Abstract
In Japan, fatty liver cases with elevated body mass index are increasing. Because of being non-malignant, these are often neglected unless accompanied by diabetes. This study investigated the risk of glucose intolerance in individuals with non-alcoholic fatty liver. We included 165 men (mean age 56.3 years; range 29–75 years) who underwent an overnight 2-day physical examination at our Health Evaluation Center. All patients underwent abdominal ultrasonography to examine fatty liver. Fasting blood glucose and 75-g glucose tolerance test (OGTT) were conducted. alanine aminotransferase (ALT) (p < 0.01) and triglyceride (p < 0.001) levels were significantly higher in the fatty liver group (FL) than in the non-fatty liver group. HbA1c, fasting blood glucose, and blood glucose level at OGTT (0 and 30 min) did not show significant differences. In the FL, OGTT was significantly elevated at 60 min(p < 0.01)and 120 min (p < 0.001), insulin level was significantly elevated at 0 and 30 min (p < 0.001), and glucagon level was significantly elevated at 0 min (p < 0.05) and 30 min (p < 0.01), with no significant differences between the groups at 60 and 120 min. This is the first study to demonstrate elevated glucagon levels after OGTT. Metabolic dysfunction-associated steatotic liver disease (MASLD) requires treatment for insulin resistance with glucagon dysregulation likely associated with its pathogenesis.
Keywords: MAFLD, Glucagon, A 75 g oral tolerance test
Subject terms: Gastroenterology, Health care
Introduction
In Japan, many people undergo annual health check-ups at their workplaces or by local governments. Although the number of fatty liver cases with increased body mass index (BMI) and abnormal alanine aminotransferase levels has been increasing in Japan since the outbreak of COVID-191, fatty liver disease is often overlooked unless complicated by diabetes or hyperlipidemia, as it is not considered malignant. Fatty liver disease can be divided into alcoholic and non-alcoholic forms. The individuals in this study had Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), which is a fatty liver disease without a history of alcohol consumption (20 g/day or less in men and 10 g/day or less in women). Liver diseases caused by other factors, such as viral hepatitis, were excluded. The prevalence of MAFLD is approximately 24% worldwide and approximately 25% in Japan2. The majority of MAFLD cases are nonalcoholic fatty liver (NAFL), of which 2–3% are Metabolic Dysfunction Associated Steatohepatitis (MASH). MAFLD is a common cause of MASH, which may progress to cirrhosis or liver cancer3. NAFL is becoming the most important cause of cirrhosis and liver cancer in the future, as viral hepatitis is decreasing and the obese population is increasing. Systemic insulin resistance increases lipolysis, leading to an influx of free fatty acids into the liver and promoting triglyceride synthesis in hepatocytes (aggravation of NAFL). Additionally, Kupffer cells are activated to phagocytose hepatocytes, which accumulate fat and undergo apoptosis, leading to chronic inflammation and fibrosis (progression to MASH). In fact, the degree of insulin resistance, as determined by glucose tolerance test results in patients with NAFL without diabetes, correlates with the degree of fibrosis on liver biopsy histology, as determined by the glucose tolerance test results4. Furthermore, diabetes has been reported as a risk factor for the progression of NAFL to MASH. Conversely, the presence of NAFL doubles the risk of developing type 2 diabetes5. Therefore, early intervention and treatment of impaired glucose tolerance are necessary to detect NAFL at an early stage and prevent its progression to MASH.
However, as mentioned above, simply noting NAFL without abnormal blood sampling data during a physical examination is not sufficient to warrant therapeutic intervention under the current circumstances, as the patient is treated as a healthy individual. The Kanazawa Medical University Health Evaluation Center conducts physical examination for approximately 4,000 patients every year, and many of them are diagnosed with fatty liver on abdominal ultrasound examination. In fact, 265 men (2,710 in total) diagnosed with an NAFL underwent abdominal ultrasound in 2019. Moreover, most patients had fasting plasma glucose (FPG) and HbA1c levels in the normal range, with no data suggesting diabetes. Although a 75 g oral glucose tolerance test (OGTT) is useful to detect insulin resistance in MAFLD cases6, the relationship between NAFL and 75 g OGTT results has not been studied in patients undergoing health check-ups. Furthermore, glucagon, along with insulin, is a hormone involved in blood glucose regulation that is suppressed after meals. In MAFLD, the plasma glucagon concentration increases, possibly indicating hepatic glucagon resistance7. To investigate the relationship between MAFLD and glucose intolerance, we divided the health check-up patients into two groups, one with fatty liver and the other without fatty liver, by abdominal ultrasonography and examined the glucose and glucagon level variation in the 75 g OGTT.
