Abstract
Cypermethrin (Cyp), a persistent synthetic pyrethroid insecticide widely used for insect control. The persistence of Cyp creates toxicity to both humans and the environment This study investigates biochar and Bacillus cereus distinct and collective effects on Cyp -contaminated soil during a 90-day incubation. This study also investigates the effects of different concentrations of Cyp (50, 100, ,500 to 1000 mg kg−1) on soil physicochemical and biological activities during a 90-day incubation period. Microbial biomass carbon and soil respiration rates decreased significantly across all cypermethrin concentrations, with the most substantial reductions observed at 1000 mg kg−1. However noticeable variations in soil enzymes and MBC over time during the entire incubation period. On 1st day, the GMean Enz and MBC rate for Cyp treatments (50, 100, ,500 to 1000 mg kg−1) ranged from 0.98 to 0.63, and 9.06, to 5.03, respectively. Under Cyp pollution, microbial biomass carbon exhibited significant decreases, with the highest inhibition (86.2%) at 1000 mg kg−1 on 1st day of incubation. Soil respiration rates dropped 77%, at 1000 mg kg−1, and Integrated biomarker response (IBR) values peaked on day 30, indicating environmental stress. Biochar and Bacillus cereus effectively facilitated the degradation of Cyp, achieving approximately 85% degradation within the first 45 days of the experiment. The combined application of biochar and Bacillus cereus increased soil pH to a neutral level from 5.9, to 7.1, reduced electrical conductivity from 1.41 µS cm− 1 to 1.20 µS cm−1, and elevated cation exchange capacity from 1.54 ± 0.04 to 6.18 C mol kg−1, while also improving organic carbon content to 3.135%. However, the dehydrogenase activity was decresed upto 47% in the combined application and all other enzymes including urasese catlayse and phostasese enzymes with Gmean enzymeatic activities were significantly improved. These findings suggest biochar and bacterial interaction for soil management to enhance soil resilience against pesticide stress.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81588-4.
Keywords: Biomarker response, Cypermethrin degradation, Microbial activities, Soil management
Subject terms: Biotechnology, Chemical biology, Microbiology, Environmental sciences
Introduction
The extensive use of the broad-spectrum synthetic pyrethroid insecticide, Cypermethrin (Cyp), has increased in recent years1 Despite their efficacy in controlling a variety of insects and pests, pose a significant environmental and health risks due to their persistence in the environment. The persistence of these pesticides creates toxicity to both humans and the environment2. Cyp contamination has been reported in different parts of the world, making it a global concern. Several studies conducted in Pakistan have documented significant levels of Cyp residues in soils and agricultural products. For instance, Cyp residues in the soils of Swat valley, ranging from 0.14 to 27.62 mg kg− 1 in rainfed areas and 0.05 to 73.75 mg kg− 1 in irrigated areas3. Similarly, Cyp contamination in vegetables like eggplant and okra in the Gujranwala and Faisalabad regions, with contamination rates of 56% and 43%, respectively which was higher 40–56% from all other studied pesticides in the reigion4. Its impact is not limited to one region, and research has shown that residues can be found in soil, water, and even food products across different countries. In China, it occupies more than 50% of the total pyrethroid market production. Its half-life ranges from 14.6 to 76.2 days in soil depending on the physicochemical properties of the soil as well as on the microbial activity of the soil5. The permissible limit of cyp in soil can vary depending on the regulatory body and the specific environmental context. However, Cyp levels in soil are considered concerning when they exceed 0.01 mg/kg to 0.1 mg/kg. Cyp leads to cyanohydrin formation, which inhibits ion channels of various biological activities and increases oxidative stress6. Cyp pollution in soil inhibits microbial biomass carbon (MBC), soil respiration, (SR) and microbial metabolic assay (qCO2). These biological activities are recognized as sensitive indicators for assessing soil quality under the influence of anthropogenic changes7. The geometric mean of enzyme activity represents the overall process of microbes and is commonly used for assessing soil health and potential toxicity8. Research has shown that herbicides like florasulam and halauxifen-methyl decrease enzyme activity after 6.5 weeks of soil contamination9. Microbiological processes such as qCO2, MBC, and SR are reliable indicators reflecting environmental disturbances, as demonstrated by the impact of fomesafen on MBC and SR under 90 days of contamination10. Additionally, the ratio of metabolic activity (qCO2), representing SR and biomass carbon, is widely utilised to assess the toxicity of pesticides11. These parameters indicate soil toxicity evaluation and a positive correlation between microbial biomass and enzyme activity highlights the interconnected nature of microbial dynamics during pesticide degradation1. Many studies have presented the adverse impacts of pesticides on individual soil properties. However, the use of integrated biomarker studies for evaluating risks associated with Cyp stress is rare. The bioindication marker or biological responses incorporating various soil enzymes, as well as biological indicators such as MBC and SR, have proven to be pronounced in measuring environmental and biological hazards in soil12. The IBR index has demonstrated effectiveness in evaluating temporary ecological threats resulting from the collective contamination of sulfonamide (antibiotics) with copper sulphate (CuSO4)13. higher values of IBR indicate higher stress levels, revealing increased toxicity of xenobiotics to soil health14. However, there is a scarcity of studies employing the IBR index to demonstrate the environmental risks associated with long-term Cyp pollution.
Various approaches, like ultrasonication, nanocomposites, and photocatalysis, have been employed to detoxify pesticides to reduce their risk and degradation15. These methods often produce secondary metabolites with higher toxicity significant costs and technical challenges16. In contrast, biochar-based bioremediation has emerged as an eco-friendly, efficient, and cost-effective alternative for eliminating pesticide residues17,18. Both bacteria and biochar contribute to reducing contaminants through adsorption and supporting soil aggregate formation, the capacities of biochar display considerable diversity19. Effective technology for treating soil pollutants involves the immobilization of microorganisms on aromatic hydrocarbons. Consequently, there is a growing importance placed on biodegradation, necessitating further exploration of untapped microorganisms for immobilization technology based on biochar for pollutant biodegradation20.
Despite the widespread endorsement of biochar, there remains a gap in our understanding of the intricate biological responses of soil microbes in Cyp-contaminated soil. Our hypothesis suggests that prolonged exposure to Cyp pollution could lead to impairments in physiochemical and biological properties. The study objective is (i) to examine the degeneracy of Cyp in agricultural soil and its impact on soil physiochemical (pH, EC, CEC, OC) and biological properties (Gmean-Enz, MBC, SR, and qCO2). (ii) calculate the risk using integrated biomarker studies (IBR/n) during Cyp degradation. (iii) Finally the Cyp degradation using synergies of biochar bacterial interaction. These investigations will enhance our understanding of the persistent behaviour of Cyp in agricultural soil and its impact on soil quality through controlled scaled experiments.
Materials and methods
Chemicals and media
β-cypermethrin (β-CP-100EC) with 95% purity, obtained from Four Brothers Pvt., Ltd., Lahore, Pakistan, was applied at a dose of 420–495 mL per hectare. The dissolution of all chemicals occurred in acetonitrile to create a stock solution with a concentration of 10 g L− 1 was kept in a closed container at a low temperature of 4 °C. Mineral salt medium (MSM) containing (NH4)2SO4, MgSO4⋅7H2O, CaCl2⋅2H2O, FeSO4⋅7H2O, Na2HPO4⋅12.H2O, KH2PO4, K2HPO4 and sodium chloride (NaCl) was used. All chemicals used in the media were purchased from Marck and Sigma Aldrich. The MSM pH was adjusted to 7.0 and subjected to autoclaving at 121 °C for 20 min. HPLC-grade methanol was used for HPLC analysis.
Isolation, identification and characterization of isolated bacterial strain
Bacterial strains used in the study were isolated with serial dilution through a streak plate technique from the rhizospheric soil of Nicotiana tabacum L (tobacco). Soil samples for the bacterial strains isolation were collected from the top 0–20 cm layer from the district Swabi, Khyber Pakhtunkhwa, Pakistan (34.0719° N, 72.4732° E). This region has more than a 15-year history of Cyp exposure to soil for tobacco plants. The collected soil sample was dried at ambient temperature then crushed, and finally filtered to achieve a particle size < 2 mm. The soil properties were then analyzed (pH, EC, TDS) using a EUTECH PC510 pH, EC, TDS meter. The dilutions of the soil samples, up to 106, were spread on minimal salt medium (MSM) containing plates. The bacterial cells were then streaked on MSM medium with 50 mg L-1 filter-sterilized pesticides content individually added (β-Cypermethrin, λ-cyhalothrin, Clorophyros, parathyrin, and Atrazine, ) followed by 30 ℃ of incubation for pure cultures.
