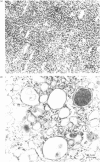
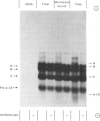
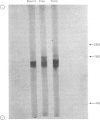
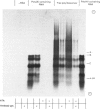
Abstract
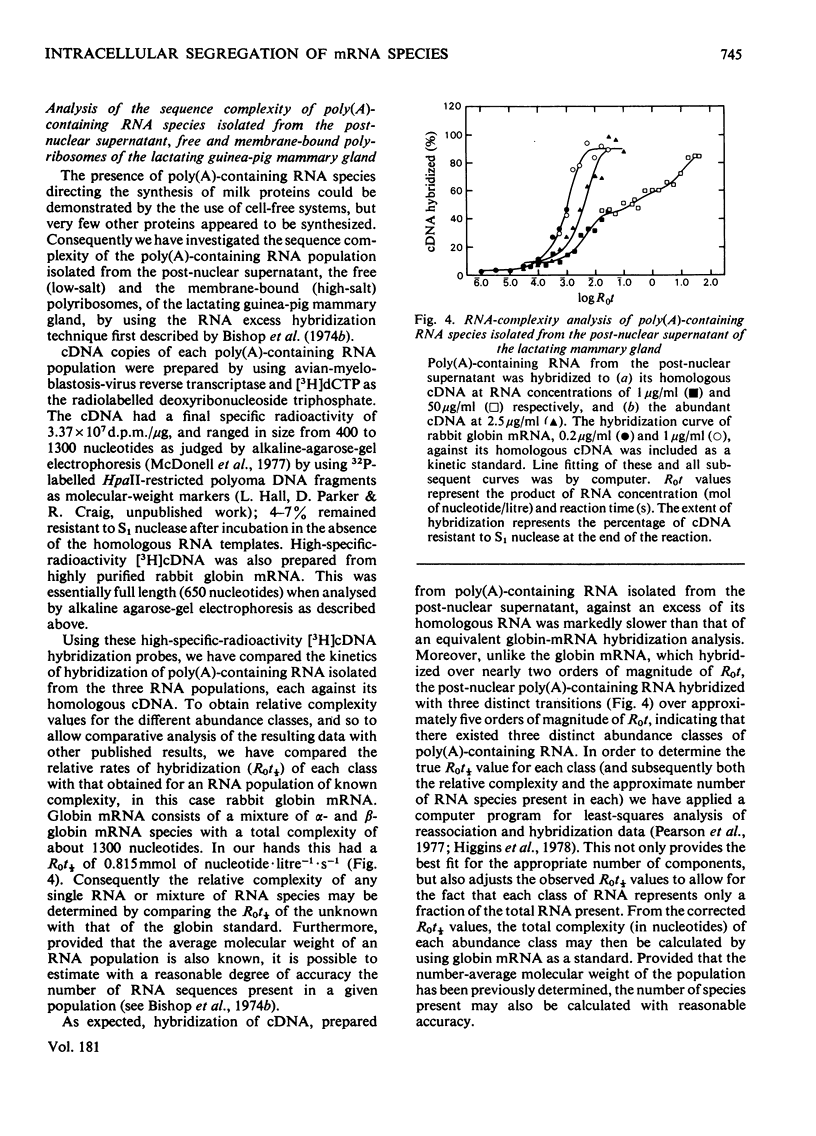
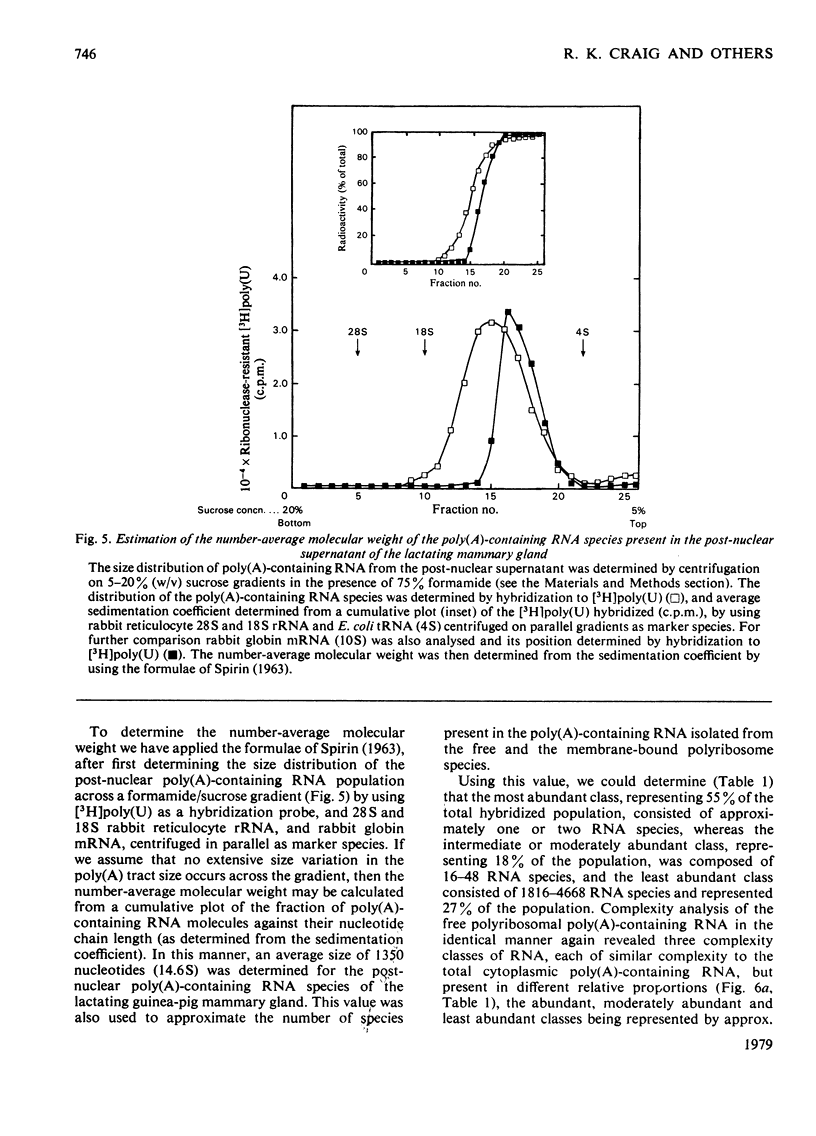
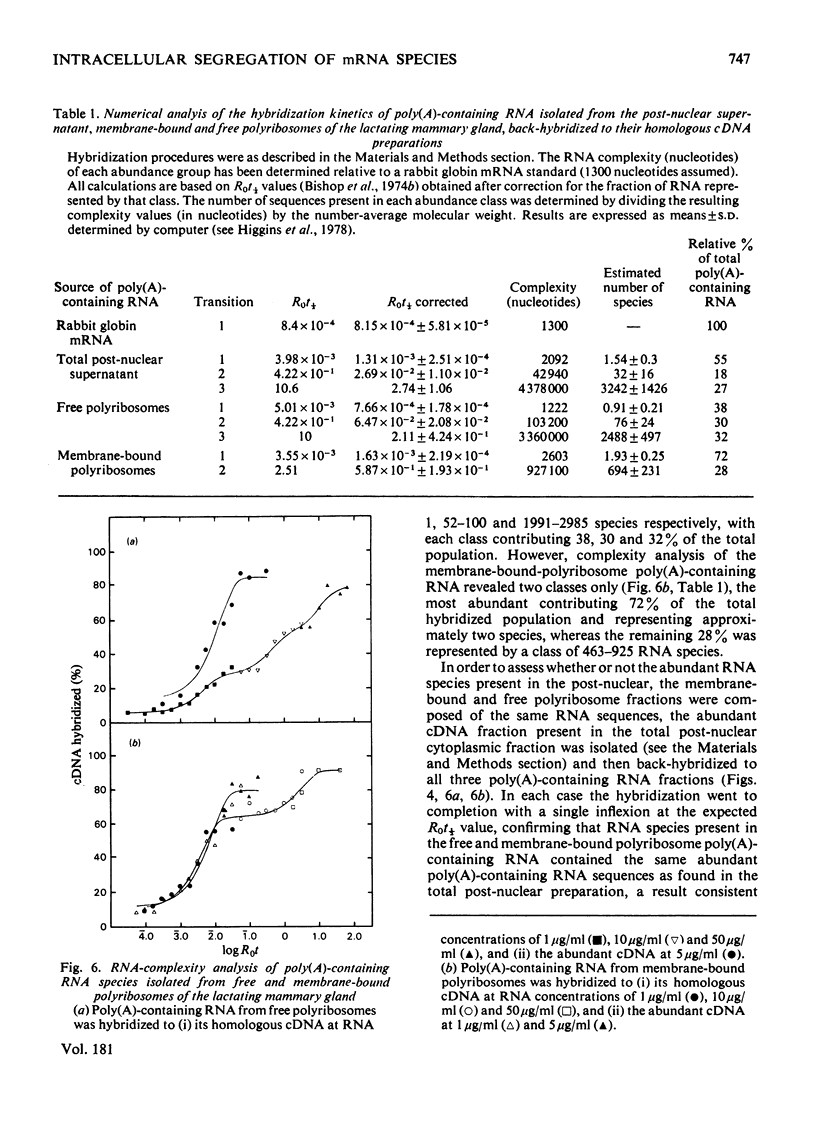
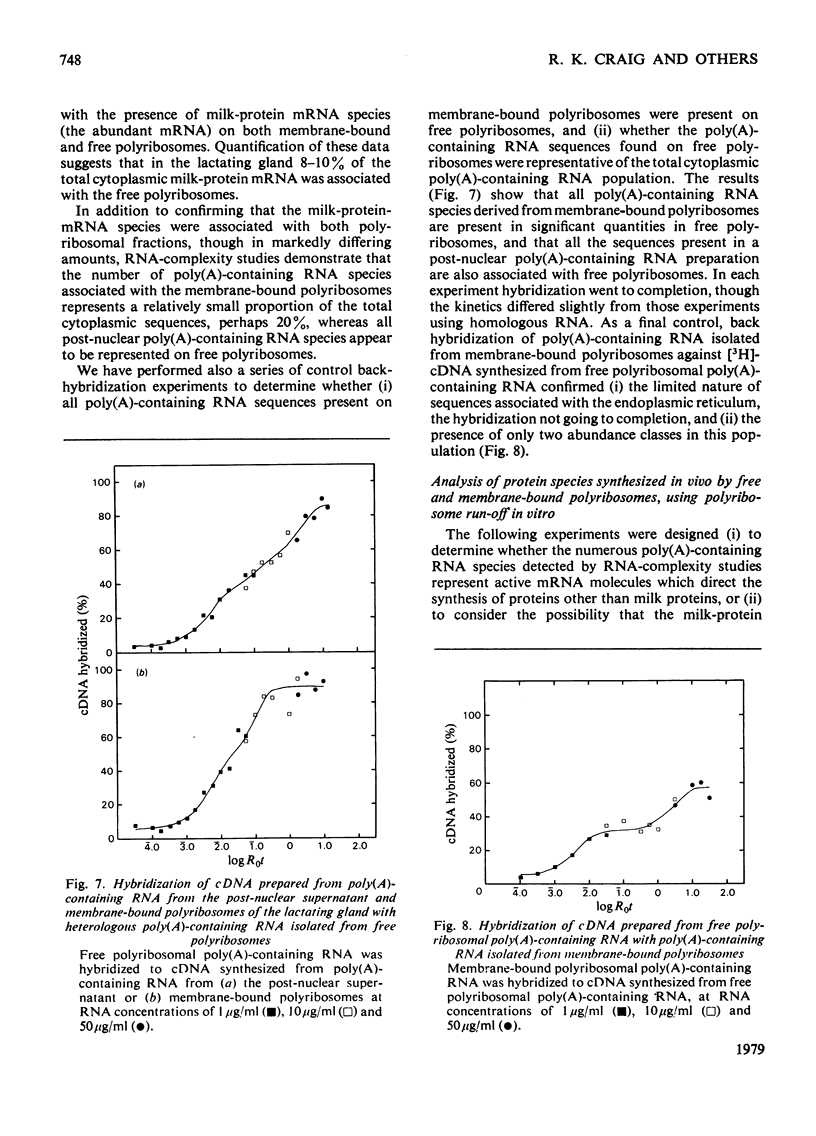
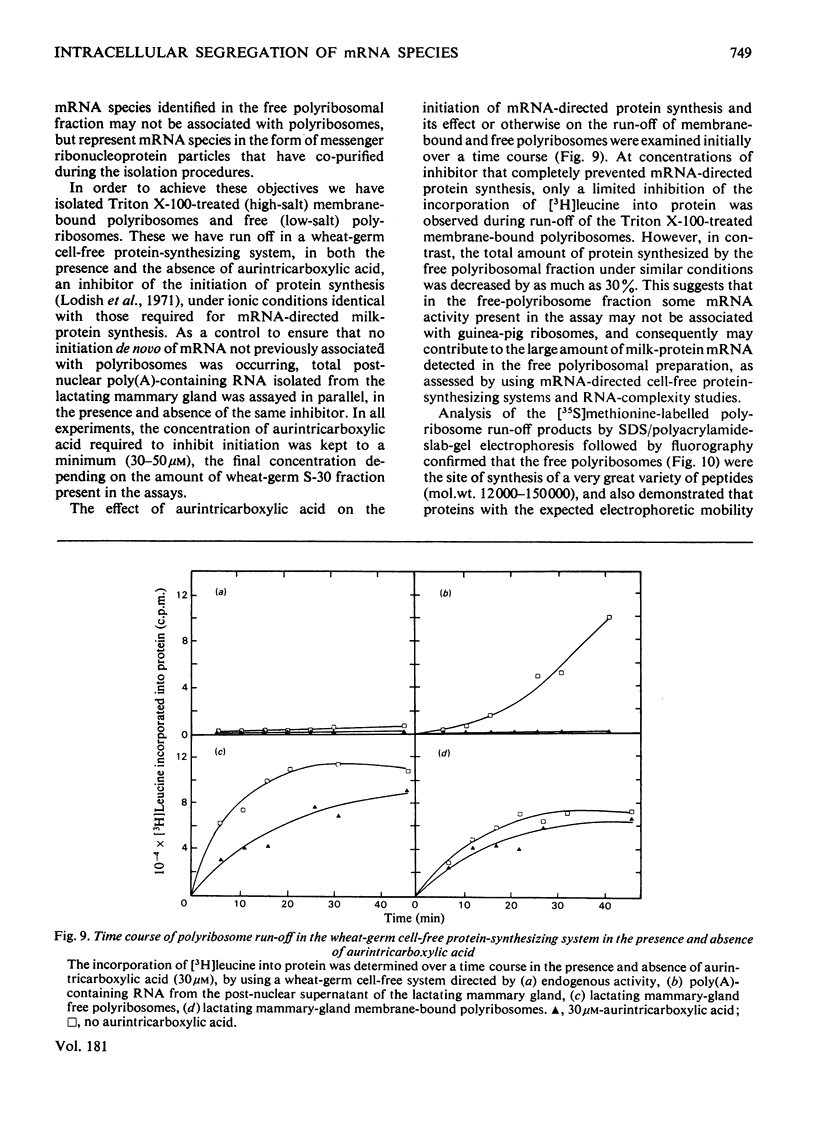
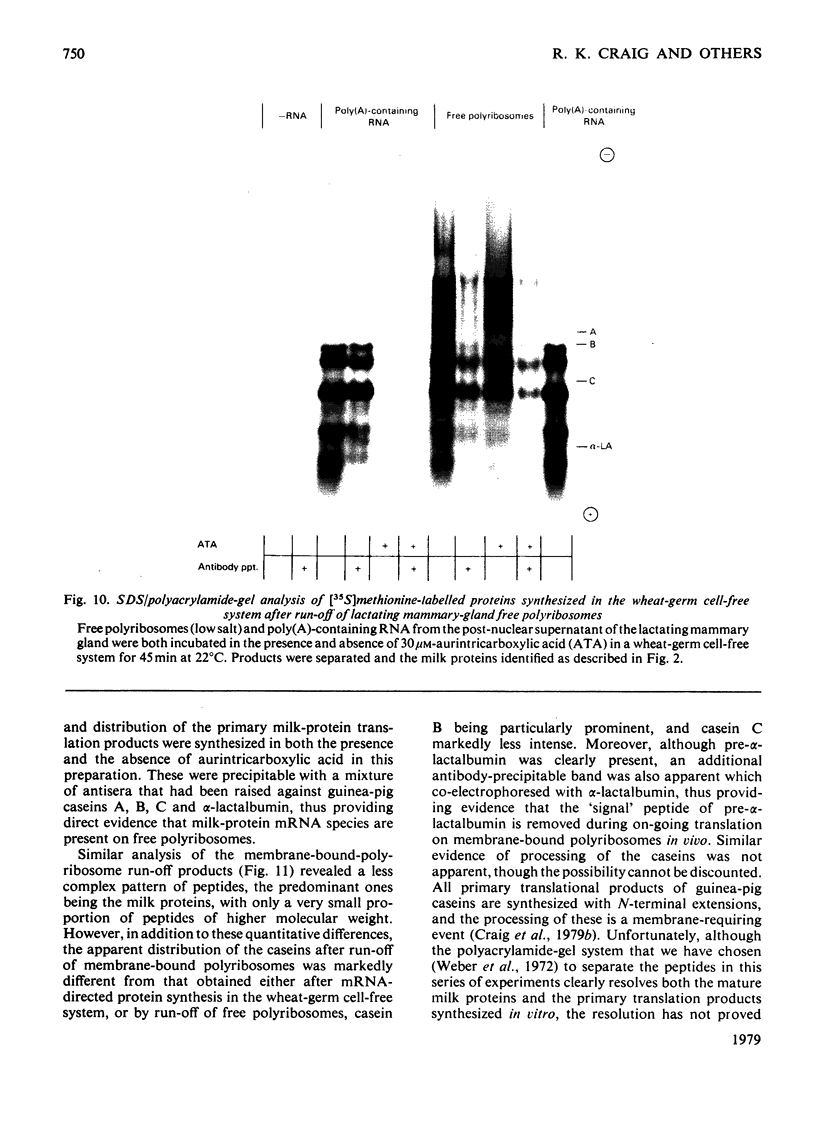
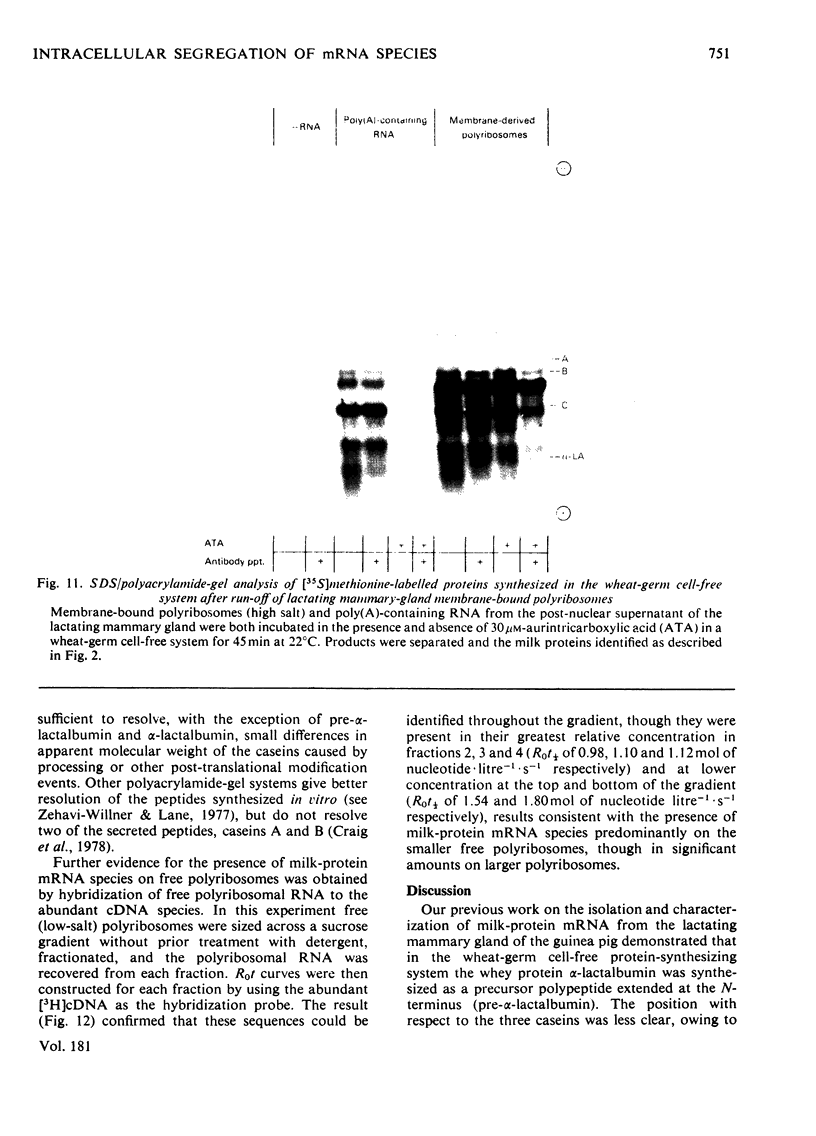
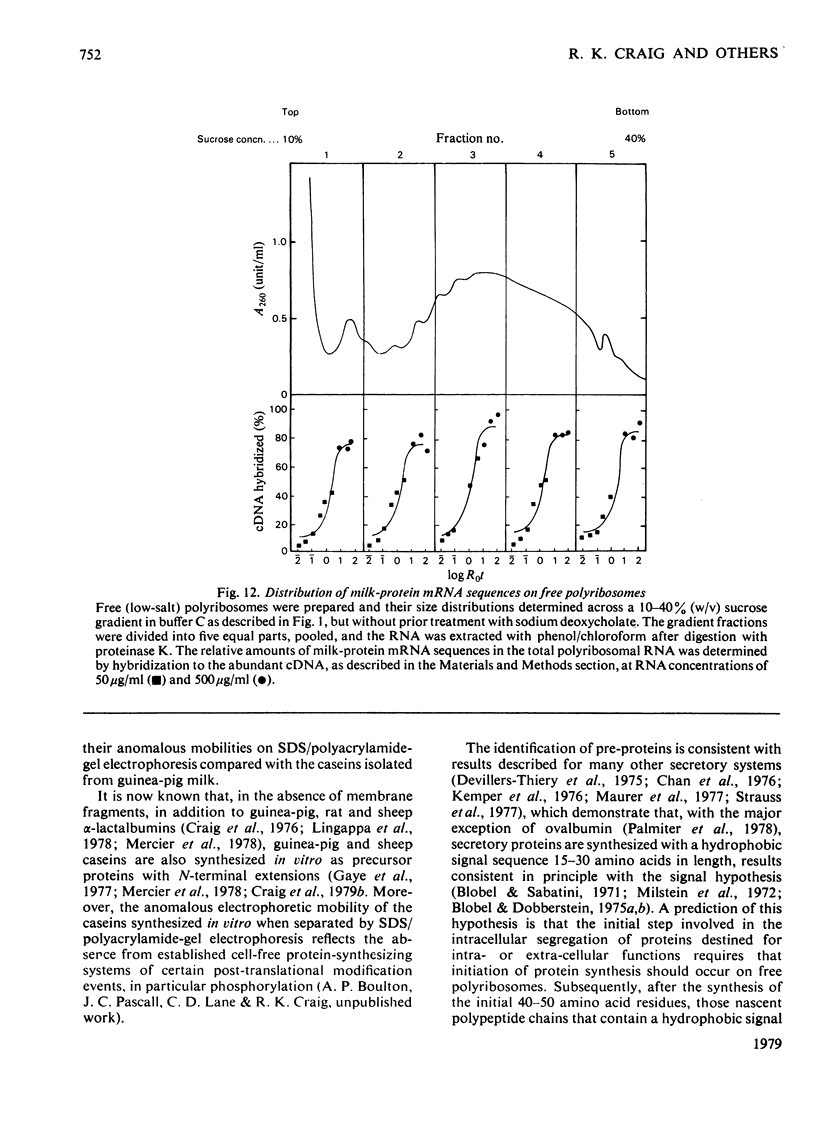