Methods
This study included 165 male patients, mean age 56.3 years (29–75 years), who underwent an overnight 2-day physical examination course at our Health Evaluation Center from October 2021 to March 2023. Approval for this study was obtained from the Institutional Review Board of Kanazawa Medical University (approval number: I634). The study was performed in accordance with relevant guidelines and regulations, and written informed consent was obtained from all participants. The patients were not treated for diabetes or hyperlipidemia. They were hepatitis B antigen-negative and hepatitis C antibody-negative and were not complicated by other liver diseases such as viral hepatitis autoimmune hepatitis, drug-induced hepatitis, or liver cancer. Abdominal ultrasound revealed a fatty liver, including hyperintense liver parenchyma, positive hepatorenal contrast, obscured intrahepatic vasculature, and deep echogenic attenuation of the liver. Daily net al.cohol intake was < 30 g (criteria for NAFLD). Blood samples were collected to measure platelets, fasting blood glucose, HbA1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-Glutamyl Trans Peptidase (γGTP), triglycerides, LDL cholesterol, non-HDL cholesterol, and 1,5 anhydro-D glucitol (1,5 AG)8as indicators reflecting postprandial hyperglycemia. A 75 g oral glucose tolerance test was performed under fasting conditions for at least 10 h, and blood glucose and glucagon (ELISA)9 were measured at 0, 30, 60, and 120 min. Insulin was measured by ELISA at 0 and 30 min. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR (fasting insulin × fasting blood glucose/405)) was calculated. FIB-4 index (age x AST (IU/l)/(platelets (109/l) x √ALT (ID/l))10 was calculated for scoring liver fibrosis. Patients were divided into four groups: (1) no fatty liver and FIB-4 index of < 1.3; (2) no fatty liver and FIB-4 index of ≥ 1.3; (3) fatty liver and FIB-4 index of < 1.3; and (4) fatty liver and index of ≥ 1.3. Significant difference tests for patient background, liver function, lipid, and glucose metabolism between the groups were performed using the median and Kruskall-Wallis tests. The risk factors related to lipid and glucose metabolism and a fatty liver were further examined by univariate logistic regression analysis. To examine the usefulness of the FIB-4 index, non-parametric multiple comparisons (Dunn’s test) were performed to determine significant differences between (1) and (2), (3), and (4).
Results
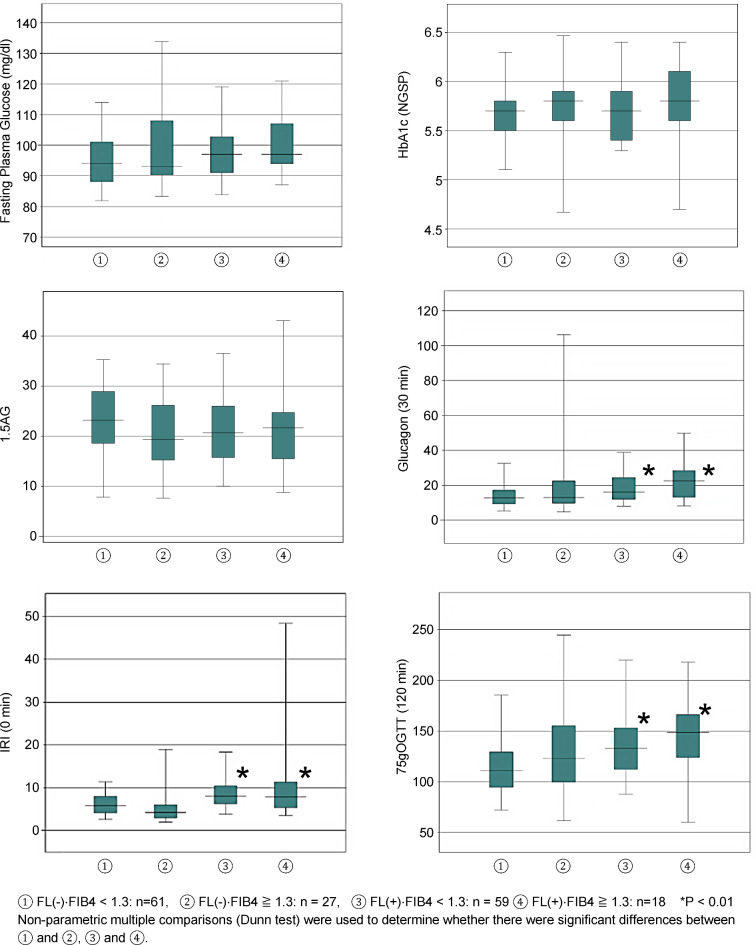
Table 1 presents the background characteristics of the 165 patients. Of the total number of patients, 88 had no fatty livers (FIB4 index:1.3>/n = 61 and 1.3≤/n = 27) and 77 had fatty livers (FIB4 index:1.3>/n = 59 and 1.3≤/n = 18). The group with fatty liver had significantly higher age (p < 0.001), body weight (p < 0.001) and BMI (p < 0.001) than the non-fatty liver group, suggesting a tendency toward older individuals and obesity in the group with a fatty liver. Table 2 displays the results of blood sampling. AST(p < 0.001), ALT(p < 0.01), and γGTP(p < 0.01) levels were significantly higher in the fatty liver group. The platelet count and triglyceride levels were significantly elevated in the fatty liver group (P < 