Tolerance capacities of the isolates were performed using the minimal inhibitory concentration (MIC) assay. Briefly, Cyp were diluted into various concentrations, 50, 100, 250, 500, and 1000, mg L− 1, in sterile MSM plates using a standard wire loop (Merck), a loopful (10 µl) of B. Cereus culture, 0.5 mL. In the MIC experiment, the initial concentration of the bacterial inoculum was approximately 3 × 106 CFU m L− 1, and the inoculum was 24 h old, prepared from an overnight culture of B. Cereus grown in nutrient broth. The Plates were incubated at 37 °C for 48 h and thereafter observed for growth to identify the effective Pesticide-resistant strains. The bacterial isolates were identified through 16s rRNA sequenced by the Department of Genome Analysis Microgen Inc. Korea. The obtained sequence was then analyzed using the BLAST search from the National Center for Biotechnology Information (NCBI), through which the database was closed to 99-100% similarities matched of different bacterial species. The species was assigned the accession number based on the 16 S rRNA sequence from GenBank21.
Biochar synthesis, properties and use as inoculum carrier for Bacillus cereus
Biochar (BC) used in the study was prepared and characterized by using the protocol of (Rehman et al.2024)22. The detailed processes are available in Supplementary material (M1). BC concentration used in the present study is 1% w/w of biochar/soil. BC was used as an individual and in combination with bacterial cells B. Cereus. The cell suspension of B. cereus was used to load biochar. For this, the cells were first inoculated in LB medium (1mL broth into 9 mL D.H2O) and cultivated in a shaking incubator set at 120 rpm for 24 h23. A final suspension was prepared to have 11.3 ± 0.23 × 106 CFU mL-1, with the OD600 of 1.0. Subsequently, the biochar was blended with the bacterial cells at 1:5 w/v (mg mL− 1). Biochar-loaded bacterial cells were used as a combined application after an 8-hour incubation period24,25.
Soil sampling and spiking with pesticides and experimental setup
Before conducting the experiments, the soil samples were analyzed to ensure they were free from any detectable levels of the pesticides under study, including Cyp, chlorpyrifos, and other commonly used pesticides. Pesticides-free soil samples were collected from the field of Kohat Agriculture Research Center. The centre spans from the latitude of 33° 35′0.24″ N and a longitude of 71.25′59.59″ E or 33.58° and 71.43°, respectively. The altitude of the study area is about 1,607 feet. Soil samples were collected from the surface soil layer (10–20 cm soil depth) at the end of Feb 2023. After removing the visible organic materials, stones and fine roots by hand. The soil was collected from multiple random spots and mixed uniformly to create a composite soil sample called parent soil. The soil sample of soil was dried in an air digital oven at 105 ℃ for 24 h and passed through a 2 mm sieve and the moisture content was measured following the protocol of (Curiel-Alegre et al., 2022). The organic carbon (OC) was determined by estimating organic carbon by measuring the weight loss after heating the soil sample to a high temperature 500–800 °C in Muffale France. The weight loss represents the loss of organic carbon. The organic carbon content is then calculated based on the following equation.
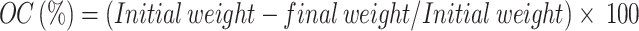 |
1 |
Cation exchange capacity (CEC) determination via ammonium acetate method involved meticulous steps for accurate results. The 5 g soil sample was sieved to ensure uniform particle size and was combined with an ammonium acetate solution at concentrations of 1 M. The mixture was allowed to equilibrate for 16 h to ensure thorough cation extraction. Subsequently, the equilibrated mixture was centrifuged at 10 x for 10 min, to effectively separate soil particles from the solution. The supernatant, containing extracted ions such as calcium (Ca²⁺), potassium (K⁺), and sodium (Na⁺), was analyzed using exchangeable cations concentrations in the extracts were measured using ASS. specifically, the Perkin-Elmer 700-ASS model. To determine CEC, the sum of exchangeable cations (Na⁺, Ca²⁺, K⁺, Mg²⁺) was calculated to ensure accurate quantification27,28. Soil pH was measured using a 1:2.5 soil-to-water and soil EC were measured using a 1:5 soil-to-water ratio with EUTECH PC510 pH, TDS, EC meter glass electrode pH meter.
For the experiment, the soil was air-dried at room temperature ( 25–30 °C) for 15 days and ground to a final particle size of 3 mm. 1% (w/w) sand was mixed to enhance the porosity. The mixture of soil and sand was then spiked with the different doses (50 mg L− 1, 100 mg L− 1, 200 mg L− 1, 250 mg L− 1, 500 mg L− 1, and 1000 mg L− 1) of cyp. This range was selected to encompass low, moderate, and high levels of contamination, facilitating a comprehensive evaluation of the efficacy of biochar and bacterial inoculum. Biochar (1% w/w of soil) and bacterial inoculum and in combination were applied. To apply the inoculant it was placed in a shaker at 25 °C for 24 h with and without biochar, 5:1 inoculum: biochar (v/w) mixture. Inoculated biochar treatments were prepared similarly, using a 50 ml bacterial culture having 12,000 bacterial cells determined by Macfralamnd solution, 10 g biochar mixture for each kg soil prepared. All inocula were added to soils with or without biochar carriers at a rate between 7 × 106 and 7 × 107 CFU g− 1 soil. About 500 g of soil will be filled in plastic pots 15 cm in length and 7 cm in width four replicates. Four treatments were conducted as follows Cyp = (Cyp spiked Soil), Cyp + B. Cereus = (Cyp spiked + Bacillus cereus), Cyp + BC = (Cyp spiked + Biochar), CS + B. Cereus + BC = (Cyp spiked + biochar + Bacillus cereus). Each treatment was set up with four replicates. These four treated soils were cultured with 70% moisture content, temperature 28 ℃ to 30 ℃ and incubation time was 45 days. Samples for physiochemical characteristics and soil enzyme activities (phosphatase, urease, dehydrogenase, ) were determined on different time intervals (day 1 to day 90), for IBR index and the overall activities were determined on day 90 of the experiment.
Cypermethrin residues determination in treatment soil
Before conducting the experiments, the soil samples were analyzed to ensure they were free from any detectable levels of the pesticides under study, including cyp.and for the extraction of Cyp residues, a 10 ± 0.01 g fresh subsoil sample with 20 mL of methyl alcohol (analytical grade, Kelong, China) was mixed. The suspension underwent ultrasonication in an ultrasonic bath for 10 min to extract Cyp. Subsequently, the suspension was centrifuged for 10 min at 10x rpm. The Cyp extraction process was 2 times repeated and the combined supernatant The resulting solution was then filtered through a 0.45 μm nylon syringe filter. The presence of Cyp in the eluent was calculated using High-Performance Liquid Chromatography (Thermo Fisher Scientific, Germany) attached to a C18 column (25 cm × 4.6 mm, Cosmosil, Japan). The mobile phase was composed of 20:80 (% v: v) of water and methanol. The wavelength for detection and temperature were set at 202 nanometers and 28–30 °C. The Cyp residue was expressed as mg kg− 1 dry weight of soil18.
Quality control of cypermethrin residues determination procdure
Preparation of standard solutions
A stock standard solution of cyp (10 mg L−1) was prepared in HPLC-grade methanol and stored in amber reagent bottles at + 4 °C. Calibration standard solutions with concentrations of 10.0, 100.0, 300.0, 500.0, 700.0, 1000.0 and 1200 mg L−1 were prepared by diluting the stock solution with the mobile phase and used for instrument calibration. Soil samples (100 g) were spiked with cyp at 100 µg L−1 and extracted three times with dichloromethane (3 × 60 mL). The organic extracts were concentrated via rotary vacuum evaporation, and the residue was reconstituted in 1.0 mL of methanol for analysis by HPLC.
Accuracy and precision
The accuracy of the method was assessed through recovery experiments. Soil samples spiked with cyp at three concentrations (10.0, 100.0, and 1000.0 µg L−1) were extracted and analyzed. The average recovery rate was found to be within the acceptable range of 85–95%. The precision was evaluated by calculating the relative standard deviation (RSD, %) of six replicate injections at the same concentrations, with RSD values below 5%, indicating good precision.
