Abstract
Emergence agitation (EA) is more commonly observed after thoracic surgeries and can lead to serious complications. This study aimed to evaluate the effectiveness of serratus anterior plane block (SAPB) combined with oxycodone for transitional analgesia in preventing EA after video-assisted thoracoscopic surgery (VATS). A total of 121 adult patients scheduled for VATS under one-lung ventilation anesthesia were enrolled and randomly divided into three groups: preoperative SAPB without opioids for transitional analgesia near the end of the surgery (SAPB + SAL group, n = 39); preoperative SAPB with sufentanil at 0.1 µg/kg for transitional analgesia (SAPB + SF group, n = 42); and preoperative SAPB with oxycodone at 0.1 mg/kg for transitional analgesia (SAPB + OCD group, n = 40). In primary outcomes, the incidences of EA in the SAPB + SAL, SAPB + SF, and SAPB + OCD groups were 38.5%, 28.6%, and 7.5% respectively. There was a statistically significant difference in EA incidence between the SAPB + OCD and SAPB + SF groups (P = 0.0136). In secondary outcomes, compared to the SAPB + SF group, the SAPB + OCD group experienced shorter tracheal extubation time [15(9, 25) min vs. 21.5(14.5, 32.5) min; P = 0.0473] and PACU stay [67.5(55.0, 85.0) min vs. 87.5(70.0, 110.0) min; P = 0.0026]; lower NRS scores at 15 min and 2 h post-extubation (P < 0.01), and higher Quality of Recovery-15 (QoR-15) scores post-surgery [113(98, 123) vs. 102(88, 112); P = 0.0122]. Our results suggest SAPB combined with oxycodone for transitional analgesia, compared with sufentanil, is more effective in preventing EA after VATS and conductive to rapid recovery postoperatively.
Trial registration: Chinese Clinical Trial Registry, identifier: ChiCTR2300077473, Date: 09/11/2023.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81801-4.
Keywords: Emergence agitation, Oxycodone, Video-assisted thoracoscopic surgery, Serratus anterior plane block, Quality of Recovery-15
Subject terms: Pain, Randomized controlled trials
Introduction
Emergence agitation (EA) is a temporary state of heightened psychomotor activity occurring upon awakening from general anesthesia, characterized by behaviors such as inconsolable agitation, aimless activity, hammering, and disorientation1,2. EA can increase the risk of complications including drainage tube dislodgement, surgical incision rupture, and bleeding. In severe cases, it may lead to hypoxemia, aspiration, falls from the bed, and cardiovascular and cerebrovascular events1. Additionally, managing EA can significantly drain the physical and mental energy of medical staff. It is reported that 31% of EA cases require additional assistance3, with severe instances necessitating the involvement of at least six PACU nurses simultaneously4.
Despite video-assisted thoracoscopic surgery (VATS) being less invasive than traditional thoracotomy5, it still generates considerable postoperative nociceptive stimuli, including incision pain, inflammatory visceral pain, and irritation from medical devices like thick double-lumen tracheal catheters and closed thoracic drainage tubes6–9. EA in adults is primarily an excessive arousal induced by internal and external stimuli during the recovery of consciousness from anesthesia1. Consequently, the higher incidence of EA in patients after thoracic surgery is often linked to these nociceptive stimuli8. To mitigate EA after VATS, both pharmacological and non-pharmacological strategies need to be explored more diligently.
According to the recently published PROSPECT methodology, serratus anterior plane block (SAPB) is recommended as Grade A for postoperative analgesia management after VATS7. SAPB is easy and safe to administer, primarily blocking the long thoracic nerve and the lateral cutaneous branches of the intercostal nerves in the serratus anterior muscle plane, effectively reducing incision pain10–12. However, SAPB may not adequately address inflammatory visceral pain or the discomfort from invasive devices. Anesthesiologists typically administer transitional opioid analgesics like sufentanil, known for its potent analgesic effects, to manage intraoperative and postoperative pain and to prevent or treat EA13,14. Studies have shown that oxycodone, compared to sufentanil, offers superior control over visceral pain following abdominal surgeries14–16 and results in quicker respiratory recovery, shorter wake and extubation times, and higher patient satisfaction after gynecological tumor surgeries17,18. However, it remains unclear whether the use of these two opioids as transitional analgesics can overcome the limitations of SAPB and effectively reduce the incidence of EA after VATS and which is better. Thus, this study aims to compare the efficacy of SAPB combined with either sufentanil or oxycodone transitional analgesia in preventing EA and improving the quality of postoperative analgesia and recovery.
Methods
This study has received approval from the Research and New Technology Ethics Committee of Yijishan Hospital, Wannan Medical College (Ethics number: (2023) Ethical Review and Research (65)), and has been registered in the Chinese Clinical Trial Registry (ChiCTR2300077473, https://www.chictr.org.cn/bin/project/edit?pid=198804, registration date: November 09, 2023). Our research adheres to the principles of Good Clinical Practice and the Helsinki Declaration. We will provide detailed information about the trial process to all participants and obtain written informed consent from them.