0.001); however, HbA1c levels showed no significant difference. The results of the association analysis between fatty liver disease and glucose metabolism-related parameters are shown in Table 3. Fasting blood glucose and 75 g glucose tolerance test at 0 min and 30 min were not significantly different between the two groups, but the values at 60 min (p < 0.01) and 120 min (p < 0.001) were significantly elevated in the group with fatty liver. The immunoreactive insulin (IRI) level was significantly elevated in the fatty liver group at 0 min (p < 0.001) and 30 min (p < 0.001). HOMA-IR was significantly elevated in the fatty liver group (p < 0.001). Glucagon levels measured during the glucose tolerance test were significantly elevated in the fatty liver group at 0 min (p < 0.05) and 30 min (p < 0.01); however, no significant differences were observed between the four groups at 60 and 120 min. 1.5 AG levels were not significantly different between the two groups (Table 3). The risk factors for fatty liver disease were examined using univariate logistic regression analysis (Table 4). The results showed that the 60- and 120-min glucose tolerance test, 0- and 30-min insulin, and 0-min glucagon were risk factors, with odds ratios (95% confidence interval) of 1.008 (1.001–1.015), 1.015 (1.006–1.024), 1.329 (1.177–1.502), 1. 019 (1.007–1.031), and 1.042 (1.010–1.074), respectively. HbA1c and 1.5 AG levels were not considered risk factors (Table 4). The association between fatty liver disease and lipid levels was examined using univariate logistic regression analysis (Table 5). The results showed that triglycerides, HDL cholesterol, and non-HDL cholesterol were associated with fatty liver with odds ratios (95% confidence intervals) of 1.013 (1.007–1.015), 1.018 (1.007–1.028), and 0.959 (0.936–0.982), respectively. LDL cholesterol levels were not significantly associated (Table 5). Finally, the FIB-4 index was calculated, and its relevance to fatty liver was examined. Patients with and without fatty livers were further divided into two groups according to the FIB-4 index value. These consisted of: (1) a non-fatty liver group: FIB4 < 1.3:61 cases; (2) a non-fatty liver group: FIB4 ≥ 1.3:27 cases; (3) a fatty liver group: FIB4 < 1.3:59 cases; and (4) a fatty liver group: FIB4 ≥ 1.3:18 cases. Nonparametric multiple comparisons (Dunn’s test) were performed to see if groups (2), (3), and (4) were significantly different from group (1). No significant differences were found in fasting blood glucose, HbA1c, and 1,5 AG between groups (2), (3), and (4) compared to group (1). The 120-min glucose tolerance test values were considerably higher in the fatty liver groups (3) and (4) than in the fatty liver group (1). IRI levels before the glucose tolerance test were markedly higher in groups (3) and (4) than in group (1). Glucagon levels were considerably higher in groups (3) and (4) than in group (1) 30 min after the glucose tolerance test (Fig. 1). In conclusion, compared to healthy individuals without fatty liver and with an FIB-4 index of < 1.3, those with fatty liver showed postprandial hyperglycemia, elevated insulin levels and elevated glucagon levels after glucose loading. It suggests that these factors are involved in fatty liver.