Limit of detection (LOD) and limit of quantification (LOQ)
The LOD was determined by spiking soil samples with Cyp at concentrations expected to produce a response 3–10 times the baseline noise. The LOD for cyp was calculated to be 3 µg L−1. The LOQ was established as the concentration that produced a signal-to-noise ratio of 10–20, with the LOQ for cyp being 10 µg L−1.
Stability test
To assess the stability of cyp in soil, two series of soil samples were prepared and stored under different conditions. One series was kept at room temperature (22 ± 1 °C) for six months, while the other series was stored in a climate chamber with temperature variations (22 ± 1 °C during the day and 4 ± 0.5 °C at night) for the same duration. No significant degradation of cyp was observed under these conditions, confirming the stability of the Cyp in soil samples during the study period.
Soil biological evaluation
At the end of the incubation experiment, soil respiration was determined by measuring the amount of CO2 produced through static incubation of soil (25 g) at 60% water holding capacity inside 1 L jars at 25 °C. CO2 produced during the 2 h period was collected in 10 ml of 0.01 M NaOH solution, which was then titrated with HCl. Microbial biomass carbon (MBC) was quantified with a solution of 0.5 M K2SO4 and organic C in the extracts was found by humid digestion with 0.066 M K2Cr2O7 at 160 °C. The metabolic quotient (qCO2) was calculated as the ratio between microbial respiration and soil microbial biomass. The quantification of microbial metabolic qCO2 serves as a crucial indicator of microbial activity in soil, reflecting the dynamic processes involved in the degradation of organic matter by microorganisms. In the context of this study, the measured qCO2 values show the impact of various treatments on soil microbial metabolic activity29,30. Soil enzymes including dehydrogenase, catalase, and urease activities were assessed based on the production of triphenyl formazan, H2O2, glucose, tyrosine, and NH3-N, respectively. However, the activities of phosphatase were determined by the release of p-nitrophenol (PNP)31.
Enzymes were measured in three replicas and expressed as the geometric mean32. The assayed enzyme activities (GMean) were calculated as in Eq. 2.
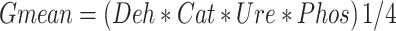 |
2 |
Where Deh, Cat, Ure, phos are dehydrogenase, catalase, urease, and phosphatase, respectively.
Integrated biomarker response (IBR)
The integrated biomarker response (IBR) index was used for risk assessment and environmental stress produced via chemical contaminants. This integrated approach provides valuable insights for assessing the toxicity of various pollutants, including Polycyclic Aromatic Hydrocarbons (PAHs), metals, and pesticides, contributing to a comprehensive understanding of the biota and environmental health33,34. We focused on four biological activities specifically, Gmean-Enz activities, MBC, SR, and qCO2 to calculate the IBR index. The computation of the IBR index required the standardization of the data (Yi), which was achieved using the following equation.
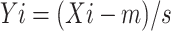 |
3 |
Here, Xi represents the average of bio-indicators for every group, while m and s denote the overall average and variability of Xi, respectively, calculated from the entire sample set.
The value
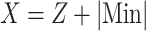 |
4 |
Here, Z = -Y or Z = Y signifies the suppression or enhancement of a marker during Cyp pollution, and Min represents the lowest value of the marker.
The IBR index was computed as.
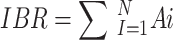 |
5 |
The IBR score was then divided by the number of all markers (n = 4) to get the IBR/n index and depends on the number of biological markers8.
Cypermethrin degrading experiment with biochar and bacteria
The air-dried soil was sieved through a 3 mm mesh and subjected to autoclaving at 121 °C for 1 h at 15 psi. This procedure was repeated three times over consecutive days to ensure the complete elimination of microbial activity. The success of the sterilization process was confirmed by conducting microbial viability tests using plate count methods, where no microbial colonies were observed after incubation Specifically, Cyp100-EC was added at the concentration of 5 mg⋅kg− 1 based on toxicity leval within the rang commonly found in Cyp contaminated soil. The B. cereus (OD 600 nm = 1) at 7 × 107 CFU mL− 1, freshly prepared inoculum, under controlled conditions was resuspended in soil along with 5% w/w biochar. To facilitate strain growth, the moisture was provided at 40%. This experimental setup was conducted in 4 replicas. The soil samples were stored in darkness in an incubator to prevent Cyp from photocatalytic reactions at 30 °C for 90 days. Data were obtained at various time intervals to extract and quantify Cyp concentrations.
Statistical analysis
The experimentations occurred with four replicas and were subjected to statistical analysis using SPSS-23 software. For comparing the means of the treatments, analysis of variance (ANOVA) was executed, followed by Duncan’s multiple range posthoc test, with a significance level set at p < 0.05.
Quality enhancement of experimental processes
In our study, we incorporated key experimental design principles such as replication, randomization, and blocking to enhance the reliability and validity of our results. These techniques were integral to minimizing variability and bias, allowing us to draw more robust conclusions. We use amber color glass reactors to prevent any photocatalytic reaction and any plastic adsorption of Cyp. Each treatment was replicated multiple times across different soil samples. This approach allowed us to account for any inherent variability in the soil properties and ensured that our findings were consistent and reliable. Different concentrations of Cyp and combinations of biochar and bacterial inoculum, were randomly assigned to the experimental units. This process helped eliminate any potential biases and ensured that differences observed between treatments were due to the treatments themselves, rather than any confounding factors. Blocking to group experimental units with similar characteristics, such as soil properties or initial contamination levels for control for known sources of variability, ensuring that our comparisons between treatments were more accurate and reflective of true treatment effects.
By integrating these design elements into our experiment, we enhanced the overall quality of our experimental processes. The results obtained are more reliable and can be confidently applied to real-world scenarios, increasing the credibility and impact of our findings.
Results
Various concentrations of cypermethrin pollution on microbial biomass carbon and soil respiration
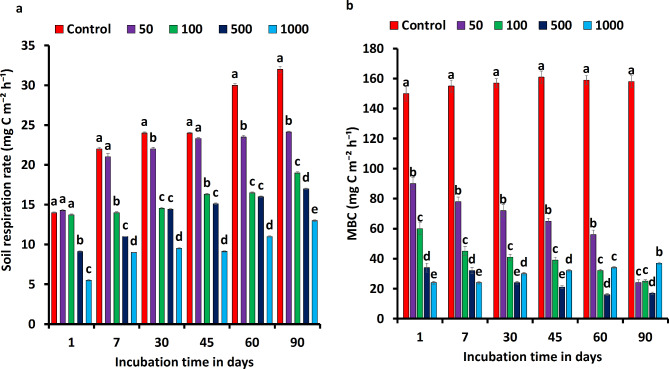
The soil respiration, on day 1, soil respiration even in the concentration of 50 mg kg− 1 exceeded that of the controlled soil., and the observing value was 8 mg C m−2 h−1, while Cyp pollution at higher concentrations of 500–1000 mg kg− 1 inhibited soil respiration significantly. Soil respiration values dropped substantially on the 7th day across all treatments. (72% decrease from 1st day) and showed gradual reductions from day 30 to 90. Intersentignigly, the Cyp impact on SR was particularly distinct in the 1000 mg kg− 1 treatment, with no significant difference observed among the other five treatments on the same day (Fig. 1a). The Microbial Biomass Content (MBC) in contaminated soil exhibited a significant decrease under Cyp pollution, with notable differences observed among CN and Cyp-treated groups with time intervals (Fig. 1b). The most substantial reduction in MBC occurred on 1st day of cyp treatment (1000 mg kg− 1), having a rate of reduction of more than 85% compared to CN. The acute toxicity of Cyp had an immediate impact on MBC, with reduction rates evident as early as day one, ranging between 58% at the start of incubation. Excitingly, the value of MBC at 50 mg kg− 1 was significantly higher than CN from 1st week to 3rd week, ranging from 20% to 105%. Beyond the 7th week, MBC was generally decreased at the end of the experiment. A gradual upturn was found in MBC from 500 mg kg− 1 to 1000 mg kg− 1 in the 2nd and 3rd months of the experiment. However, the MBC in Cyp polluted soils showed a minor upturn in 3rd month of the experiment compared to the first week of incubation.