1. Free and membrane-bound polyribosomes were isolated and the associated mRNA species characterized by cell-free protein synthesis, RNA-complexity analysis and polyribosome run-off in vitro. 2. Of the recovered polyribosomal RNA 85% was associated with membrane-bound polyribosomes and contained 87--93% of the total milk-protein mRNA species as assessed by cell-free protein synthesis or RNA-complexity analysis. 3. RNA-complexity analysis showed that the abundant (milk-protein mRNA assumed) species constituted 55% of the post-nuclear poly(A)-containing RNA population, the remainder consisting of a moderately abundant population (18%) and a low abundance population (27%). Calculations suggest that each population contained up to 2, 48 and 5000 different species respectively. 4. RNA-complexity analysis of the free polyribosomal poly(A)-containing RNA demonstrated that all the species in the post-nuclear fraction were present, though in different proportions, the abundant, moderately abundant and low-abundance groups representing 38, 30 and 32% of this population. 5. RNA-complexity analysis of the membrane-bound polyribosomal poly(A)-containing RNA revealed a more limited population, 72% consisting of the abundant (milk-protein mRNA) species, and 28% a population of up to 900 RNA species. 6. Polyribosome run-off confirmed that milk-protein mRNA was associated with the membrane-bound and free polyribosomes, but represented only a small fraction of the total protein synthesized by the latter. 7. Comparative analysis of milk proteins synthesized in mRNA-directed cell-free systems, or by run-off of free and of membrane-bound polyribosomes, is consistent with the interpretation that in vivo the initiation of protein synthesis occurs on free polyribosomes, followed by the attachment of a limited population to the endoplasmic reticulum. After attachment, but before completion of peptide synthesis, the detachable N-terminal peptide sequence of one of these(pre-alpha-lactalbumin) is removed. 8. The results are discussed in terms of the mechanisms involved in the intracellular segregation of mRNA species in the lactating guinea-pig mammary gland.