Table 1.
Clinical characteristics.
| FIB-4 index | Fatty liver(-) | Fatty liver(+) | p-value | ||
|---|---|---|---|---|---|
| 1.3> | 1.3≦ | 1.3> | 1.3≦ | ||
| N | 61 | 27 | 59 | 18 | |
| Age (years) | 51 | 66* | 50** | 68* | < 0.001 |
| (33–72) | (56–75) | (29–75) | (57–74) | ||
| Weight (kg) | 67.4 | 66.5 | 74.9** | 73.5 | < 0.001 |
| (53.2–95.7) | (44.6–82.1) | (58.3–101.2) | (61.8–99.0) | ||
| BMI | 22.8 | 22.9 | 24.8*/** | 25.6*/** | < 0.001 |
| (17.6–27.8) | (18.2–28.0) | (20.4–31.8) | (21.9–32.7) | ||
Upper: Median; lower: range(maximum–minimum); p-value: Kruskall-Wallis test.
*vs. FL(-)/1.3>,**vs. FL(-)/1.3<.
BMI, Body Mass Index.
Table 2.
Clinical characteristics.
| FIB-4 index | Fatty liver(-) | Fatty liver(+) | p-value | ||
|---|---|---|---|---|---|
| 1.3> | 1.3≦ | 1.3> | 1.3≦ | ||
| AST(U/L) | 19 | 21 | 21 | 27* | < 0.001 |
| (13–41) | (16–50) | (14–51) | (19–76) | ||
| ALT(U/L) | 20 | 19 | 27*/** | 32*/** | < 0.01 |
| (7–43) | (13–52) | (10–125) | (11–132) | ||
| γGTP(U/L) | 26 | 25 | 36 | 45* | < 0.01 |
| (11–189) | (12–82) | (10–704) | (19–112) | ||
| Platelets | 258 | 208 | 268** | 224* | < 0.001 |
| (170–395) | (133–331) | (208–462) | (145–265) | ||
| Triglycerides | 92 | 90 | 129* | 153* | < 0.001 |
| (35–290) | (37–217) | (51–785) | (42–930) | ||
| LDL-C | 122 | 120 | 127 | 126 | 0.3178 |
| (65–182) | (63–190) | (49–190) | (73–194) | ||
| HDL-C | 62 | 62* | 53 | 54 | < 0.01 |
| (34–100) | (38–103) | (33–88) | (39–94) | ||
Upper: Median; lower: range (maximum–minimum); p-value: Kruskall-Wallis test.
*vs. FL(-)/1.3>, **vs. FL(-)/1.3<.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGTP, γ-Glutamyl Trans Peptidase; LDL-C, Low Density Lipoprotein cholesterol; HDL-C, High Density Lipoprotein cholesterol.
Table 3.
Glucose tolerance related indicators.