Fig. 1.
Responses of microbial activities to Cyp pollution for 90 days incubation in (a) Soil respiration rate and (b) microbial biomass carbon (MBC); The different lower case letters indicate significant differences at p < 0.05 levels using the one-way ANOVA Duncan test were applied where N = 4.
Integrated assessment of cypermethrin toxicity via integrated biomarker response index
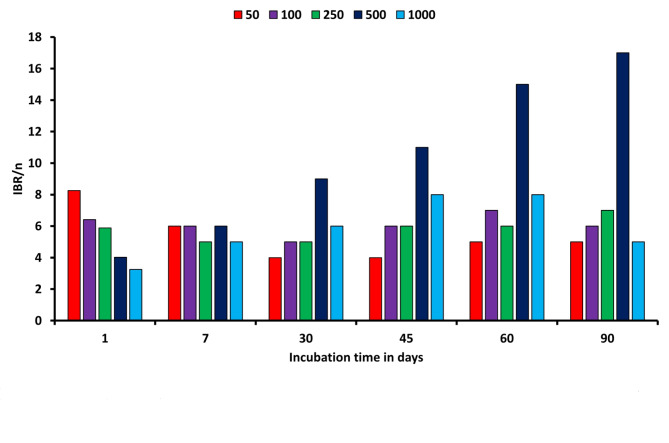
The sensitivity of bioindicators, involving Gmean-Enz, MBC, SR, and qCO2, was high at a higher amount of Cyp. For all bioindicators, there a noticeable variations in soil enzymes and MBC over time during the entire incubation period. On 1st day, the GMean Enz and MBC rate for Cyp treatments (50-1000 mg kg− 1) ranged from 0.98 to 0.63, and 9.06, to 5.03, respectively. Subsequently, these scores increased by 60% on day 60 and ultimately declined to 20% on day 90. The influences of Gmean-Enz, MBC, SR, and metabolic properties of various Cyp treatments reached their maximum levels on day 30 (Fig. 2). The IBR/n ratios exhibited statistically significant ranging from 8.3 to 3.3 for Cyp concentrations between 50 and 1000 mg kg− 1 on 1st day and from 4 to 8 on days 30 to 45 for 50 mg kg− 1 and 1000 mg kg− 1.
Fig. 2.
Changes of IBR/n values for each contamination concentration along with Cypdegeneracy.
Isolation, identification, and characterization of cypermethrin degrading bacterial strain
Bacterial strains were isolated from the rhizospheric soil of the tobacco field in Swabi, Khyber Pakhtunkhwa. due to its demonstrated potential to enhance the growth of microorganisms in a medium containing pesticides, (individually added) specifically Cyp (750 ppm), Clorophyros (350 ppm), Atrazine (700 ppm), and Parathyrin (500 ppm) (Figure S1). Due to the higher tolerant capacity, we forward the strains with Cyp experiments. The subsequent analysis of the partial 16 S rRNA sequence, conducted through the online BLAST program, revealed that the selected strain belonged to the genus Bacillus. This taxonomic assignment was further validated by the high degree of similarity observed in the sequence, with a 99% match to Bacillus cereus. The sequence information has been officially deposited in the GenBank database under the accession number JX276537.1.
Biochar yield, proximal, and chemical characteristics
The biochar yield at 550 °C was 69 ± 3.5%, with 5.75 ± 0.05% for mobile matter, 65.00 ± 0.01% for resident matter, and 27.25 ± 0.05% for ash content (Table S1). The biochar exhibited a pH of 12.53, and its electrical conductivity measured 40 µS cm− 1. The BET surface area was determined to be 45.30 m² g−1, with a pore size of 0.133 cm³ g−1 and an average pore diameter of 1.50 nm.
Soil physico-chemical characteristics
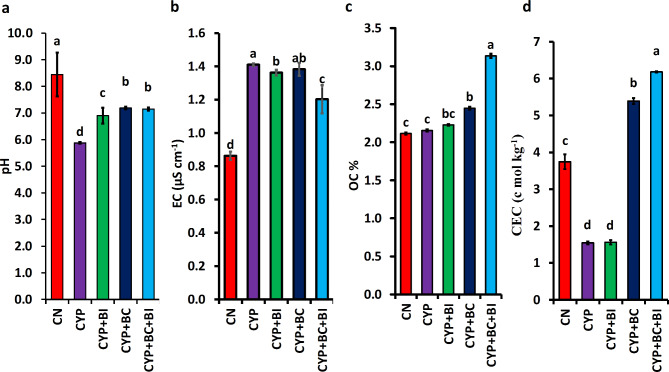
Adding Cyp induced changes in soil physicochemical properties, including pH, electrical conductivity (EC), organic carbon (OC), and cation exchange capacity (CEC), but amending the contaminated soil with biochar, B. cereus, or their combinations effectively mitigated the negative impacts. The addition of Cyp lowered the pH of the control soil (CN) from 8.4 to 5.9, making the soil acidic. The addition of biochar in the Cyp-contaminated Soil (CS) helped enhance the pH to a neutral range (~ 7.2), while bacterial inoculation raised it to 6.9. In the combined application of Biochar and B. cereus in CS (CS + BC + B. cereus) soil pH was maintained at a neutral pH (7.1) (Fig. 3a). The EC of CN soil increased from 0.86 µS cm− 1 to 1.41 µS cm− 1 after cyp contamination. However, the bacterial inoculum and biochar reduced the soil EC enhanced by cyp contamination, which was 1.36 µS cm− 1, and 1.38 µS cm− 1, respectivaly. The combination of bacteria and biochar in CS further lowered the EC to 1.20 µS cm−1 (Fig. 3b). The Organic content of CN soil (2.11 ± 0.01%) did not change significantly (p < 0.05) after Cyp-contamination (2.15 ± 0.01%). While both biochar (2.44 ± 0.01%) and bacterial inoculum (2.22 ± 0.01%) slightly increased the value, these changes were not statistically significant. However, a statistically significant increase (3.135 ± 0.03%) was observed in the combined application of biochar and bacterial inoculum in Cyp-contaminated soil (Fig. 3c). Cyp-contamination reduced the CEC value from 3.74 ± 0.02 C mol kg− 1 to 1.54 ± 0.04 C mol kg− 1. The addition of biochar significantly increased CEC to 5.43 ± 0.1 mol kg−1, while bacterial amendment did not cause a significant change in the CEC value (1.56 ± 0.1 mol kg− 1). The combined application enhanced the CEC value to 6.18 ± 0.02 C mol kg− 1 (Fig. 3d).
Fig. 3.
The physiochemical properties of soil (a) pH (b) EC, (c) OC, and (d) CEC, different treatments are CN (control) Cyp ( Cyp contaminated soil ) BC (biochar) BC + BI (Combination of biochar and bacterial inoculum). Error bars represent the standard error from 4 replicates measurements. Asterisks represent significant differences within a group according to Duncan’s test (p < 0.05).
Microbial biomass carbon
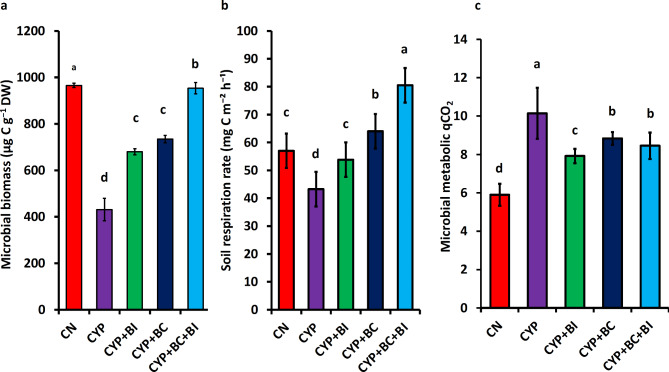
In the control treatment, microbial biomass carbon was 965.5 µg C g− 1 DW and in the Cyp treated soil microbial biomass carbon was 431 µg C g− 1 DW in 10–20 cm depth. The use of bacterial inoculum (B. Cereus) significantly increased the MBC in Cyp treated soil at 679.75 µg C g− 1 DW in 10–20 cm depth. However, significant increases (p < 0.05) were found in microbial biomass carbon by biochar application and microbial biomass carbon was determined at 734.25 µg Cg− 1 DW in 10–20 cm depth. Interestingly the microbial biomass carbon rate was significantly increased in the combined application of biochar and bacterial inoculum (Bacillus cereus) which was 953.25 µg C g− 1 as shown in Fig. 4a.
