et al. Changes in neurotransmitter levels and insomnia after traumatic brain injury. J Shanxi Med Univ. 2014;45(10):976–978. [Google Scholar]
- 52.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13(9):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mu P, Huang YH. Cholinergic system in sleep regulation of emotion and motivation. Pharmacol Res. 2019;143:113–118. [DOI] [PubMed] [Google Scholar]
- 54.Yating W, Lu S, Xian W, et al. Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: a randomized control trial. Brain Sci. 2022;12(10):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. [DOI] [PubMed] [Google Scholar]
- 56.Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo-Liang H, Rui W, Yu-Ping Y, Rui S, Shu-Hui Z, Ze-Dong C. Electricity for atherosclerosis rabbit intestinal flora and the effects of serotonin. J Liaoning Univ Tradit Chin Med. 2022;24(01):18–21. [Google Scholar]
- 58.Wei-Wei G, Zai-Ming C, Yin L. Effects of melatonin on serum 5-HT, BDNF and sleep quality in patients with neurodermatitis with sleep disorders. Mod Pract Med. 2021;33(05):608–610. [Google Scholar]
- 59.Junjie W, Shiqi G, Cameron L, et al. Melatonin as an antioxidant agent in stroke: an updated review. Aging Dis. 2022;13(6):1823–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyungseon C, Jeong LY, Seonyoung P, Kyung JN, Sun SH. Efficacy of melatonin for chronic insomnia: systematic reviews and meta-analyses. Sleep Med Rev. 2022;66:101692. [DOI] [PubMed] [Google Scholar]
- 61.Danli L, Yuxin L, Huipeng H, et al. Melatonin offers dual-phase protection to brain vessel endothelial cells in prolonged cerebral ischemia-recanalization through ameliorating ER stress and resolving refractory stress granule. Transl Stroke Res. 2022;14(6):910–928. [DOI] [PubMed] [Google Scholar]
- 62.Carrión MÁB, Rol MA. Melatonin as a mediator of the gut microbiota–host interaction: implications for health and disease. Antioxidants. 2023;13(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chibaatar E. Study on the mechanism of N-acetyl-5-methoxytryptophan (melatonin) inhibiting the neuroinflammatory response of microglia BV2 by down-regulating the release of high mobility group Box1 (HMGB-1) [thesis]. 2020. [Google Scholar]
- 64.Leandro DRL, Vinicius TL, LdB C, Monica LA, Sergio T, Helena H. Effects of supplementation with Lactobacillus probiotics on insomnia treatment. Altern Ther Health Med. 2021;27(S1):178–184. [PubMed] [Google Scholar]
- 65.Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu M, Mao W, Wu H, et al. Transient plasticity response is regulated by histone deacetylase inhibitor in oxygen-glucose deprivation condition. Pharmacol Rep. 2023;75(5):1200–1210. [DOI] [PubMed] [Google Scholar]
- 68.Riemann D, Espie CA, Altena E, et al. The European Insomnia Guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res. 2023;32(6):e14035. [DOI] [PubMed] [Google Scholar]
- 69.Lu L, Yu-Qing W. Analysis of gut microbiota changes in dopamine receptor DRD5 null mice based on high-throughput sequencing. Int J Lab Med. 2023;44(13):1537–1540+1546. [Google Scholar]
- 70.Andrea B, Andrea Z, Gusmeo CD, et al. Peripheral brain-derived neurotrophic factor (BDNF) in insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2022;67:101738. [DOI] [PubMed] [Google Scholar]
- 71.Sergio S, Karla M, Ricardo R, et al. Insomnia impairs both the Pro-BDNF and the BDNF levels similarly to older adults with cognitive decline: an exploratory study. Int J Mol Sci. 2023;24(8):7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeong S, Chokkalla AK, Davis CK, Vemuganti R. Post-stroke depression: epigenetic and epitranscriptomic modifications and their interplay with gut microbiota. Mol Psychiatry. 2023;28(10):4044–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kristine KJ, Caspar B, Simon H, Ernst NR, Peter L, Suzette S. Gut microbiota variations in patients diagnosed with major depressive disorder-A systematic review. Brain Behav. 2021;11(7):e02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neda H, Shirin R, Majid M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. 2019;24(6):1–10. [DOI] [PubMed] [Google Scholar]
- 75.Fang Y. Clinical efficacy evaluation and mechanism of Jiawei Jiaotai pill in the treatment of irritable bowel syndrome with heart-kidney dysfunction and liver stagnation and spleen deficiency [dissertation]. 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.