Abstract
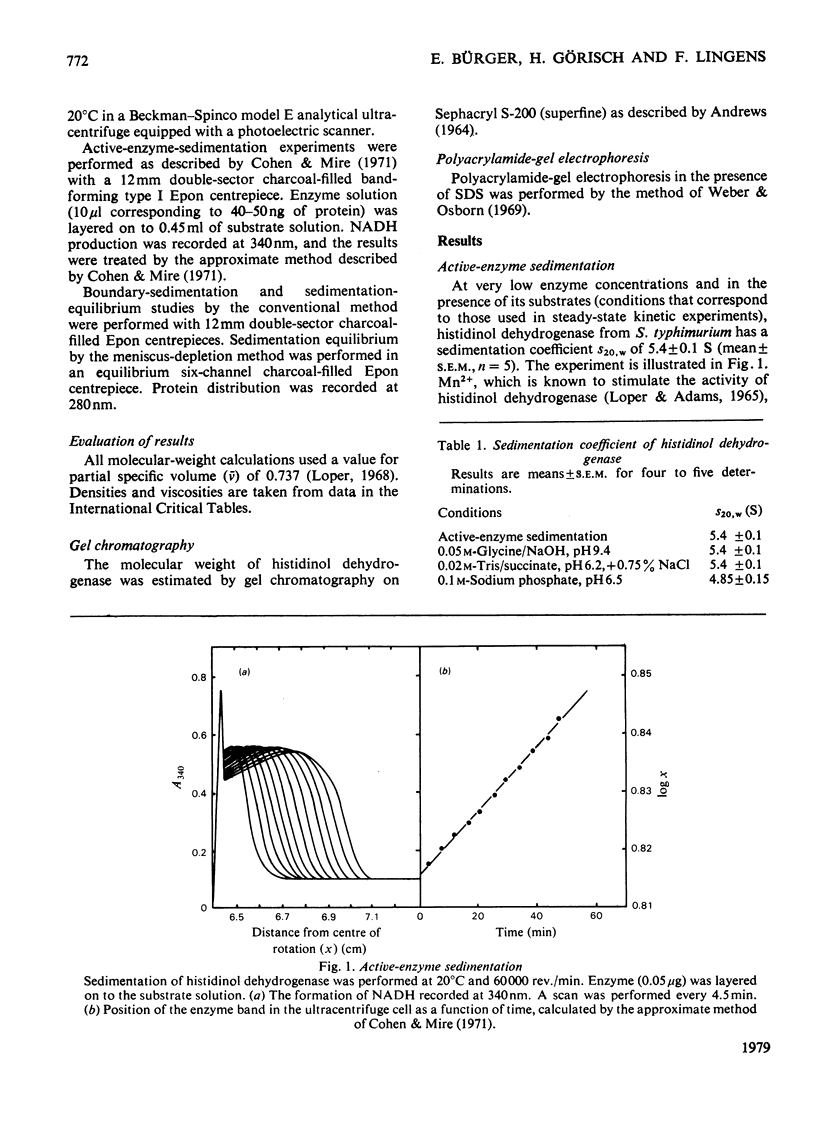
The active-enzyme-sedimentation procedure was used to identify the catalytically competent form of histidinol dehydrogenase (EC 1.1.1.23) isolated from Salmonella typhimurium. At pH 9.4 the active species has a sedimentation coefficient S20,W of 5.4S, indicating that the dimer with a mol.wt. of approx. 83 000 is the enzymically active form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. G., Firca J. R., Loper J. C. Histidinol dehydrogenase from salmonella typhimurium and Escherichia coli. Purification, some characteristics and the amino acid sequence around a reactive thiol group. Biochim Biophys Acta. 1977 Aug 23;493(2):429–440. doi: 10.1016/0005-2795(77)90199-4. [DOI] [PubMed] [Google Scholar]
- Cohen R., Mire M. Analytical-band centrifugation of an active enzyme-substrate complex. 1. Principle and practice of the centrifugation. Eur J Biochem. 1971 Nov 11;23(2):267–275. doi: 10.1111/j.1432-1033.1971.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Greeb J., Atkins J. F., Loper J. C. Histidinol dehydrogenase (his D) mutants of Salmonella typhimurium. J Bacteriol. 1971 May;106(2):421–431. doi: 10.1128/jb.106.2.421-431.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Yourno J. Proteolytic release of a histidinol dehydrogenase fragment from the double enzyme histidinol dehydrogenase-imidazolylacetol-phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Apr 10;246(7):2203–2206. [PubMed] [Google Scholar]
- LOPER J. C., ADAMS E. PURIFICATION AND PROPERTIES OF HISTIDINOL DEHYDROGENASE FROM SALMONELLA TYPHIMURIUM. J Biol Chem. 1965 Feb;240:788–795. [PubMed] [Google Scholar]
- Loper J. C. Histidinol dehydrogenase from Salmonella typhimurium. Crystallization and composition studies. J Biol Chem. 1968 Jun 25;243(12):3264–3272. [PubMed] [Google Scholar]
- Pauly H. E., Pfleiderer G. D-glucose dehydrogenase from Bacillus megaterium M 1286: purification, properties and structure. Hoppe Seylers Z Physiol Chem. 1975 Oct;356(10):1613–1623. doi: 10.1515/bchm2.1975.356.2.1613. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Bruni C. B. Properties of a fused protein formed by genetic manipulation. Histidinol dehydrogenase-imidazolylacetol phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Mar 25;246(6):1806–1813. [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yourno J. Composition and subunit structure of histidinol dehydrogenase from Salmonella typhimurium. J Biol Chem. 1968 Jun 25;243(12):3277–3288. [PubMed] [Google Scholar]
- Yourno J., Ino I. Purification and crystallization of histidinol dehydrogenase from Salmonella typhimurium LT-2. J Biol Chem. 1968 Jun 25;243(12):3273–3276. [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]