| FIB-4 index | Fatty liver(-) | Fatty liver(+) | p-value | ||
|---|---|---|---|---|---|
| 1.3> | 1.3≦ | 1.3> | 1.3≦ | ||
| FPG | 94 | 93 | 97 | 97 | 0.1975 |
| (76–119) | (88–137) | (83–120) | (87–121) | ||
| Glucose | 95 | 96 | 99 | 100 | 0.2610 |
| (75 g OGTT 0 min) | (81–125) | (83–141) | (83–120) | (85–118) | |
| Glucose | 157 | 177 | 171 | 184 | 0.0139 |
| (75 g OGTT 30 min) | (114–218) | (121–260) | (119–214) | (112–234) | |
| Glucose | 156 | 184 | 188* | 224* | < 0.01 |
| (75 g OGTT 60 min) | (60–281) | (100–270) | (88–281) | (145–265) | |
| Glucose | 111 | 123 | 133* | 149* | < 0.001 |
| (75 g OGTT 120 min) | (68–224) | (61–247) | (75–257) | (60–218) | |
| IRI | 5.8 | 4.2 | 8*/** | 7.8 | < 0.001 |
| (75 g OGTT 0 min) | (2.3–13) | (1.9–20.4) | (3.5–19.2) | (3.5–48.4) | |
| IRI | 33.6 | 25.7 | 44** | 53.4 | < 0.001 |
| (75 g OGTT 30 min) | (7.5–202.5) | (7.5–89.8) | (16.1–240.6) | (21.3–167.7) | |
| IRI/BS | 0.55 | 0.3 | 0.57** | 0.59** | < 0.01 |
| (0.07–7.25) | (0.07–0.88) | (0.13–3.81) | (0.22–2.21) | ||
| 1.5AG | 23.2 | 19.3 | 20.7 | 21.7 | 0.2076 |
| (4.6–40.1) | (7–34.7) | (8.7–37.4) | (8.8–43.1) | ||
| Glucagon | 24.1 | 18.1 | 26.6** | 27.6 | < 0.05 |
| (75 g OGTT 0 min) | (10.5–47.3) | (7.9–45.7) | (4–62.9) | (14.4–46.4) | |
| Glucagon | 12.8 | 13 | 16.1** | 22.6** | < 0.01 |
| (75 g OGTT 30 min) | (3.5–42.9) | (4.7–114.6) | (8–43.7) | (8.2–19.9) | |
| Glucagon | 9.05 | 9.5 | 12.3 | 13.3 | 0.0143 |
| (75 g OGTT 60 min) | (3.5–28.8) | (3.5–98.8) | (3.9–32.9) | (4.2–38.4) | |
| Glucagon | 9.4 | 10.5 | 10.1 | 11.6 | 0.3426 |
| (75 g OGTT 120 min) | (1.6–30.4) | (3.5–77.1) | (3.8–22.7) | (6.3–50.7) | |
| HOMA-R | 1.27 | 1 | 1.68*/** | 1.93*/** | < 0.001 |
| (0.58–3.24) | (0.40–7.85 | (0.53–4.91) | (0.98–12.67) | ||
| HbA1c | 5.7 | 5.8 | 5.7 | 5.8 | 0.2426 |
| (5.1–6.3) | (4.6–6.5) | (5.1–6.4) | (4.7–6.4) | ||
Upper: Median; lower: range(minimum–maximum); p-value: Kruskall-Wallis test.
*vs. FL(-)/1.3>, **vs. FL(-)/1.3<.
FPG, Fasting plasma glucose; 75 g OGTT, a 75 g oral glucose tolerance test; IRI, immunoreactive insulin; IRI/BS, initial insulin secretion; 1.5 AG, 1–5 anhydro-D glucitol;.
HOMA-R, Homeostatic Model Assessment for Insulin Resistance.
Table 4.
Risk factors associated with fatty liver in glucose metabolism.
| P | OR | 95% CI | |
|---|---|---|---|
| 75 g GTT (0 min) | 0.26 | 1.019 | 0.986–1.052 |
| 75 g GTT (30 min) | 0.081 | 1.012 | 0.999–1.025 |
| 75 g GTT (60 min) | < 0.05 | 1.008 | 1.001–1.015 |
| 75 g GTT (120 min) | < 0.05 | 1.015 | 1.006–1.024 |
| IRI (0 min) | < 0.05 | 1.329 | 1.177–1.502 |
| IRI (30 min) | < 0.05 | 1.019 | 1.007–1.031 |
| IRI/BS | 0.236 | 1.332 | 0.829–2.142 |
| 1.5 AG | 0.388 | 0.982 | 0.941–1.024 |
| HbA1c | 0.358 | 1.592 | 0.591–4.285 |
| Glucagon (0 min) | < 0.05 | 1.042 | 1.010–1.074 |
| Glucagon (30 min) | 0.109 | 1.025 | 0.994–1.057 |
| Glucagon (60 min) | 0.548 | 1.009 | 0.980–1.038 |
| Glucagon (120 min) | 0.585 | 0.991 | 0.959–1.024 |
Univariate logistic regression analysis.
Table 5.
Risk factors associated with fatty liver in lipids.
| P | OR | 95% CI | |
|---|---|---|---|
| Triglyceride | < 0.05 | 1.013 | 1.007–1.019 |
| LDL-Chol | 0.053 | 1.012 | 1.000–1.023 |
| nonHDL-Chol | < 0.05 | 1.018 | 1.007–1.028 |
| HDL-Chol | < 0.05 | 0.959 | 0.936–0.982 |
Univariate logistic regression analysis.