Inclusion criteria: Patients aged 18–65 scheduled for VATS under general anesthesia using one-lung ventilation with a double-lumen tube, and classified as American Society of Anesthesiologists (ASA) grade I–II.
Exclusion criteria: Patients with contraindications to SAPB, those suffering from chronic pain, cognitive impairment, opioid abuse or allergies, and patients expected to require admission to the intensive care unit (ICU) post-surgery.
Patients who met the inclusion criteria were recruited the day before surgery. They were introduced to the Numerical Rating Scale (NRS)5,19 for pain assessment to ensure their complete understanding of the scoring system. The NRS scores range from 0 to 10, with 0 indicating no pain, 1–3 indicating mild pain, 4–6 indicating moderate pain, and 7–10 indicating severe pain. The patients were randomly assigned to three groups in a 1:1:1 ratio using a computer-generated number table as follows: SAPB after anesthesia induction without opioid transitional analgesia before the end of surgery (SAPB + SAL group), SAPB combined with sufentanil at 0.1 µg/kg for transient analgesia (SAPB + SF group), and SAPB combined with oxycodone at 0.1 mg/kg for transient analgesia (SAPB + OCD group). To maintain the blinding of outcome assessments, the group assignments of the patients were placed in opaque envelopes and managed by the anesthesiologist in charge.
The general anesthesia protocol proceeded as follows: upon entering the operating room, patients had peripheral veins cannulated and were infused with Lactated Ringer’s solution at a rate of 5–6 ml/kg/h. Vital signs such as electrocardiogram readings, blood pressure, pulse oxygen saturation (SpO2), and body temperature were routinely monitored. Anesthesia was induced following pre-oxygenation, administering midazolam at 0.02 mg/kg, sufentanil at 0.6 µg/kg, etomidate at 0.25 mg/kg, and cisatracurium benzoate at 0.25 mg/kg in sequence. Three minutes post-induction, a double-lumen endotracheal tube was inserted using a video laryngoscope, with correct placement confirmed by fiberoptic bronchoscopy. Throughout the surgery, anesthesia and analgesic depth were maintained with propofol at 4–6 mg/kg/h and remifentanil at 0.2–0.4 µg/kg/h, while muscle relaxation was ensured by continuous administration of cisatracurium benzoate at 0.1–0.15 mg/kg/h. Ephedrine (6 mg) and atropine (0.5 mg) were administered as necessary to manage intraoperative hypotension and bradycardia. Mechanical ventilation settings were adjusted by the anesthesiologist based on the patient’s condition to maintain SpO2 above 90% and end-tidal carbon dioxide partial pressure within 35–45 mmHg.
SAPB was administered following protocols from previous studies12,20. With the patient in a supine position and the arm abducted to 90 degrees, the skin was sterilized using either iodophor or 75% medical alcohol. The ultrasound probe was initially positioned sagittally in the mid-axillary line of the thoracic cage. Identifying the fifth rib in this location involved counting ribs downward and laterally. The latissimus dorsi (superficial and posterior), teres major (superior), and serratus anterior muscles (deep and inferior) were located over the fifth rib. Using an in-plane technique, a needle was advanced and 30 ml of 0.33% ropivacaine was injected under continuous ultrasound guidance, deep to the serratus anterior muscle.
The VATS proceeded with patients in the lateral position. Cisatracurium benzenesulfonate infusion was stopped roughly 20 min before surgery concluded. Approximately 15 min before the end of the procedure, the anesthesiologist opened the assigned opaque envelope and administered oxycodone, sufentanil, or saline intravenously according to the assigned group. All groups underwent sputum aspiration under deep anesthesia, and both propofol and remifentanil were discontinued upon admission to the PACU, where the awakening process began.
Criteria for tracheal extubation included the patient regaining basic consciousness and spontaneous respiratory function, with a tidal volume greater than 6 ml/kg, a respiratory rate between 10 and 20 breaths per minute, and an oxygen saturation above 93% at 60% oxygen concentration. If a patient was not ready for extubation but exhibited significant agitation, mechanical ventilation or assisted breathing was continued after administering a static dose of 1 mg/kg propofol until successful extubation.
Patient behavior was monitored using the Riker Sedation-Agitation Scale (RSAS)1,21 throughout the emergence phase. Once consciousness was regained, pain assessment was conducted using the NRS. To prevent moderate pain, an intravenous analgesia pump set at 3 ml/h delivering a mixture of 100 µg sufentanil and 100 mg flurbiprofen ester diluted in 100 ml saline was used for all groups. As patients were ready to leave the PACU, the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)1,22 was used to check for emergence delirium (ED) in those diagnosed with EA.
