Abstract
Background
Androgenic alopecia (AGA) is commonly known as male patterned baldness. A high level of dihydrotestosterone (DHT) plays a significant role in AGA development. Inhibition of the enzyme steroid 5-alpha reductase (S5AR), responsible for converting testosterone into DHT, has been shown to delay the progression of AGA. Teak (Tectona grandis L.f) leaf extract exhibited a potent S5AR inhibitory activity. To prove the effectiveness and safety of teak leaf extract as a hair growth promotor, a double-blind, randomized placebo-controlled trial was conducted.
Methods
Eighty-one AGA subjects were randomly assigned to receive either a hair tonic containing 1% teak leaf extract (HT-teak), 5%minoxidil (positive control), or a placebo administered twice daily, for 24 weeks. Efficacy was assessed through target area hair count (TAHC), anagen-to-telogen ratio (A/T), hair shedding every 4 weeks, and patients’ subjective assessments of hair regrowth were assessed at the end of the experiment. Data was analyzed using repeated measure ANOVA.
Results
Both the HT-teak and minoxidil groups exhibited a significant increase in TAHC and A/T, along with a decrease in hair shedding compared to baseline values. Conversely, the placebo group showed no observable signs of hair regrowth. Furthermore, the HT-teak group reported the highest satisfaction scores, and there were no indications of skin irritation or systemic effects on sexual dysfunction and palpitation after 24 weeks of HT-teak application.
Conclusion
Teak leaf extract, as incorporated in HT-teak, demonstrates potential as an alternative mild hair growth promoter for individuals with AGA, offering both efficacy and safety.
Trial registration
This study was retrospectively registered on International Standard Randomised Controlled Trial Number (ISRCTN.com); ISRCTN24541842 (registered on January 8, 2024).
Keywords: androgenic alopecia, hair growth, tectona grandis l.f, teak, 5-alpha reductase inhibitor, clinical trial
Introduction
Androgenic alopecia (AGA) commonly known as male patterned baldness, is characterized by hair thinning mainly at the vertex and temporal areas of the scalp. The pathogenesis of AGA is still debated, but the excessive level of dihydrotestosterone (DHT), is considered one significant cause for AGA. DHT is a more potent androgen that exhibits an affinity for binding with androgen receptors (ARs) and is converted from testosterone by the enzyme 5-alpha reductase (S5AR). 1 When binding to the androgen receptors, this promotes the miniaturization of hair follicles by disturbing the normal hair cycle which is classified into 3 phases: anagen (growth phase), catagen (regression phase), and telogen (resting phase). 2 The AGA pathogenesis hypothesis involves the increasing level of pro-inflammatory cascade, including reactive oxygen species, COX-2 signalling, IL-1, and TNF-α. 3 Therefore, the S5AR inhibitor, finasteride, which is orally administered, as well as the topical vasodilator, minoxidil, are the first-line drugs for AGA treatment approved by the United States Food and Drug Administration (FDA). Both finasteride and minoxidil have shown positive results in hair regrowth application. 4 However, long-term oral use of finasteride may lead to systemic adverse effects on the male reproductive system 5 and skin irritation has also been reported after applying minoxidil. 6 Therefore, alternative hair growth promotors, which might be safer, have gained attention in recent research.7,8
Tectona grandis L.f, commonly known as teak, is a prominent timber species endemic to Southeast Asia. Teak wood is highly valued, but the leaves have no economic value. However, teak leaves have demonstrated various biological properties, such as wound healing, 9 as an antidiabetic,10,11 and as a treatment for anti-hemolytic anemia. 12 Traditionally, teak seeds have been used as a hair tonic in India. Jaybhaye, D. et al 13 found that petroleum-ether extract of teak seeds could stimulate hair growth in albino mice. Recently, various parts of teak had been evaluated for S5AR inhibitory activity and the results revealed that teak leaves have the highest activity against S5AR, alongside other biological activities relevant to hair regrowth, including anti-testosterone effects on Human Follicle Dermal Papilla Cells (HFDPCs) and the inhibition of interleukin-1β (IL-1β) secretion. 14 Furthermore, the diterpenes, namely (+)-Eperua-8,13-dien-15-oic acid (cpd.1), and (+)-Eperua-7,13-dien-15-oic acid (cpd.2), were isolated from teak leaf extract and exhibited S5AR inhibitory activity with IC50 values of 14.19 ± 2.87 µM, and 14.65 ± 0.31 µM. 15
To underscore the potential of teak leaf extract as an alternative topical hair growth promotor, the effectiveness and safety of a hair tonic containing 1% teak leaf extract (HT-teak) were evaluated compared to 5% minoxidil and a placebo, through a randomized double-blind controlled trial.