Fig. 1.
No significant differences were found in fasting blood glucose, HbA1c, and 1,5 AG between groups (2), (3), and (4) compared to group (1). The 120-min glucose tolerance test values, IRI levels before the glucose tolerance test, and glucagon levels (OGTT 30 min) were significantly higher in the fatty liver groups (3) and (4) than in the fatty liver group (1). IRI: immunoreactive insulin.
Discussion
The study results from individuals undergoing physical check-ups showed that the group with fatty liver detected by abdominal ultrasonography was more frequently complicated by abnormal glucose metabolism compared to the non-fatty liver group, even in the absence of a formal diagnosis of diabetes. In particular, postprandial hyperglycemia, which is a high 120-min value in a glucose tolerance test, is strongly associated with a fatty liver, even if fasting blood glucose and HbA1c levels are not elevated. In addition, IRI was markedly higher in the fatty liver group in the 0- and 30-min glucose tolerance tests, and HOMA-IR was also elevated, suggesting that the fatty liver group was at a risk of insulin resistance. Glucagon levels were also higher in the fatty liver group 30 min after glucose loading. In fact, it has been reported that glucagon levels are markedly elevated and sustained in individuals with type 2 diabetes compared to healthy individuals after a glucose tolerance test11. Additionally, hepatic glucagon resistance causes the dysregulation of lipid and amino acid/protein metabolism in MAFLD, leading to excessive fat accumulation, hyperglucagonemia, and increased oxidative stress, which may contribute to MAFLD deterioration7. In this study, glucagon levels decreased in patients with diabetes mellitus, even in those without a diagnosis of diabetes, and glucagon depletion was delayed, suggesting that glucagon may be involved in the progression of MAFLD from an earlier stage. Recently, the efficacy of glucagon-like peptide-1 (GLP-1) receptor agonists has been reported for the treatment of MASH/MAFLD, as they promote insulin secretion, improve hepatic glucose metabolism, and suppress appetite by inhibiting glucagon secretion12,13. In addition, it has been reported that GLP-1 receptor agonists reduced not only blood glucose but also ALT, body weight, and the AST/platelet ratio in patients with type 2 diabetes and MAFLD12. Furthermore, animal studies have reported that GLP-1 receptor agonists improve steatohepatitis14. The fact that the present study also showed an increase in glucagon in the state of simple fatty liver without complications of diabetes suggests that administration of GLP-1 receptor agonists in the uncomplicated state of diabetes may treat MAFLD at an early stage, indicating that administration of GLP-1 receptor agonists in the absence of diabetes mellitus may be able to treat MAFLD early.
However, since an association between insulin resistance and the development of sarcopenia has recently been reported15and taking GLP-I inhibitors has been reported to further increase the risk of sarcopenia16, the effect on muscle health should also be considered when prescribing drug therapy for fatty liver aimed at weight loss.
Health examinations commonly evaluate glucose intolerance mainly based on fasting blood glucose and HbA1c values. In contrast, in this study, the 120-min glucose tolerance test value was considerably elevated in the group with fatty liver whose fasting blood glucose and HbA1c values were not elevated. It indicates that in the hidden stage of diabetes that is not diagnosed in the health examination, postprandial hyperglycemia was thought to play a remarkable role in the formation of fatty liver. Fatty liver is caused by hyperglycemia and hyperinsulinemia as well as increased triglyceride synthesis in the liver, as free fatty acids entering the liver are a source of triglycerides. Moreover, hyperinsulinemia and endoplasmic reticulum stress suppress the production of very low density lipoprotein17. It has also been reported that insulin resistance associated with the formation of fatty liver may have an inflammatory background. Insulin resistance is associated with a decrease in omentin, a type of adiphoktin18, and an elevated uric acid to HDL cholesterol ratio is useful as a new indicator of type 2 diabetes and metabolic syndrome19–21. In addition, type 2 diabetes and obesity have been reported to be associated with mean platelet volume (MPV) as a new inflammatory marker22, and MPV has also been suggested to be associated with hepatitis B-related liver fibrosis23. In this study, we did not examine markers that link insulin resistance to inflammation. We would like to determine in the future whether inflammatory markers are elevated before the diagnosis of diabetes mellitus.