Fig. 4.
Effects of Biochar (BC) and bacterial Inoculum (BI) Bacillus Cereus amendments on (a) microbial biomass carbon, (b) soil respiration rate and (c) microbial metabolic (qCO2) in Cyp contaminated soil (CS) control CN soil. Values are mean of n = 4± S.E. Statistically significant different values are represented by different lowercase letters according to Duncan’s test (p < 0.05).
Soil basal respiration rate
Soil basal respiration rates (53 mg C m−2 h−1 DW) decreased due to Cyp-contamination (26 mg C m−2 h−1) but increased after amending the soil with biochar and bacterial inoculum The bacterial inoculation increased the basal respiration rates to 56.26 mg C m−2 h−1 which further increased to 61 mg C m−2 h−1 in biochar amendments. The combined application of Biochar and B. cereus in CS (CS + BC + B. cereus) substantially enhanced soil basal respiration rates to 76 mg C m−2 h−1 (Fig. 4b). These findings suggest the potential of biochar and bacterial inoculation to mitigate the impact of Cyp-contamination on soil microbial activity, highlighting promising avenues for sustainable soil management practices.
Microbial metabolic qCO2
The baseline microbial metabolic qCO2 in control soil (CN) was recorded at 5.9, indicating the natural level of carbon dioxide release associated with microbial processes (Fig. 4c). In the presence of Cyp-contamination(CS), the qCO2 level surged to 10.1, signifying a notable increase in microbial metabolic activity induced by the stress of the contaminant. With the introduction of Bacillus cereus, the qCO2 was 7.9. This suggests a restorative effect on microbial metabolic processes, potentially mitigating the adverse impact of Cyp (Fig. 4c). The addition of biochar resulted in a qCO2 value of 8.8, indicating a moderated but increased microbial metabolic activity. However, The combined application of biochar and Bacillus cereus yielded a qCO2 value of 8.5. This synergistic approach demonstrates a balanced enhancement of microbial metabolic activity, potentially contributing to the remediation of Cyp-contaminated soil.
Soil enzymatic analysis
Phosphatase, catalase, urease, and dehydrogenase, were measured to characterize the dynamic changes of microbial activities induced by soil amended with biochar and B. Cereus. Biochar and B. Cereus addition showed a significant promotion of all selected enzyme activities. However, Dehydrogenase activity was reduced by 34% in the combined application of biochar-bacterial interaction in contaminated soil. The biochar-bacterial combination produced highly significant effects on enzyme activities, compared with the Cyp-contaminated soil treatment, the treatment promoted the activities of all the studied enzymes Table 1.
Table 1.
Soil enzymes in contaminated soils (CS), amended with bacteria inoculum B. Cereus (BI), biochar (BC), and biochar + bacteria inoculum (BC + BI).
| Soil enzymes (mg g− 1 24 h− 1) | CS | CS + BI | CS + BC | CS + BC + BI |
|---|---|---|---|---|
| Alkaline Phosphatase | 4.6 ± 0.93 c | 6.12 ± 0.79 b | 6.31 ± 0.86 b | 8.13 ± 1.01 a |
| Catalase | 3.4 ± 0.03 bc | 4.9 ± 0.06 b | 4.5 ± 0.21c | 6.5 ± 0.41 a |
| Urease | 54.5 ± 0.98 c | 77.9 ± 1.33 b | 77.9 ± 1.13 b | 218.6 ± 1.95 a |
| Dehydrogenase | 0.45 ± 0.02bc | 0.47 ± 0.02 b | 0.45 ± 0.01b | 0.35 ± 0.03 a |
All measurements are taken at pH = 7. Values are the mean ± SE where n = 5. Different latter indicates difference at significance level using one-way Annova (Duncan) test (op < 0.05), and n = 4 ± SD. Where a is highly signficant b is significant and c is lower significantly different.
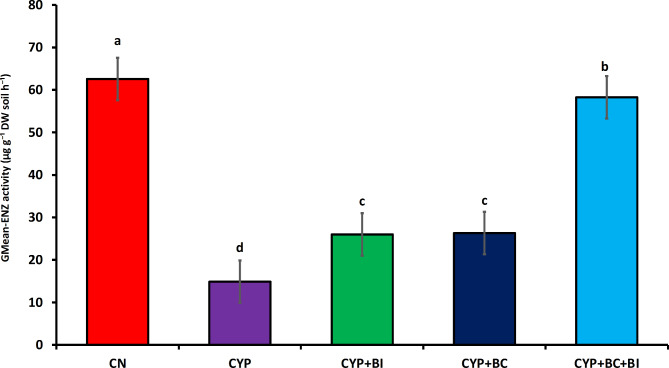
The GMean-EN value for the CN treatment started at 62.56 µg g−1 DW soil h−1. and remained relatively stable over the experimental period. In Fig. 5 the CS treatment, the initial GMean-EN value was 14.87, showing a slight decrease in this value by the end of the experiment. The CS + BI treatment exhibited an initial GMean-EN value of 25.98 µg g−1 DW soil h−1. For the CS + BC treatment, the GMean-EN values started at 26.31 µg g−1 DW soil h−1. and showed a consistent trend, reaching this value by the end of the experimental period. The CS + BC + BI treatment displayed a Gmean-Enz value of 58.26 µg g−1 DW soil h−1., which remained relatively stable throughout the experiment. The Gmean-Enz value for CN started at 62.57 µg g−1 DW soil h−1 and remained stable. The GMean-EN value showed a decrease of 76.20% with Cyp Concentration. However, the Biochar and bacterial amendment increased the Gmean-Enz by 56%.
Fig. 5.
Effects of Biochar (BC) and bacterial Inoculum (BI) Bacillus Cereus amendments on soil Gmean-Enz in Cyp contaminated soil. Values are mean of n = 4± S.E. Statistically significant values are represented by lowercase letters according to Duncan’s test (p < 0.05).
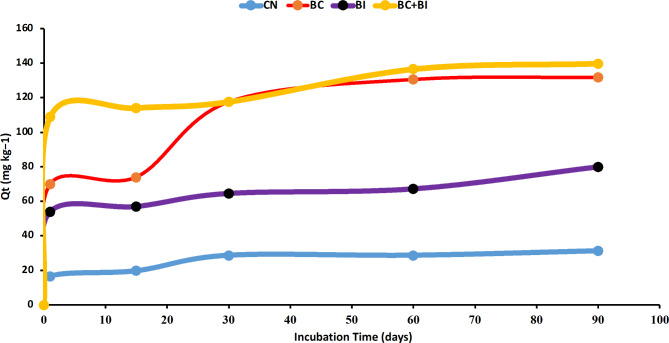
Cypermethrin degrading experiment with biochar and bacteria
In Fig. 6, it is evident that nearly 85% of Cyp is obtained within the first 45 days, reaching adsorption equilibrium by day 30. This rapid adsorption of Cyp on biochar signifies a swift adsorption process. Subsequently, the experimental data underwent simulation using the Elovich and intra-particle diffusion models. As depicted in Table 2 the Elovich model effectively replicates the cyp-degradation rate, exhibiting a high coefficient of determination (r2 > 0.999).
Fig. 6.
Cyp Degradation experiments for 90 days incubation in soil under different treatment Control no amendment only Cyp (CN), Biochar (BC), and bacterial Inoculum Bacillus Cereus (BI) and Combination of biochar and Bacillus Cereus (BC + BI). Where Qt (mg kg− 1) is the the quantity of Cyp degraded over time.
Table 2.