Full text
PDF




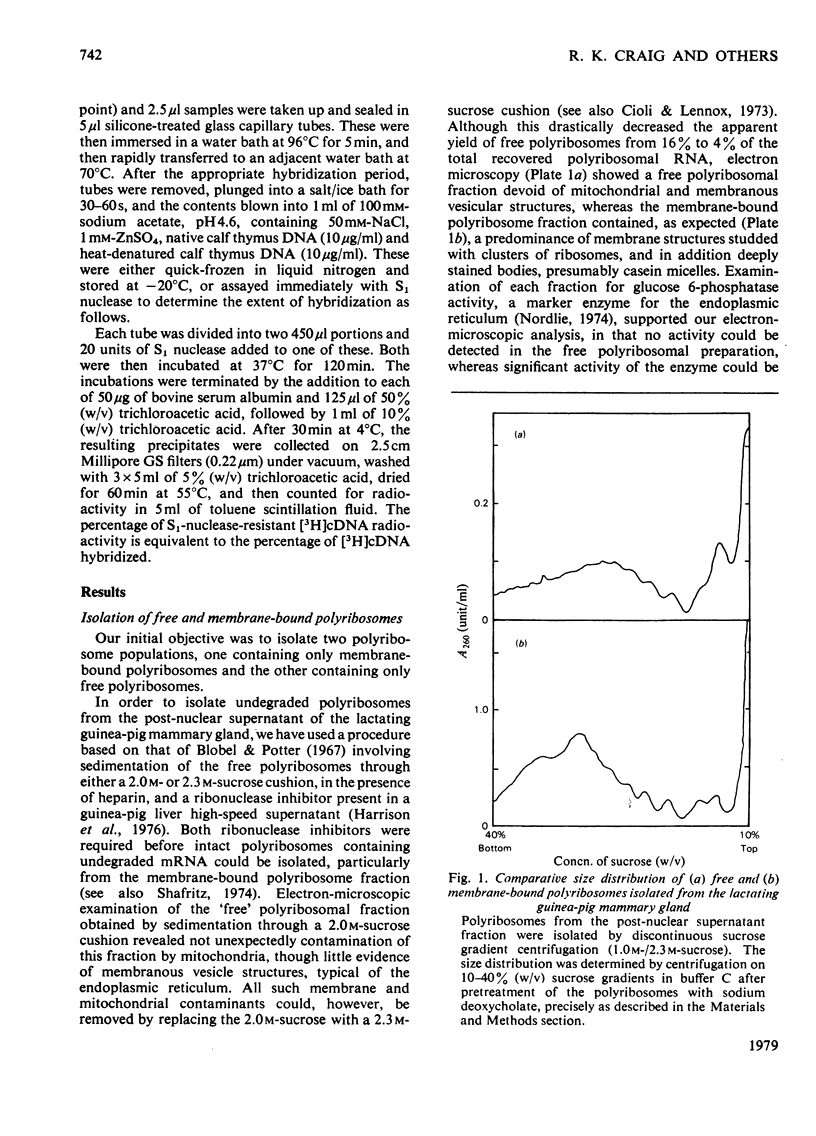
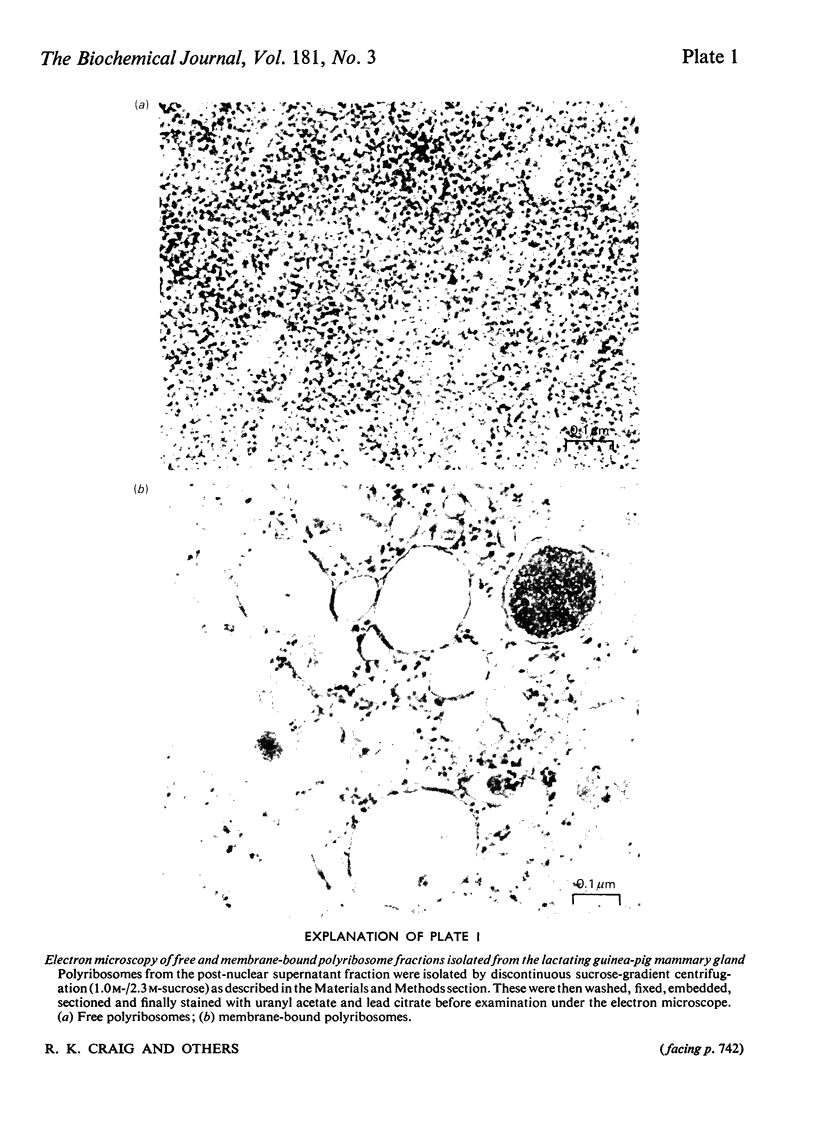

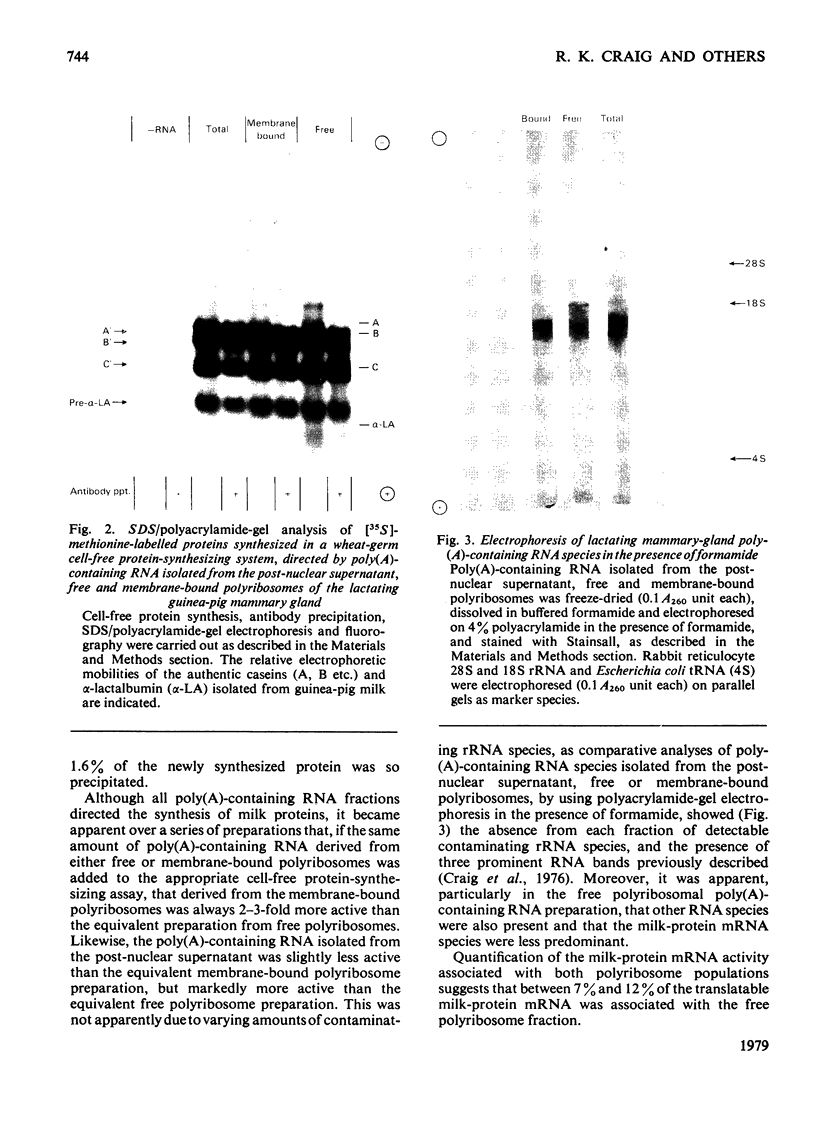




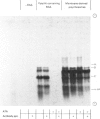
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Berridge M. V., Evans W. H. Biogenesis of plasmalemmal glycoproteins. Intracellular site of synthesis of mouse liver plasmalemmal 5'-nucleotidase as determined by the sub-cellular location of messenger RNA coding for 5'-nucleotidase. Biochim Biophys Acta. 1975 Oct 15;407(3):325–337. doi: 10.1016/0005-2787(75)90100-8. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brew K. The complete amino-acid sequence of guinea-pig -lactalbumin. Eur J Biochem. 1972 May 23;27(2):341–353. doi: 10.1111/j.1432-1033.1972.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Bishop J. O. Two classes of messenger RNA in cultured rat cells: repetitive sequence transcripts and unique sequence transcripts. J Mol Biol. 1974 Dec 25;90(4):649–663. doi: 10.1016/0022-2836(74)90530-0. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioli D., Lennox E. S. Immunoglobulin nascent chains on membrane-bound ribosomes of myeloma cells. Biochemistry. 1973 Aug 14;12(17):3211–3217. doi: 10.1021/bi00741a011. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaye P., Gautron J. P., Mercier J. C., Hazé G. Amino terminal sequences of the precursors of ovine caseins. Biochem Biophys Res Commun. 1977 Dec 7;79(3):903–911. doi: 10.1016/0006-291x(77)91196-2. [DOI] [PubMed] [Google Scholar]
- Harrison O. S., Craig R. K., Campbell P. N. Isolation and characterization of messenger ribonucleic acid species for guinea-pig milk proteins from free and membrane-bound polyribosomes. Biochem Soc Trans. 1976;4(2):340–341. doi: 10.1042/bst0040340. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Hicks S. J., Drysdale J. W., Munro H. N. Preferential synthesis of ferritin and albumin by different populations of liver polysomes. Science. 1969 May 2;164(3879):584–585. doi: 10.1126/science.164.3879.584. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Parker M. G., Herries D. G. Effects of testosterone on sequence complexity of polyadenylated RNA from rat seminal vesicle. Eur J Biochem. 1978 Nov 15;91(2):327–334. doi: 10.1111/j.1432-1033.1978.tb12683.x. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Distribution of casein mRNA between free and membrane-bound polysomes during induction of lactogenesis in the rabbit. Mol Cell Endocrinol. 1977 Apr;7(2):125–135. doi: 10.1016/0303-7207(77)90062-4. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Ernst M. D., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry. 1976 Jan 13;15(1):15–19. doi: 10.1021/bi00646a003. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., Chan K. M., Feigelson P. Translational control of hepatic alpha2u globulin synthesis by growth hormone. Cell. 1978 Nov;15(3):743–750. doi: 10.1016/0092-8674(78)90260-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Mackinlay A. G., West D. W., Manson W. Specific casein phosphorylation by a casein kinase from lactating bovine mammary gland. Eur J Biochem. 1977 Jun 1;76(1):233–243. doi: 10.1111/j.1432-1033.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., Gorski J., McKean D. J. Partial amino acid sequence of rat pre-prolactin. Biochem J. 1977 Jan 1;161(1):189–192. doi: 10.1042/bj1610189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mechler B., Vassalli P. Membrane-bound ribosomes of myeloma cells. I. Preparation of free and membrane-bound ribosomal fractions. Assessment of the methods and properties of the ribosomes. J Cell Biol. 1975 Oct;67(1):1–15. doi: 10.1083/jcb.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J. C., Haze G., Gaye P., Petrissant G., Hue D., Boisnard M. Amino terminal sequence of the precursor of ovine alpha-lactalbumin. Biochem Biophys Res Commun. 1978 Nov 29;85(2):662–670. doi: 10.1016/0006-291x(78)91213-5. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Effect of estrogen on the sequence and population complexity of chick oviduct poly(A)-containing RNA. J Biol Chem. 1976 Jun 25;251(12):3738–3748. [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C. Metabolic regulation by multifunctional glucose-6-phosphatase. Curr Top Cell Regul. 1974;8(0):33–117. doi: 10.1016/b978-0-12-152808-9.50009-2. [DOI] [PubMed] [Google Scholar]
- Okuyama A., McInnes J., Green M., Pestka S. Distribution of MOPC-315 light chain messenger RNA in free and membrane-bound polyribosomes. Biochem Biophys Res Commun. 1977 Jul 11;77(1):347–351. doi: 10.1016/s0006-291x(77)80203-9. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. A small particulate component of the cytoplasm. J Biophys Biochem Cytol. 1955 Jan;1(1):59–68. doi: 10.1083/jcb.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. G., Mainwaring W. I. Effects of androgens on the complexity of poly(A) RNA from rat prostate. Cell. 1977 Oct;12(2):401–407. doi: 10.1016/0092-8674(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. Differences in size, structure and function of free and membrane-bound polyribosomes of rat liver. Evidence for a single class of membrane-bound polyribosomes. Biochem J. 1977 Oct 15;168(1):1–8. doi: 10.1042/bj1680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M. The synthesis of serum proteins on attached rather than free ribosomes of rat liver. Biochem Biophys Res Commun. 