To date, when fatty liver is detected during health check-ups, the main focus of guidance has been on lipid control, particularly triglyceride control. High insulin and blood glucose levels are believed to be contributing factors.
However, performing a glucose tolerance test during every physical examination is unrealistic, and hidden postprandial hyperglycemia may often go undetected when fasting blood glucose and HbA1c levels are normal. Therefore, we thought that 1,5 AG might be useful as a value that reflects glucose metabolism even earlier than HbA1c. 1,5 AG is a sugar alcohol whose structure is similar to that of glucose and is mainly consumed in the diet; 99% of 1,5 AG is reabsorbed in the renal tubules in normal conditions. Under normal conditions, the concentration of 1,5 AG in the blood remains constant. However, increased urinary glucose excretion owing to postprandial hyperglycemia, for example, inhibits reabsorption, resulting in decreased blood 1,5 AG levels8. In this study, 1,5 AG was measured in all patients and significant differences (P < 0.05) were found between the two groups; however, no significant differences were found between the group with fatty liver and non-fatty liver groups (Table 4). This may be due to many individuals reducing their food intake in the days leading to the check-ups, making 1,5 AG an unreliable indicator of postprandial hyperglycemia during the examinations.
Recently, the FIB-4 index (age x AST(IU/l)/(platelets(109/l) x √ALT(ID/l)) has been recommended as one of the screening tests to examine whether liver fibrosis develops from NAFL to MASH10.
A low cut-off value of 1.30 or less for the FIB-4 index indicates a low probability of liver fibrosis, whereas a high cut-off value of 2.67 or more indicates a high probability of fibrosis, and referral to a specialist is recommended24.
In this study, the FIB-4 index was measured in all individuals, and no individual had an FIB-4 index of 2.67 or higher. We further divided the groups with and without fatty liver into two groups, one with an FIB-4 index of < 1.3 and the other with an FIB-4 index of ≥ 1.3, with a boundary of 1.325, which is said to have a low potential for liver fibrosis, for a total of four groups.
The results showed that the 120-min glucose tolerance test, IRI (0 min), and glucagon (30 min) were considerably higher in the “with fatty liver, FIB-4 index < 1.3” and “with fatty liver, FIB-4 index > 1.3” groups than in the “non-fatty liver, FIB-4 index < 1.3” group (Fig. 1).
Regarding insulin resistance and MAFLD, previous studies have reported that insulin resistance modulates CYP7B1 and promotes the transition to MASH by causing the accumulation of oxysterols26.
Our results also indicate that for patients with a fatty liver and an FIB-4 index of 1.3 or higher, even if not accompanied by diabetes mellitus, aggressive intervention such as diet and exercise guidance is necessary when postprandial hyperglycemia owing to insulin hypersecretion or sustained high glucagon levels is observed, even if insulin resistance is not suspected. In addition, the “fatty liver and FIB-4 index < 1.3” group, which is acceptable for follow-up observation, also showed a marked increase in blood glucose level at 120 min after the meal, and therefore, regardless of the FIB-4 index value, when a fatty liver is detected, aggressive intervention is necessary because postprandial hyperglycemia is considered to be a background cause.
In this study, individuals with fatty livers were divided into four groups: those with and without fatty liver, and more or less than 1.3 of FIB-4 index. In the fatty liver group, even if fasting blood glucose and HbA1c levels were normal, elevated blood glucose(120 min), IRI(0 min), and glucagon levels(30 min) after glucose loading were observed in the OGTT.
Reports indicate a higher prevalence of MAFLD in patients with diabetes mellitus complications owing to insulin resistance and that they are more likely to progress to MASH27; however, NAFL and glucose intolerance/glucagon levels have not been studied in healthy individuals. This study indicated that the presence of fatty liver (NAFL) in individuals with normal fasting blood glucose and HbA1c levels is associated with impaired glucose tolerance and glucagon metabolism, which may also influence the development of diabetes mellitus.