The primary outcomes evaluated were the incidence of EA (if RSAS ≥ 5) and oversedation (if RSAS ≤ 3). Secondary outcomes included changes in mean arterial pressure (MAP) and heart rate (HR), tracheal extubation time, PACU stay duration, pain scores within 24 h post-surgery, the incidence of respiratory depression, and quality of recovery 24 h post-surgery, which was assessed using the QoR-15 scale. This scale evaluates the overall recovery experience covering five domains: pain, mental state, emotional state, independence, and comfort.
Sample size calculation and statistical analysis
Using PASS 15 software, the sample size was calculated based on the incidence of EA. In a preliminary study involving 15 patients per group, the incidence rates of EA were 40% in the SAPB + SAL group, 28% in the SAPB + SF group, and 7% in the SAPB + OCD group. Assuming a Type I error of 0.05 and a Type II error of 0.2 (corresponding to 80% power to detect this difference), 33 patients were required in each group. To account for a potential 10% loss to follow-up, at least 37 cases were eventually included in each group.
Data analysis was conducted using SPSS 27.0 (IBM Corp, Armonk, NY, USA) and Graphpad Prism 8.0. All data were summarized as mean ± standard deviation (SD), median (interquartile range: 25–75%), or count (percentage), as appropriate. The Kolmogorov–Smirnov test was used to assess the distribution of data. For normally distributed data, differences between study groups were analyzed using Tukey’s multiple comparisons test, while the Kruskal–Wallis test was used for non-normally distributed data. Categorical data were compared using Fisher’s exact test or the Chi-square test as appropriate.
Results
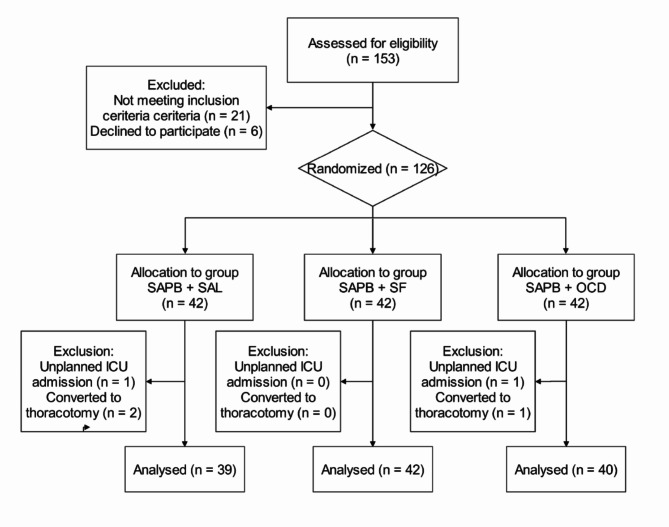
From November 30, 2023, to April 12, 2024, 126 patients were randomized into the SAPB + SAL, SAPB + SF, and SAPB + OCD groups. Any deviations from the inclusion criteria were handled as per the exclusion criteria (Fig. 1).
Fig. 1.
CONSORT (Consolidated Standards of Reporting of Trials) flow diagram of participant recruitment and treatment.
The baseline characteristics of the participants and operation-related variables are summarized in Table 1. There were no statistically significant differences in these characteristics among the three groups.
Table 1.
Baseline characteristics of the patients and operation-related
| Group SAPB + SAL (n = 39) | Group SAPB + SF (n = 42) | Group SAPB + OCD (n = 40) | P value | |
|---|---|---|---|---|
| Age (year) | 55.2 ± 8.2 | 54.6 ± 8.9 | 54.8 ± 8.7 | 0.9399 |
| Gender (male/female) | 14/25 | 12/30 | 16/24 | 0.5443 |
| BMI (kg/m2) | 24.0 ± 3.0 | 23.8 ± 3.9 | 24.0 ± 3.2 | 0.9101 |
| ASA (I/II) | 7/32 | 9/33 | 8/32 | 0.9254 |
| Hypertension (n) | 9 (23.1%) | 7 (16.7%) | 9 (22.5%) | 0.7297 |
| Recent smoking (n) | 5 (12.8%) | 7 (16.7%) | 4 (10.0%) | 0.6698 |
| History of social drinking (n) | 1 (2.6%) | 2 (4.8%) | 3 (7.5%) | 0.5987 |
| Type of surgery (n) (We/Se/Lo) | 2/12/25 | 5/7/30 | 3/8/29 | 0.5011 |
| Sufentanil consumption (µg) | 40.8 ± 5.9 | 40.7 ± 6.7 | 40.2 ± 6.0 | 0.8916 |
| Duration of surgery (min) | 95.9 ± 29.9 | 94.4 ± 29.5 | 96.5 ± 30.1 | 0.9496 |
| Duration of anesthesia (min) | 119.3 ± 30.5 | 118.3 ± 30.6 | 119.7 ± 29.8 | 0.9762 |
Values are presented as mean ± SD, number (%). ASA American Society of Anesthesiologists, BMI body mass index, We Wedge resection, Se segment resection, Lo lobectomy resection.