Constant parameters calculated from the Elovich, and Intraparticle diffusion, models for cyp-degradation or adsorption in control soil (CN), biochar (BC), bacterial inoculum (BI) and combination of BC and BI (BC + BI).
| Treatments | Overall adsorption | Elovich | Intraparticle diffusion | ||||
|---|---|---|---|---|---|---|---|
| qt | α | β | R 2 | kd | C | R 2 | |
| CN | 31.4 | 3.3 | 15 | 0.83 | 1.8 | 15 | 0.87 |
| BC | 131.8 | 15 | 61 | 0.74 | 9 | 58 | 0.84 |
| BI | 80 | 4.7 | 50.7 | 0.61 | 3 | 49 | 0.66 |
| BC + BI | 139.8 | 6.5 | 104.2 | 0.55 | 4 | 101 | 0.59 |
This suggests that the interaction between Cyp and biochar or microbes involves chemical interaction between ligands and Cyp. Supplementary Figure S2 presents the corresponding adsorption curves for Table 2 of the Elovich model. Table 2 provides insight into the adsorption or degradation stages, aligning with the intra-particle diffusion model (r2 = 0.991). The adsorption curves are provided now in Figure S3. This observation indicates that film diffusion governs the adsorption during the initial 15 days, while intra-particle diffusion becomes the predominant mechanism after 15 days until reaching adsorption equilibrium. In the control soil, the Cyp was also reduced but it was very slow and very little due to the soil particles however the adsorption equilibrium pattern was the same in the control soil where No amendment was added.
Discussion
Identifying Bacillus cereus as the strain of interest is particularly significant, given the diverse capabilities and ecological roles associated with this bacterial genus. Bacillus species are known for their versatile metabolic activities including the ability to degrade organic pollutants and promote plant growth through various mechanisms35,36. The strain’s resilience and effectiveness in the presence of multiple pesticides also underscore its potential applicability in agricultural and environmental bioremediation contexts37 Furthermore, the deposition of the strain’s sequence in GenBank enhances the accessibility of this valuable genetic information for the scientific community, facilitating future research, comparative analyses, and potential biotechnological applications. Overall, the selection and characterization of the Bacillus cereus strain contribute to our understanding of the microbial diversity in tobacco field rhizospheric soil and open avenues for exploring its practical applications in sustainable agriculture and environmental management. Supplementary Table S1 provides comprehensive insights into the characteristics of the biochars studied, encompassing yield, proximate composition, and chemical analysis.
The impacts of Cyp contamination on the biochemical properties, including enzyme activity, and microbiological indices such as microbial biomass carbon (MBC), soil respiration (SR), and microbial metabolic activities qCO2 is crucial for monitoring the adverse impacts of pesticide dissipation on soil health. The decrease in microbial biomass carbon under Cyp contamination indicates a negative influence on the overall microbial community, potentially leading to a decrease in microbial growth and biomass38. The impact on MBC reflects the disturbance of microbial populations due to the presence of Cyp19,39. In Fig. (1a and 4b) a significant reduction in soil respiration in the presence of Cyp contamination was found. This reduction in SR highlights the adverse effects of Cyp on microbial functions and disruption of microbial activity. The Cyp treatments resulted in notable reductions in Gmean-EN and microbial biomass carbon, particularly at the start of incubation, showing significantly low compared to other time intervals. The lower enzyme production in Cyp-contaminated soil is due to the direct toxic effect of Cyp on the microorganisms responsible for enzyme production and cyneds groups which are the by-product of Cyp-inhibiting enzymes40,41. The pesticide interferes with the microbial cell membranes, cellular respiration, and other vital processes, leading to a decay in the metabolic processes of these organisms18. Consequently, the production and secretion of enzymes are hindered Similar results were presented by Herenandez et al.(2018)42 with chlorpyrifos, dimethoate, and phosalone. Cyp caused a significant rise in BR for the 50 mg kg−1 treatment on 1st day, possibly attributed to the acclimatization of native microbial response, to adjustments in cellular processes. This can be attributed to the adaptation process under Cyp stress43. Consistent with previous studies, initial decreases in urease and acid phosphatase activities, followed by recovery, were observed in soils treated with 1,3-dichloropropene and chlorantraniliprole44,45. The potential explanation lies in Cyp acting as a carbon source for soil microbes, augmenting the microbial biomass. Microbes use Cyp as organic carbon sources, for energy production through respiration. In this scenario, the higher microbial biomass leads to increased soil respiration rates as more microbes respire and release carbon dioxide. However, apparent trends emerged after 60 days, revealing declines in GMean-EN, microbial biomass carbon, and SR under Cyp stress. This phenomenon can attributed to the generation of harmful byproducts, such as paraoxon, diethyl thiophosphate, and p-nitrophenol, recognized for their persistence in soil and causing toxicity to soil microorganisms11,46. Gmean-Enz for Cyp degeneracy was comparatively lower than observed in instant pollution8. This difference might be due to prolonged application of pesticides, potentially leading to decreased soil quality, degradation, and microbial processes over the long term44,47.
The gradual dissipation of Cyp residues in the soil emerged as a prominent feature, exhibiting a rapid decline over the 90-day experimental period, ultimately leading to complete degradation by the study’s conclusion (Fig. 6). The Elovich equation hinted at the complexity of the adsorption process, portraying it as a multistep phenomenon involving chemical reactions at the surface In the context of Cyp adsorption, the Elovich model provides insights into the mechanisms involved in the interaction between Cyp molecules and the adsorbent surface and bacterial producing legends such as different types of enzymes. It suggests that the adsorption of Cyp is not a simple, one-step process but involves multiple stages of chemical reactions, possibly including the formation of chemical bonds between Cyp and the adsorbent. Similarly, the intraparticle diffusion model reveals information about the rate-limiting step within the Cyp and biochar bacterial interaction. If intraparticle diffusion is a significant factor, it implies that the adsorbate molecules are diffusing into the pores of the bacterial cell or biochar pores, and the rate of this intraparticle diffusion process influences the overall adsorption kinetics. In the context of soil studies, where these models find application, it was observed that the adsorption or degradation equilibrium for Bacillus cereus was attained by day 30. Notably, this equilibrium was relatively slower in comparison to scenarios involving biochar or combined applications, potentially influenced by hydrogen bonding and π-π interactions. The combination of biochar with the bacteria creates synergistic effects, providing multiple interaction sites for adsorbate molecules. This enhances the overall adsorption capacity, the increased specificity and complexity of interactions can contribute to a slower attainment of equilibrium. The slower equilibrium observed in scenarios involving hydrogen bonding and π-π interactions, particularly with biochar, is a result of the enhanced specificity, strength, and complexity of these interactions. These interactions contribute to a more efficient adsorption process, they also introduce additional molecular requirements and alignment considerations, slowing down the attainment of equilibrium48. The environmental fate of pyrethroid pesticides, particularly Cyp, is typically steered by abiotic degradation processes, including photolysis, oxidation, hydrolysis, plus bioremediation. The study, conducted under conditions excluding light exposure, indicated that the dissipation of Cyp was primarily influenced by hydrolysis and biodegradation, with photodegradation playing a subordinate role49. The pH of the soil emerged as a pivotal factor affecting the hydrolysis of Cyp, with faster hydrolysis rates expected under alkaline conditions. This is attributed to the nucleophilic activity of OH⁻ ions, which facilitate the hydrolytic transformation into other metabolites2,43. In addition to hydrolysis, the involvement of microorganisms in Cyp degradation was evident. Microorganisms exhibited the ability to utilize Cyp as a carbon source, adapting to challenging environmental conditions2. Remarkably, very low residues of Cyp were detected in Cyp-treated soil after the incubation periods in combined application treatment. Despite the absence of detectable Cyp residues, it is imperative to acknowledge the potential toxicity risks to the quality of soil during biodegradation. Often, the absence of residues in the soil can lead to the undervaluation of toxicity risks associated with Cyp degradation2,17,50.
The use of biochar, and bacteria, interaction is explored for their potential to alleviate the negative impacts of Cyp. The cumulative soil respiration rate in Cyp -contaminated soil exhibited a notable decrease. This decline in respiration is attributed to variations in topsoil minerals sources, with substantially higher minerals compared to the Cyp-contaminated soil. SR and biomass carbon were significantly elevated (43–92% and 87–186% higher, respectively) in the biochar-treated soil, aligning with findings indicating a close relationship between basal activity, microbial biomass, and nutrient availability2,43,51. Soil electrical conductivity (EC) and moisture, pivotal factors governing microbial activity, were crucial considerations in evaluating SR. The SR exhibited strong correlations with EC and pH throughout the study. Despite a lower soil pH, the SR surpassed those in soils with higher pH, underscoring the impact of soil contamination on microbial composition and organic matter. Sandy soils, particularly those with low water-holding capacity, are vulnerable and the challenge is addressed by biochar and expanded shale, clay, and slate (ESCS) to enhance water retention in rhizospheric soil quality52 The application of biochar and microbial interaction significantly augmented soil respiration rates by 65% in Cyp -contaminated soil. However, the impact of biochar and bacteria on soil SR was comparatively lower. Increased soil respiration was likely due to microbial decomposition of applied biochar, accelerated decomposition of soil organic matter, and heightened root respiration. The contribution of biochar and microbial interaction to cumulative soil respiration rates was estimated at 5.4% 43. Supporting Table S1 explains the alkaline nature of the biochar attributed to alkali metals present on the biochar surface, contributing to the formation of hydroxide. The BET surface area 45.30 and average pore diameter (1.50) suggest a substantial surface available for adsorption and interaction. the pore size and the average pore diameter highlight the porous nature of the biochar, indicative of potential adsorption and catalytic capabilities53.