Materials and Methods
Study Design
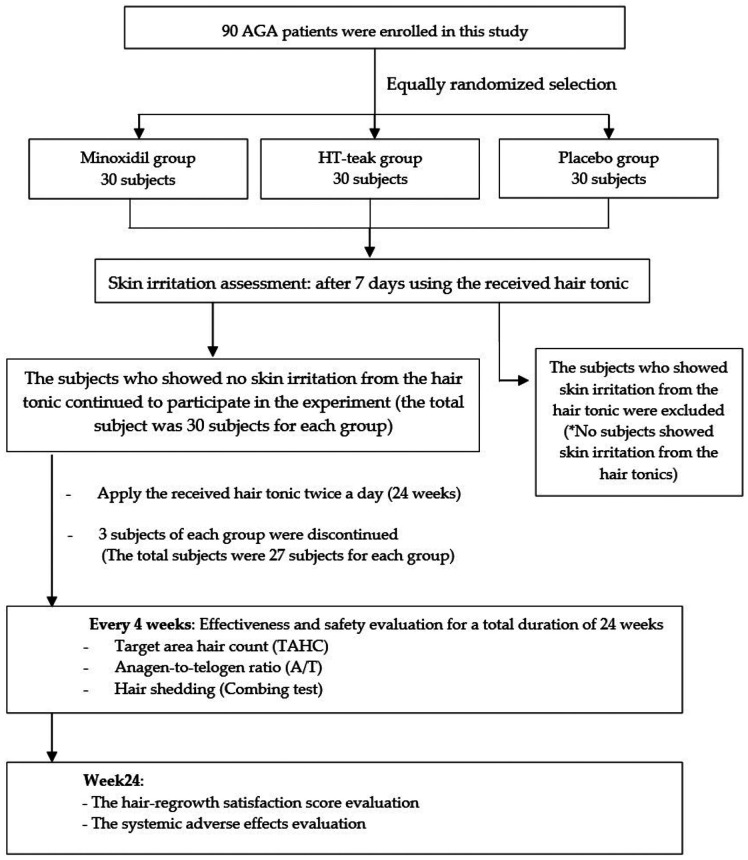
This study was a double-blind, randomized placebo-controlled trial in 90 AGA subjects (Clinical registration; ISRCTN24541842). The evaluation process was performed every 4 weeks for 24 weeks at the Cosmetics and Natural Products Research Center (CosNat), Naresuan University. The protocol was approved by Naresuan University Institutional Review Board (NU-IRB), IRB number P10123/64. The subjects were randomly divided equally to receive 1% teak leaf extract (HT-teak), 5% minoxidil (positive control), or placebo. Randomization was processed through the function of Microsoft Excel. The subjects had to apply 3–5 drops (0.5 ml) of the received product twice a day (morning and night) at the scalp vertex and the temporal scalp. Each subject was assigned a Unicode blinded to the subjects and investigators when enrolled, and all subjects received the same shampoo formula for washing their hair at least once a day. At the end of study period user satisfaction questionnaires were completed by the subjects, and interviews conducted by the assigned dermatologist were held to ascertain the occurrences of systemic side effects, such as the male sexual dysfunctions and palpitations. The study evaluation protocol is concisely outlined in Figure 1.
Figure 1.
Flow diagram illustrating the subject enrollment and study evaluation protocol.