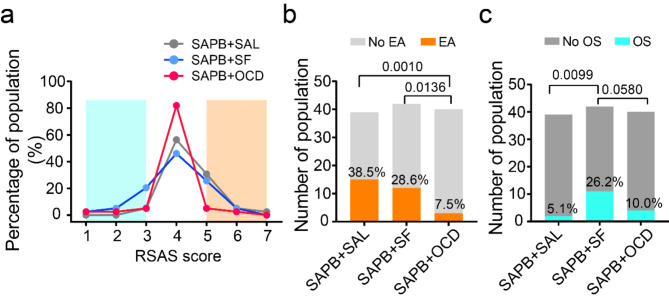
The primary outcome measured the distribution of RSAS score from 1 to 7 across each group, as depicted in Fig. 2a. The incidence of EA was significantly lower in the SAPB + OCD group compared to the SAPB + SAL group (7.5% vs. 38.5%; relative risk (RR) = 5.128; 95% confidence interval (CI), 1.771–15.75; P = 0.0010) and the SAPB + SF group (7.5% vs. 28.6%; RR = 3.810; 95% CI, 1.273–11.99; P = 0.0136) (Fig. 2b). Additionally, the incidence of oversedation was higher in the SAPB + SF group compared to the SAPB + SAL group (26.2% vs. 5.1%; RR = 0.1958; 95% CI, 0.050–0.719; P = 0.0099), and marginally higher than in the SAPB + OCD group (26.2% vs. 10.0%; RR = 2.619; 95% CI, 0.9707–7.355; P = 0.0580). However, there was no significant difference between the SAPB + OCD and SAPB + SAL groups in terms of oversedation (10.0% vs. 5.1%; RR = 2.619; 95% CI, 0.9707–7.355; P = 0.6752) (Fig. 2c).
Fig. 2.
The primary outcome. (a) The line chart presents the percentage of population corresponding to sedation-agitation scale (RSAS) in three groups; The orange shaded part represents emergence agitation, and the turquoise part represents oversedation. OCD oxycodone, SAL saline, SAPB anterior serratus plane block, SF sufentanil. (b,c) The diagrams present the incidence of emergence agitation (EA) (b) and incidence of oversedation (OS) (c); The Chi-square test was used. RSAS criterions1,21: “1” as minimal or no response to noxious stimuli; “2” as arousal to physical stimuli but non-communicative; “3” as difficult to arouse but awakens to verbal stimuli or gentle shaking; “4” as calm and follows commands; “5” as anxious or physically agitated but calms to verbal instructions; “6” as requires restraint and frequent verbal reminding of limits; and “7” as attempting to remove tracheal tube or catheters, or striking at staff.
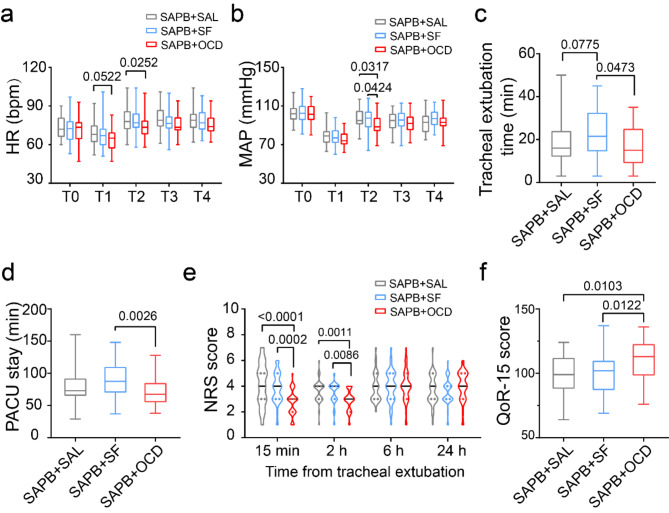
In the secondary outcomes, MAP and HR showed no significant differences among the three groups during the anesthesia recovery period, except immediately before and after tracheal extubation (P < 0.05) (Fig. 3a,b). Compared to the SAPB + SF group, tracheal extubation time was shorter in the SAPB + OCD group [15 (9, 25) min vs. 21.5 (14.5, 32.5) min; P = 0.0473] and the SAPB + SAL group [16 (12, 24) min vs. 21.5 (14.5, 32.5) min; P = 0.0775] (Fig. 3c). PACU stay was significantly shorter in the SAPB + OCD group compared to the SAPB + SF group [67.5 (55.0, 85.0) min vs. 87.5 (70.0, 110.0) min; P = 0.0026] (Fig. 3d). The NRS score at 15 min post-extubation was significantly lower in the SAPB + OCD group compared to both the SAPB + SAL group [3 (2, 3) vs. 4 (3, 5); P < 0.0001] and the SAPB + SF group [3 (2, 3) vs. 4 (3, 5); P = 0.0002]. At 2 h, the NRS score in the SAPB + OCD group remained lower than in the SAPB + SAL [3 (2, 4) vs. 4 (3, 4); P = 0.0011] and the SAPD + SF groups [3 (2, 4) vs. 4 (3, 4); P = 0.0086]. However, at 6 and 24 h post-surgery, there were no significant differences in pain score among the groups (P > 0.05) (Fig. 3e). The 24-h Quality of Recovery-15 (QoR-15) score was higher in the SAPB + OCD group compared to the SAPB + SAL group [113 (98, 123) vs. 99 (88, 112); P = 0.0103] and the SAPB + SF group [113 (98, 123) vs. 102 (88, 112); P = 0.0122] (Fig. 3f).