1968 Jun 28;31(6):845–850. doi: 10.1016/0006-291x(68)90528-7. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Formation of membrane-bound polyribosomes. J Mol Biol. 1972 Apr 14;65(3):413–422. doi: 10.1016/0022-2836(72)90198-2. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., O'Neal D. L., McHugh J. E., Comstock J. P. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978 Jan 24;17(2):290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Roy A. K. Androgen-dependent synthesis of aplha-2u globulin in the rat: role of the pituitary gland. J Endocrinol. 1973 Feb;56(2):295–301. doi: 10.1677/joe.0.0560295. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 6. Release of enzymes and ribonucleic acid from ribonucleoprotein particles. J Biophys Biochem Cytol. 1960 Jul;7:631–644. doi: 10.1083/jcb.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A. Protein synthesis with messenger ribonucleic acid fractions from membrane-bound and free liver polysomes. Translation characteristics of liver polysomal ribonucleic acids and evidence for albumin production in a messenger-dependent reticulocyte cell-free system. J Biol Chem. 1974 Jan 10;249(1):81–88. [PubMed] [Google Scholar]
- Shuster R. C., Houdebine L. M., Gaye P. Studies on the synthesis of casein messenger RNA during pregnancy in the rabbit. Eur J Biochem. 1976 Dec;71(1):193–199. doi: 10.1111/j.1432-1033.1976.tb11106.x. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Hynes N., Groner B., Schütz G. Frequency distribution of messenger sequences within polysomal mRNA and nuclear RNA from rat liver. Eur J Biochem. 1977 Jul 1;77(1):141–151. doi: 10.1111/j.1432-1033.1977.tb11652.x. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Donohue A. M., Bennett C. D., Rodkey J. A., Alberts A. W. Rat liver preproalbumin: in vitro synthesis and partial amino acid sequence. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1358–1362. doi: 10.1073/pnas.74.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Yap S. H., Strair R. K., Shafritz D. A. Distribution of rat liver albumin mRNA membrane-bound and free in polyribosomes as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5397–5401. doi: 10.1073/pnas.74.12.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. D., Birnie G. D. Complexity and specificity of polysomal poly(A+) RNA in mouse tissues. Biochemistry. 1976 Jun 29;15(13):2823–2829. doi: 10.1021/bi00658a019. [DOI] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Drake R. L., Munro H. N. Distribution of ferritin mRNA and albumin mRNA between free and membrane-bound rat liver polysomes. Biochim Biophys Acta. 1977 Jan 20;474(2):234–244. doi: 10.1016/0005-2787(77)90198-8. [DOI] [PubMed] [Google Scholar]