Consequently, the interaction between biochar and microbes may have influenced the bacterial composition of (soil organic matter) SOM. Cyp contamination affected soil respiration rates, with the application of ash notably increasing the root weight2,51. The addition of nutrients through biochar and microbial application likely enhanced respiration rates, suggesting a positive effect on microbial activity due to increased nutrient availability. Despite a tendency for basal respiration to decrease in Cyp -contaminated soil, the interaction of biochar and microbes led to an increase in basal respiration rates. This rise was mirrored by microbial biomass C, indicating a strong correlation between the two in both biochar and bacterial treatments. The transfer of nutrients from biochar to mineral soil promoted the growth of microorganisms, enhancing microbial biomass54. This approach proves beneficial in regions with low precipitation, enhancing water-holding capacity and nutrient availability while facilitating the degradation of Cyp and reducing environmental stress. Changes in Gmean-Enz activity can reflect variations in MBC responding to environmental disruptions8. The enzyme is considered a reliable criterion for measuring total microbial activity and demonstrated a positive relationship between soil enzymes and microbial biomass carbon when compost was added31. Native microbes use organic compounds as their source of energy and carbon increases enzyme secretion to meet their life needs through proliferation. However, under stressful conditions, microbes might prioritize energy consumption for cell maintenance over proliferation, leading to soil respiration and biomass of microbes18,41.
Effectively evaluating the risk linked to the degeneracy of Cyp on soil microbial ecology involves considering microbial activities in conjunction with the IBR/n index. The positions of individual bioindicators visually depict the environmental injuriousness of Cyp degeneracy in the ecosystem through IBR/n results for varying Cyp doses Fig. 2. The escalation in IBR/n values correlates with elevated Cyp concentrations, signifying an intensified biotoxicity risk to microorganisms over the long term. Our findings are consistent with studies on microplastics and atrazine dose-dependent rise in E. fetida stress, and the significant time-dependent nature of IBR/n values became apparent30. On 1st day the scores of IBR/n were lower than those from the week to 45 days. The temporary decrease is attributed to soil particles and Cyp toxicity on Gmean-Enz and microbes47. However, this protective effect was short-term throughout the complete evolution period, as the harmful outcomes of Cyp upon microorganisms prevailed. This is evidenced by the IBR/n scores remaining pretty high compared to 1st day at the end of the experiment, despite the absence of any residue of Cyp in the soil.
Conclusion
Bacillus cereus demonstrated enhanced pesticide tolerance, promoting growth in Cyp-contaminated media. The introduction of biochar into Cyp-contaminated soil increased pH (5.9 to 7.2), decreased electrical conductivity, and positively impacted soil organic content (2.15–3.135%). Cation exchange capacity surged from 1.54 to 6.18 C mol kg− 1, and soil respiration rate rose from 26 to 76 mg C m-² h− 1. Microbial biomass carbon increased from 38.34 to 65.78 µg C g−1 DW. Enzymatic activities were promoted, indicating improved soil health. Moreover an initial decrease, Cyp residues rapidly declined, becoming undetectable after 90 days. The study highlights a comprehensive improvement in soil properties and microbial activities through biochar and Bacillus cereus interaction in Cyp-contaminated soil.
The study provides a comprehensive evaluation of the synergistic effects of biochar and Bacillus cereus in remediating Cyp -contaminated soils. By demonstrating significant improvements in soil physicochemical properties, microbial biomass, and enzymatic activities, this research offers a practical and environmentally sustainable approach to mitigating the adverse effects of pesticide contamination. The findings have important implications for enhancing soil health and productivity, particularly in agricultural systems exposed to pesticide stress. Moreover, the ability to effectively degrade Cyp within 90 days underscores the potential of this method as a viable strategy for large-scale bioremediation efforts, contributing to sustainable agricultural practices and environmental protection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to the Higher Education Commission (HEC), Pakistan for providing funds under the International Research Support Initiative Program (IRSIP) to work in the Subsurface Engineering and Analysis Lab at the Department of Civil and Environmental Engineering, University of California Los Angeles (UCLA) and The UWA Institute of Agriculture and School of Agriculture and Environment the University of Western Australia, Perth, WA, 6001 Australia. We are also thankful for the technical support of the State Key Laboratory of Marine Food Processing & Safety Control, College of Food Sciences and Engineering, Ocean University of China. We are thankful to Hubei Key Laboratory of Energy Storage and Power Battery, School of Mathematics, Physics and Optoelectronic Engineering, Hubei University of Automotive Technology, Shiyan 442002 China for providing biochar characterizations. We are also thankful to the Department of Chemical and Biomolecular Engineering, at the University of California Los Angeles, especially for providing spectroscopic and chromatographic characterization of the samples.
Author contributions
Hamid Rehman, Ziafat Rehman, Tonoy K. Das, Maha Rehman, Basit Ahmed Khan, Sunny Nandi: Conceptualization, Methodology, Software, Visualization, Investigation, Writing- Original draft preparation. Khurshid Ahmad, Sanjay K. Mohanty, Wasif ur Rehman: Data curation, Validation, Supervision, Resources, Writing - Review & Editing. Rehan Naeem, Mohit Bajaj, Milkias Berhanu Tuka: Project administration, Supervision, Resources, Writing - Review & Editing.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rehan Naeem, Email: dr.rehan@kust.edu.pk.
Milkias Berhanu Tuka, Email: milkias.berhanu@aastu.edu.et.
References
- 1.Fu, H. et al. Advances in organophosphorus pesticides pollution: current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mat. vol424, 127494 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Raj, A. & Kumar, A. Recent advances in assessment methods and mechanism of microbe-mediated chlorpyrifos remediation. Environ. Res.214, 114011 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Mohammad, N., Rasul, M. M., Nafees, M. & Jan, M. R. Residues of cypermethrin and endosulfan in soils of Swat valley. Soil. Environ.28, 2074–9546 (2009). [Google Scholar]
- 4.Amjad, A. et al. Dietary intake assessment of pyrethroid residues from okra and eggplant grown in peri-urban areas of Punjab. Pakistan J. Environ. Sci. Pollut Res. Vol27, 39693–39701 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Chen, S., Geng, P., Xiao, Y. & Hu, M. Bioremediation of β-cypermethrin and 3-phenoxybenzaldehyde contaminated soils using streptomyces aureus HP-S-01. Appl. Microbiol. Biotechnol.94, 505–515 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Rehman, H. et al. Curcumin attenuates Hepato-, and Nephrotoxicity Induced by Cypermethrin through inhibition of oxidative stress in male albino rabbits. Res. Square v 1 (2021).