Fig. 3.
The positive secondary outcome. (a,b) The box diagram presents the heart rate (a) and mean arterial pressure (b) corresponding to time course in the group SAPB + SAL, group SAPB + SF and group SAPB + OCD; the Tukey’s multiple comparisons test was used. T0 pre-anesthesia, T1 pre-extubation, T2 post-extubation 5 min, T3 post-extubation 10 min, T4 post-extubation 30 min, HR heart rate, MAP mean arterial pressure; (c) The box diagram presents the time from discontinue anesthesia to tracheal extubation in three groups; the Kruskal–Wallis test was used. (d) The box diagram presents the time staying in postanesthesia care unit (PACU). The NRS scores range from 0 to 10, with 0 indicating no pain, 1–3 indicating mild pain, 4–6 indicating moderate pain, and 7–10 indicating severe pain. The Kruskal–Wallis test was used. (e) The violin diagram presents the pain score against the numerical rating scale (NRS) within 24 h after tracheal extubation, the Kruskal–Wallis test was used. (f) The box diagram presents the score against quality of requirements-15 (QoR-15) in postoperative 24 h; the Kruskal–Wallis test was used.
Other secondary outcomes showed no significant differences among the three groups and are summarized in Table 2.
Table 2.
The other secondary outcome.
| Group SAPB + SAL (n = 39) | Group SAPB + SF (n = 42) | Group SAPB + OCD (n = 40) | P value | |
|---|---|---|---|---|
| Chest tube indwelling time (day) | 3 (2–4) | 3 (2–3) | 3 (2–3) | 0.9714 |
| Postoperative length of stay (day) | 4 (3–5) | 4 (4–5) | 4 (4–5) | 0.6153 |
| Vomiting and nausea (n) | 10 (25.6%) | 15 (35.7%) | 13 (32.5%) | 0.6109 |
| Respiratory depression (n) | 4 (10.3%) | 7 (16.7%) | 4 (10.0%) | 0.5824 |
| CRBD (n) | 7 (17.9%) | 4 (9.5%) | 5 (12.5%) | 0.5278 |
Values are presented as median (Q1, Q3) or number (%).
CRBD catheter-related bladder discomfort.
Discussion
The study showed that the SAPB combined with oxycodone for transitional analgesia is more effective in preventing EA after VATS, resulting in shorter tracheal extubation times and PACU stays, as well as higher QoR-15 score at 24 h, compared with the SAPB combined with sufentanil for transitional analgesia.
Adult EA can cause serious harm to patients and caregivers. Effective tools for diagnosing and grading EA are crucial for evaluating the effectiveness of EA prevention. In this study, the Riker Sedation-Agitation Scale (RSAS), the first scoring system proven to be reliable in critically ill adult patients1,21, was selected to evaluate EA. RSAS rates awareness and agitation based on seven behaviors, with the main advantage of providing clear criteria for observation to minimize subjectivity1. A score of 4 was classified as the optimal state of anesthesia emergence, a score of ≥ 5 was defined as EA, and a score of ≤ 3 was defined as oversedation.
EA and excitatory ED can be challenging to differentiate due to their similar behaviors. However, there are still some differences between the two, including: EA happens during consciousness recovery, while Excitatory ED occurs with ongoing cognitive issues after consciousness returns. Excitatory ED also has lucid intervals, absent in EA22,23. Considering the above, we believe that what we observed is EA rather than excitatory ED. Moreover, the results of CAM-ICU also supported our opinion.
The total incidence of EA after VATS in the 121 patients included in the study was approximately 25%, higher than in non-cardiac surgery (19%)24. Despite deliberately avoiding the use of inhaled anesthetics, which may increase EA25, the study suggests that EA is still common after VATS, even though VATS is much less invasive than thoracotomy. Fortunately, the incidence of EA varied significantly among different preventive measures in this study (38.5%, 28.6%, and 7.5%), indicating that effective interventions can prevent EA after VATS.