However, this study was limited to individuals who underwent physical examinations, resulting in an insufficient broad sample for a comprehensive analysis. Furthermore, as the study included only men and excluded women, it remains unclear whether these findings apply to populations in other gender. Future studies should aim to expand the scope of coverage to address this limitation. Although the combination of MAFLD and liver insulin resistance predisposes to diabetes28, this study is the first to show that glucagon levels are elevated after a glucose tolerance test. MAFLD has been reported to require treatment for insulin resistance29. In order to evaluate the glucagon dysregulation in the risk of MAFLD, we plan to include a larger sample size in future studies.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author contributions
RO and YN made substantial contributions to the conception and design of the work. KF made significant contribution to the analysis of data. MI, AW, HK, and RI contributed significantly to the acquisition of data. KI made significant contribution to have drafted the work and substantively revised it. All authors have approved the submitted version and any substantially modified version that involves the authors’ contributions to the study.All authors have agreed both to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the authors were not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This study was supported by JSPS KAKENHI (grant number: JP21K10483).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because of privacy laws but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsubara, R. et al. A survey of patients with gout during COVID-19 pandemic. Gout Uric Nucleic Acids. 45, 141–147 (2021). [Google Scholar]
- 2.Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol.15, 11–20 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Eguchi, Y. et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J. Gastroenterol.47, 586–595 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology52, 1836–1846 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Ballestri, S. et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol.31, 936–944 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Kimura, Y. et al. Clinical usefulness of glucose tolerance test in nonalcoholic fatty liver disease patients without overt diabetes mellitus. Kanzo51, 586–588 (2010). [Google Scholar]
- 7.Galsgaard, K. D. The vicious circle of hepatic glucagon resistance in non-alcoholic fatty liver disease. J. Clin. Med.9, 40–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akanuma, Y., Morita, M., Fukuzawa, N., Yamanouchi, T. & Akanuma, H. Urinary excretion of 1,5-anhydro-D-glucitol accompanying glucose excretion in diabetic patients. Diabetologia31, 831–835 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Wewer Albrechtsen, N. J. et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 57, 1919–1926 (2014). [DOI] [PubMed]
- 10.Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver diseases. Clin. Gastroenterol. Hepatol.7, 1104–1112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo, T. et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme-linked immunosorbent assay. J. Diabetes Investig. 7, 324–331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohki, T. et al. The effectives of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci. World J. 496453 (2012). (2012). [DOI] [PMC free article] [PubMed]
- 13.Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet387, 679–690 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Trevaskis, J. L. et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol.302, G762–G772 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Liu, Z. J. & Zhu, C. F. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr.15, 46 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massimino, E. The impact of glucose-lowing drugs on Sarcopenia in type 2 diabetes:current evidence and underlying mechanisms. Cells10, 1958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ota, T., Gayet, C. & Ginsberg, H. N. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest.118, 316–332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aktas, G. & Alcelik, A. Association between omentin levels and insulin resistance in pregnancy. Exp. Clin. Endocrinol.122, 163–166 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Kosekli, M. A. et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev. Assoc. Med. Bras.67, 459–554 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Kosekli, M. A. & Gulali, A. Serum uric acid to HDL cholesterol ratio is associated with diabetic control in new onset type 2 diabetic population. Acta Clin. Ctoat. 62, 277–282 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocak, M. Z. et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev. Assoc. Med. Bras. () 65, 9–15 (2019). (1992). [DOI] [PubMed]
- 22.Gulali, A. et al. Mean platelet volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med. J.7, 650–653 (2018). [Google Scholar]
- 23.Kosekli, M. A. Mean platelet volume and platelet to lymphocyte count ratio are associated with hepatitis B-related liver fibrosis. Eur. J. Gastroenterol. Hepatol.34, 423–327 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Rinella, M. E. & Sanyal, A. J. Management of NAFLD: a stage-based approach. Nat. Rev. Gastroenterol. Hepatol.13, 196–205 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Yoneda, M. et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J. Gastroenterol.48, 1051–1060 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Kakiyama, G. et al. Insulin resistance dysregulates CYP7B1 leading to oxysterol accumulation: a pathway for NAFL to NASH transition. J. Lipid Res.61, 1629–1644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii, H., Kawada, N. & Japan Study Group of Nafld Jsg-Nafld. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int. J. Mol. Sci.21, 3863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu, W. et al. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: insulin resistance between hepatic and peripheral tissues. Front. Pharmacol.9, 1566 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seko, Y. et al. Insulin resistance increases the risk of incident type 2 diabetes mellitus in patients with non-alcoholic fatty liver disease. Hepatol. Res.48, E42–E51 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because of privacy laws but are available from the corresponding author on reasonable request.