- 7.Khan, A. H. A. et al. Sustainability of phytoremediation: Post-harvest stratagems and economic opportunities for the produced metals contaminated biomass. J. Envion Manag. 326, 116700 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Tao, K. et al. Ecotoxicity of parathion during its dissipation mirrored by soil enzyme activity, microbial biomass and basal respiration. Chemosphere311, 137116 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee, S., Tripathi, S., Mukherjee, A. K., Bhattacharyya, A. & Chakrabarti, K. Persistence of the herbicides florasulam and halauxifen-methyl in alluvial and saline alluvial soils, and their effects on microbial indicators of soil quality. Eur. J. Soil. Biol.73, 93–99 (2016). [Google Scholar]
- 10.Wu, X., Xu, J., Dong, F., Liu, X. & Zheng, Y. Responses of soil microbial community to different concentration of fomesafen. J. Hazard. Mater.273, 155–164 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Karpun, N. N. et al. Side effects of traditional pesticides on soil microbial respiration in orchards on the Russian Black Sea coast. Chemosphere275, 130040 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Sanchez, W., Burgeot, T. & Porcher, J. M. A novel ‘Integrated Biomarker Response’ calculation based on reference deviation concept. Environ. Sci. Pollut Res.20, 2721–2725 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Yang, R. et al. Dose and time-dependent response of single and combined artificial contamination of sulfamethazine and copper on soil enzymatic activities. Chemosphere250, 126161 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Huang, Y. et al. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ. Res.194, 111661 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Uniyal, S., Sharma, R. K. & Kondakal, V. New insights into the biodegradation of chlorpyrifos by a novel bacterial consortium: process optimization using general factorial experimental design. Ecotoxicol. Environ. Saf.209, 111885 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Bolan, S. et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ.886, 163968 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Bhatt, P., Zhou, X., Huang, Y., Zhang, W. & Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mat. vol411, 125026 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Bhatt, P. et al. Indigenous bacterial consortium-mediated cypermethrin degradation in the presence of organic amendments and Zea mays plants. Environ. Res.212, 113137 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Xiang, L. et al. Integrating Biochar, Bacteria, and plants for sustainable remediation of soils contaminated with Organic pollutants. Environ. Sci. Technol.56, 16546–16566 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, M. et al. Efficient bio-remediation of multiple aromatic hydrocarbons using different types of thermotolerant, ring-cleaving dioxygenases derived from Aeribacillus pallidus HB-1. Bioresour Technol.398, 130472 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Park, S. L. et al. Improvement of polyhydroxybutyrate (PHB) plate-based screening method for PHB degrading bacteria using cell-grown amorphous PHB and recovered by sodium dodecyl sulfate (SDS). Int. J. Biol. Macromol.177, 413–421 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Rehman, H. et al. Electromagnetic biochar: A novel material for cadmium adsorption from industrial wastewater. Int. J. Environ. Sci. Technol.21, 1756 (2023). [Google Scholar]
- 23.Huang, J., Xiao, Y. & Chen, B. Nutrients removal by Olivibacter Jilunii immobilized on activated carbon for aquaculture wastewater treatment: ppk1 gene and bacterial community structure. Bioresour Technol.370, 128494 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Sun, D., Hale, L. & Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils52, 515–522 (2016). [Google Scholar]
- 25.Rehman, H. et al. Synergistic biochar and Serratia marcescens tackle toxic metal contamination: A multifaceted machine learning approach. J. Environ. Manag.370, 122575 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Curiel-Alegre, S. et al. Evaluation of biostimulation, bioaugmentation, and organic amendments application on the bioremediation of recalcitrant hydrocarbons of soil. Chemosphere307, 135638 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Razzaghi, F., Arthur, E. & Moosavi, A. A. Evaluating models to estimate cation exchange capacity of calcareous soils. Geoderma400, 115221 (2021). [Google Scholar]
- 28.Farhangi-Abriz, S. & Ghassemi-Golezani, K. Improving electrochemical characteristics of plant roots by biochar is an efficient mechanism in increasing cations uptake by plants. Chemosphere313, 137365 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Huang, Y. et al. Heavy metal-tolerant bacteria bacillus cereus BCS1 degrades pyrethroid in a soil–plant system. J. Hazard. Mater.461, 132594 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Yang, Y. et al. Interactions between soil organic matter chemical structure and microbial communities determine the spatial variation of soil basal respiration in boreal forests. Appl. Soil. Ecol.183, 104743 (2023). [Google Scholar]
- 31.Arif, M. S. et al. Fresh and composted industrial sludge restore soil functions in surface soil of degraded agricultural land. Sci. Total Environ.619–620, 517–527 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Gallego, S. et al. Ecotoxicological impact of the antihypertensive valsartan on earthworms, extracellular enzymes and soil bacterial communities. Environ. Pollut275, 116647 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Devin, S., Burgeot, T., Giambérini, L., Minguez, L. & Pain-Devin, S. The integrated biomarker response revisited: Optimization to avoid misuse. Environ. Sci. Pollut Res.21, 2448–2454 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Graw, S. et al. Multi-omics data integration considerations and study design for biological systems and disease. Mol. Omics. 17, 170–185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng, M., Li, K., Yan, Y. J., Huang, F. & Peng, D. Enhanced cadmium removal by growing Bacillus cereus RC-1 immobilized on different magnetic biochars through simultaneous adsorption and bioaccumulation. Environ. Sci. Pollut Res.29, 18495–18507 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Hussain, S. et al. Enhancement effect of AgO nanoparticles on fermentative cellulase activity from thermophilic Bacillus subtilis Ag-PQ. J. Gen. Eng. Biotechnol.21, 151 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mushtaq, M. U. et al. Enhanced uptake of cd, cr, and Cu in Catharanthus roseus (L.) G.Don by Bacillus cereus: Application of moss and compost to reduce metal availability. Environ. Sci. Pollut Res.27, 39807–39818 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Han, B. et al. Microbial community evolution and individual-based model validation of biofilms in single-stage partial nitrification/anammox system. Bioresour Technol.397, 130463 (2024). [DOI] [PubMed] [Google Scholar]
- 39.Ogura, A. P. et al. A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. J. Environ. Manage.300, 113753 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Lu, S. et al. Effect of light intensity on nitrogen transformation, enzymatic activity, antioxidant system and transcriptional response of Chlorella pyrenoidosa during treating mariculture wastewater. Bioresour Technol.397, 130465 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Birolli, W. G. & da Silva, B. F. & Rodrigues Filho, E. Biodegradation of the pyrethroid cypermethrin by bacterial consortia collected from orange crops. Environ. Res.215, 114388 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Hernandez, J. C., Ríos, J. M. & Attademo, A. M. Response of digestive enzymes and esterases of ecotoxicological concern in earthworms exposed to chlorpyrifos-treated soils. Ecotoxicolo27, 890–899 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Malla, M. A., Dubey, A., Kumar, A., Yadav, S. & Kumari, S. Modeling and optimization of chlorpyrifos and glyphosate biodegradation using RSM and ANN: Wlucidating their degradation pathways by GC-MS based metabolomics. Ecotoxicol. Environ. Saf.252, 114628 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Wu, M. et al. Temporal dynamics of the compositions and activities of soil microbial communities post-application of the insecticide chlorantraniliprole in paddy soils. Ecotoxicol. Environ. Saf.144, 409–415 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Montiel-Rozas, M. M., Hurtado-Navarro, M., Díez-Rojo, M. Á., Pascual, J. A. & Ros, M. Sustainable alternatives to 1,3-dichloropropene for controlling root-knot nematodes and fungal pathogens in melon crops in Mediterranean soils: Efficacy and effects on soil quality. Environ. Pollut. 247, 1046–1054 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Wołejko, E., Jabłońska-Trypuć, A., Wydro, U., Butarewicz, A. & Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides – A review. Appl. Soil. Ecol.147 (09), 006 (2020). [Google Scholar]
- 47.Wang, Z. et al. Long-term as contamination alters soil enzyme functional stability in response to additional heat disturbance. Chemosphere229, 471–480 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Liao, X., Kang, H., Haidar, G., Wang, W. & Malghani, S. The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: A meta-analysis. Geoderma411, 115692 (2022). [Google Scholar]
- 49.Huang, Y. et al. Efficient biodegradation of multiple pyrethroid pesticides by Rhodococcus pyridinivorans strain Y6 and its degradation mechanism. Chem. Eng. J.469, 143863 (2023). [Google Scholar]
- 50.Huang, H. et al. Effects of pyrolysis temperature, feedstock type and compaction on water retention of biochar amended soil. Sci. Rep.11, 7419 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barathi, S., Sabapathi, N., Kandasamy, S. & Lee, J. Present status of insecticide impacts and eco-friendly approaches for remediation-a review. Environ. Res. vol240, 117432 (2024). [DOI] [PubMed] [Google Scholar]
- 52.Das, T. K. et al. Use of expanded shale, clay, and slate aggregates and biochar in the clear zone of road infrastructures for sustainable treatment of stormwater. J. Clean. Prod.428, 139443 (2023). [Google Scholar]
- 53.Khan, B. A. et al. Effectiveness of the engineered pinecone-derived biochar for the removal of fluoride from water. Environ. Res.212, 113540 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Li, Y. et al. The combined effect of titanium dioxide nanoparticles and cypermethrin on male reproductive toxicity in rats. Environ. Sci. Pollut Res.30, 22176–22187 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.