The PROSPECT methodology for VATS, recently published, recommended regional analgesic techniques such as paravertebral block (PVB) and erector spinae plane block (ESPB), with SAPB as a second choice7,20. However, we chose SAPB in this study instead of PVB and ESPB because of its advantages, such as no special requirements on the patient’s position, clear visualization of the puncture needle under ultrasound, and fewer complications such as pneumothorax and puncturing the intercostal artery12. These advantages make it feasible to implement SAPB after the induction of general anesthesia to reduce patient discomfort and panic. We chose the deep layer injection because it is technically easier, particularly in obese patients, and provides more easily recognizable sono-anatomy compared to superficial injection20. Additionally, some studies support that local anesthetics diffuse more easily and provide a wider area of block with deep layer injection7,26. Postoperative local pain tests validated the effect of SAPB in this study.
Our study found a high incidence of EA (38.5%) in the SAPB + SAL group, indicating that while SAPB can provide better regional analgesia, it may lack effectiveness on other nociceptive stimuli related to EA, including catheter stimulation and neuroinflammatory response8. This finding suggests that it is necessary to add transitional analgesics after VATS, especially in patients who are maintained with remifentanil during the operation. Transitional analgesia with opioid receptor agonists is generally preferred in clinical practice. In this study, we compared the effects of 0.1 mg/kg of oxycodone with a 1 µg/kg equivalent dose of sufentanil15.
Our study shows, for the first time, that SAPB combined with oxycodone for transitional analgesia significantly decreases the incidence of EA after VATS, but not sufentanil, indicating that sufentanil’s role in preventing EA after VATS is inferior to oxycodone’s. We speculate the reasons may be as follows: ① Oxycodone can better inhibit tracheal catheter irritation. It is well known that double-lumen tubes may cause greater irritation and choking in patients during the recovery period, and may induce a strong cardiovascular response. Consistent with previous studies15, our study shows that patients with oxycodone exhibited less choking and fewer sharp fluctuations in MAP and HR before and after extubation. ② Oxycodone has a better effect on inhibiting inflammatory visceral pain. The injury to viscera, parietal pleura, and lung during VATS may cause a persistent inflammatory response, and the thoracic drainage tube exacerbates inflammatory visceral pain with respiratory activity during recovery. Previous studies have suggested that oxycodone is better than sufentanil at inhibiting visceral pain and serum inflammatory factors15. The lower NRS score in the SAPB + OCD group within 15 min and 2 h after extubation supports our understanding. There was no difference between groups in NRS score at 6 h and beyond, possibly because oxycodone was administered as a single injection, at which point the drug may have reached an ineffective concentration.
Moreover, the study proves that oxycodone transitional analgesia did not lead to oversedation as sufentanil did. This may be attributed to the unique sedative effects of oxycodone. Previous studies have found that the sufentanil group has lower BIS values and higher respiratory depression than the equal-effect oxycodone group27. Our results were consistent with this, showing an oversedation incidence of 26.2% in the SAPB + SF group and only 10% in the SAPB + OCD group. The incidence of respiratory depression was higher in the SAPB + SF group, but there was no statistical significance between the two groups.
The significant differences in the incidence of EA and oversedation between sufentanil and oxycodone in this study may be due to different opioid receptor mechanisms. Oxycodone activates both µ-receptors and κ-receptors, although it has a lower affinity for κ-receptors28,29. In contrast, sufentanil selectively activates µ-receptors and has little effect on κ-receptors, which are significantly related to visceral pain30,31. Therefore, oxycodone for transitional analgesia after VATS may provide more comprehensive postoperative acute pain relief than sufentanil. However, in this study, we cannot attribute the benefits solely to oxycodone and must consider the combined effect of SAPB. The advantages of oxycodone over sufentanil are likely based on its ability to inhibit predominantly visceral pain. In this study, shorter tracheal extubation times, shorter PACU stays, and higher 24-h QoR-15 scores are likely due to the combined effects of both SAPB and oxycodone. A higher 24-h QoR-15 score indicates a better recovery experience as perceived by the patients themselves32.
There are some limitations to our study. First, due to the small sample size, we did not include more groups to verify the effects and side effects of different doses of oxycodone or to compare non-opioid drugs such as dexmedetomidine and non-steroidal analgesics recommended in the PROSPECT methodology for VATS, which could help explore better preventive strategies. Second, since the primary outcome of this study was the incidence of EA, we did not collect blood markers of inflammatory factors, sympathetic nervous system activity, or hypothalamic-pituitary-adrenal axis-related hormones to substantiate evidence associated with inflammatory visceral pain. Third, our study did not utilize depth of anesthesia monitoring, which is generally regarded as beneficial for postoperative recovery in certain patients. Nevertheless, we are confident that this omission did not affect our outcomes, since none of the three patient cohorts underwent this monitoring. Moreover, the utility and dependability of anesthesia depth monitoring continue to be a topic of ongoing discussion within the medical community33–35.
In conclusion, SAPB combined with oxycodone for transitional analgesia can effectively prevent EA after VATS without leading to more side effects such as oversedation and respiratory depression, which is conducive to rapid postoperative recovery of patients. However, more studies with larger sample sizes should be conducted to justify the findings of our study, as relevant research is insufficient and our study has limitations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ASA
American Society of Anesthesiologists
- BIS
Bispectral index
- BMI
Body Mass Index
- CAM-ICU
Confusion Assessment Method for the Intensive Care Unit
- CI
Confidence Interval
- CRBD
Catheter-Related Bladder Discomfort
- EA
Emergence Agitation
- ED
Emergence Delirium
- ESPB
Erector Spinae Plane Block
- HR
Heart Rate
- ICU
Intensive Care Unit
- Lo
Lobectomy resection
- MAP
Mean Arterial Pressure
- NRS
Numerical Rating Scale
- OCD
Oxycodone
- OS
Oversedation
- PACU
Postanesthesia Care Unit
- PVB
Paravertebral Block
- QoR-15
Quality of Recovery-15
- RR
Relative Risk
- RSAS
Riker Sedation-Agitation Scale
- SAL
Saline
- SAPB
Serratus Anterior Plane Block
- SD
Standard Deviation
- Se
Segment resection
- SF
Sufentanil
- SpO2
Pulse Oxygen Saturation
- T0
Pre-anesthesia
- T1
Pre-extubation
- T2
Post-extubation 5 min
- T3
Post-extubation 10 min
- T4
Post-extubation 30 min
- VATS
Video-Assisted Thoracoscopic Surgery
- We
Wedge resection
Author contributions
Experiment design: YLW, YQC Data collection/analysis: TW, YLW, QBW, ZJH, HC, WC, LLZ, JKH Writing of paper: YLW, TW, YQC, WC, HC Revision of paper: All authors.
Funding
This study was supported by funding from Anhui University Research Project (No. 2023AH040252, 2023AH051745), Natural Science Foundation of Anhui Province (No. 2208085MC55), Wannan Medical College Research Fund Project (No. WK2022F03), the Key Project of Anhui Province ‘Leading the Charge with Open Competition’, and Anhui Province Clinical Key Specialty Construction Project.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The protocol was approved by the Scientific Research and New Technology of Wannan Medical College Yijishan Hospital IRB (Ethical Approval Number: 2023.65) on June 20, 2023. All methods were performed in accordance with relevant guidelines and regulations and with CONSORT recommendations. The study was registered in the Chinese Clinical Trial Registry (Identifier: ChiCTR2300077473, Date: 09/11/2023). Before participation, all patients and/or their legal guardians provided written informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Wang, Qiu-Bo Wang and Zi-Jun Hou contributed equally to this work.
Contributor Information
Yu-Long Wang, Email: dr_wangyulong@163.com.
Yong-Quan Chen, Email: chenyq263@163.com.
References
- 1.Tolly, B. et al. Adult emergence agitation: a veteran-focused narrative review. Anesth. Analg.132, 353–364. 10.1213/ane.0000000000005211 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Safavynia, S. A., Arora, S., Pryor, K. O. & García, P. S. An update on postoperative delirium: clinical features, neuropathogenesis, and perioperative management. Curr. Anesthesiol. Rep.8, 252–262 (2018). [PMC free article] [PubMed] [Google Scholar]
- 3.Munk, L., Andersen, G. & Møller, A. M. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol. Scand.60, 1059–1066. 10.1111/aas.12717 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Hudek, K. Emergence delirium: a nursing perspective. AORN J.89, 509–516. 10.1016/j.aorn.2008.12.026 (2009) (quiz 517 – 509). [DOI] [PubMed] [Google Scholar]
- 5.Bendixen, M., Jørgensen, O. D., Kronborg, C., Andersen, C. & Licht, P. B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol.17, 836–844. 10.1016/s1470-2045(16)00173-x (2016). [DOI] [PubMed] [Google Scholar]
- 6.Holbek, B. L., Petersen, H., Kehlet, R., Hansen, H. J. & H. & Fast-track video-assisted thoracoscopic surgery: future challenges. Scand. Cardiovasc. J.50, 78–82. 10.3109/14017431.2015.1114665 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Feray, S., Lubach, J., Joshi, G. P., Bonnet, F. & Van de Velde, M. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia77, 311–325. 10.1111/anae.15609 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, A., Huang, J., Schroeder, D., Sprung, J. & Weingarten, T. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br. J. Anaesth.121, 1052–1058. 10.1016/j.bja.2018.07.017 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Sun, K. et al. Moderate-severe postoperative pain in patients undergoing video-assisted thoracoscopic surgery: a retrospective study. Sci. Rep.10, 795. 10.1038/s41598-020-57620-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, J. Q., Yang, X. L., Gu, H., Chai, X. Q. & Wang, D. The role of Serratus Anterior Plane Block during in video-assisted thoracoscopic surgery. Pain Ther.10, 1051–1066. 10.1007/s40122-021-00322-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, M. H. et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia73, 1260–1264. 10.1111/anae.14424 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Blanco, R., Parras, T., McDonnell, J. G. & Prats-Galino, A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia68, 1107–1113. 10.1111/anae.12344 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Zeng, K., Long, J., Li, Y. & Hu, J. Preventing postoperative cognitive dysfunction using anesthetic drugs in elderly patients undergoing noncardiac surgery: a systematic review and meta-analysis. Int. J. Surg.109, 21–31. 10.1097/js9.0000000000000001 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, X. et al. Comparison of oxycodone and sufentanil in patient-controlled intravenous analgesia for postoperative patients: a meta-analysis of randomized controlled trials. Chin. Med. J. (Engl). 136, 45–52. 10.1097/cm9.0000000000002259 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An, Y. et al. Preemptive oxycodone is superior to equal dose of sufentanil to reduce visceral pain and inflammatory markers after surgery: a randomized controlled trail. BMC Anesthesiol. 19, 96. 10.1186/s12871-019-0775-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Y. et al. Oxycodone versus other opioid analgesics after laparoscopic surgery: a meta-analysis. Eur. J. Med. Res.26. 10.1186/s40001-020-00463-w (2021). [DOI] [PMC free article] [PubMed]
- 17.Kim, N. S. et al. Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: a prospective, randomized, double-blind study. Medicine (Baltim).96, e6286. 10.1097/md.0000000000006286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang, S. J. et al. Oxycodone vs sufentanil in patient-controlled intravenous analgesia after gynecological tumor operation: a randomized double-blind clinical trial. J. Pain Res.13, 937–946. 10.2147/jpr.S236933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdel, N. et al. Systematic review of endometriosis pain assessment: how to choose a scale? Hum. Reprod. Update. 21, 136–152. 10.1093/humupd/dmu046 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Finnerty, D. T. et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br. J. Anaesth.125, 802–810. 10.1016/j.bja.2020.06.020 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Riker, R. R., Picard, J. T. & Fraser, G. L. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit. Care Med.27, 1325–1329. 10.1097/00003246-199907000-00022 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Evered, L. et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth.121, 1005–1012. 10.1016/j.bja.2017.11.087 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez, B. A. et al. Post-anaesthesia care unit delirium: incidence, risk factors and associated adverse outcomes. Br. J. Anaesth.119, 288–290. 10.1093/bja/aex197 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Card, E. et al. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br. J. Anaesth.115, 411–417. 10.1093/bja/aeu442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo, J. Y. et al. Effect of total intravenous anesthesia vs volatile induction with maintenance anesthesia on emergence agitation after nasal surgery: a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg.145, 117–123. 10.1001/jamaoto.2018.3097 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piracha, M. M., Thorp, S. L., Puttanniah, V. & Gulati, A. A tale of two planes: deep versus superficial serratus plane block for postmastectomy pain syndrome. Reg. Anesth. Pain Med.42, 259–262. 10.1097/aap.0000000000000555 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Zhang, J. et al. Preemptive anti-stress response effects of oxycodone versus sufentanil for patients undergoing cardiac valve replacement—a randomized controlled trial. Clin. Pharmacol. Drug Dev.9, 321–329. 10.1002/cpdd.764 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Nielsen, C. K. et al. Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain132, 289–300. 10.1016/j.pain.2007.03.022 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Raff, M. et al. Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain Therapy8, 19–39. 10.1007/s40122-019-0122-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengupta, J. N., Su, X. & Gebhart, G. F. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology111, 968–980. 10.1016/s0016-5085(96)70064-1 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Simonin, F. et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J.17, 886–897. 10.1093/emboj/17.4.886 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myles, P. S. et al. Systematic review and consensus definitions for the standardised endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br. J. Anaesth.120, 705–711. 10.1016/j.bja.2017.12.037 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Ni, K. et al. Paradox of age: older patients receive higher age-adjusted minimum alveolar concentration fractions of volatile anaesthetics yet display higher bispectral index values. Br. J. Anaesth.123, 288–297. 10.1016/j.bja.2019.05.040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wildes, T. S. et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA321, 473–483. 10.1001/jama.2018.22005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deschamps, A. et al. Electroencephalography-guided anesthesia and delirium in older adults after cardiac surgery: the ENGAGES-Canada Randomized Clinical Trial. JAMA332, 112–123. 10.1001/jama.2024.8144 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.