Subjects
The sample size was calculated based on the previous research study. 16 Employing a significance level of 0.05 and a test power of 80%, the accepted sample size was set at 25 subjects per group, resulting in a total of 75 subjects. To account for potential attrition, with an anticipated 20% loss of follow-up, the total sample size was adjusted to 90 subjects. Inclusion criteria were (1) men with AGA described by Hamilton-Norwood type II to V, (2) aged between 20–60 years old. The subjects were excluded if they (1) had scalp lesions; (2) were sensitive to any hair growth promotors or minoxidil; (3) used other hair-regrowth products 3 months and/or minoxidil 6 months before enrolling in this project; (4) took systemic steroids for more than 14 days within the 2 months before baseline evaluation; (5) had a medical history of radiation on the scalp; (6) had chronic diseases such as kidney disease, heart disease, or uncontrolled blood pressure. All subjects were enrolled by the assigned dermatologist. The subjects who had an allergy to the products used were withdrawn. The subjects were not allowed to use other hair growth products and were required to apply at least 80% of the provided products. This was a requirement that was checked every 4 weeks. The subjects were mandated to sign a consent form before participating in the study.
Test Preparation
Raw Materials and Extraction
Teak leaves were harvested from Nakhon Nayok Province, Thailand. The plant material was authenticated by Assistant Prof. Dr Pranee Nangngam from the Faculty of Science at Naresuan University. A voucher specimen (number 05721) was kept at the Department of Biology, Faculty of Science, Naresuan University, Phitsanulok, Thailand. The appropriate permissions for the collection and use of plant material were followed by the Plant Varieties Protection Act B.E. two thousand and five hundred and forty-two (1999) of Thailand. Teak leaves were dried, ground to powder and were extracted through cold ethanol maceration. The power-solvent ratio was 1:8 and the maceration time was 60 min. The teak leaf extract contained 20% w/w of cpd.1% and 9% cpd.2 analysed using validated high-performance liquid chromatography (HPLC) condition (Supplementary data). 15
HT-Teak Preparation and Other Interventions
The component of HT-teak was predominantly water, presenting itself as a liquid solution. The formulation contained 1% of the teak leaf extract as an active ingredient.
The interventions were prepared in three variations, (1) an HT-teak (2) 5% minoxidil, and (3) a placebo, which was a base of the HT-teak. These hair tonics were packaged in identical packaging with different codes.
Stability Study
Triplicate samples of HT-teak were kept at accelerating condition (50°C) for 4 weeks. The remains of the cpd.1 and cpd.2 were evaluated by HPLC. 15 The shelf life was calculated using the Q10 method.17,18
Skin Irritation Assessment
Skin irritation assessment was adapted from the patch test, 17 modified to involve applying the products to the target area for 7 days. The severity of irritation was classified using a skin irritation score.17,18 The assessment was conducted by dermatologist. Subjects who experienced skin irritation such as dryness, redness, rash, and other common indicators of irritation due to the received hair tonic were excluded.
Efficacy Evaluation
Target Area Hair Count (TAHC) and Anagen-to-Telogen Ratio (A/T)
The permanent ink dot was marked at the center of the 1 cm2 target area on the scalp to evaluate Novellus hair/cm2 using macro-photograph trichoscopy Leviacam®. Assessments were conducted at baseline and subsequently every 4 weeks for 24 weeks. Trichoscopy software generated data for TAHC, anagen hair, and telogen hair, which counted and categorized hair types based on their length and thickness in the microphotographs of the target scalp area. This data was normalized into means of percentage change of TAHC and A/T ratio which were investigated and compared between baseline and at weeks 4, 8, 12, 16, 20, and 24.
Hair Shedding (Combing Test)
Subjects gently combed their hair from vertex to temporal regions on both the left and right sides of the scalp, using the same type of comb. Hair shedding was counted by an investigator who was blinded to the product assignments. The mean of difference change in hair shedding was compared across baseline and weeks 4, 8, 12, 16, 20, and 24.
Adverse Events
Dermatological lesions, including redness, rash, itch, and dryness on the scalp were examined every 4 weeks until the end of the study using the same skin irritation score mentioned above. Systemic adverse events related to the male reproductive system and palpitations were also evaluated through interviews. These evaluations were conducted by the assigned dermatologist.
Satisfaction Score Evaluation
At the end of the study (week 24), subjects completed user satisfaction questionnaires independently. The questionnaires utilized a 7-point scale: −3 indicating significantly worse, −2 moderately worse, −1 minimally worse, 0 no change, + 1 minimally improved, + 2 moderately improved, and +3 significantly improved. The mean scores were calculated and compared between the groups.
Statistical Analysis
The percentage changes of TAHC, A/T ratio, and different changes of hair shedding were expressed as mean ± standard errors (SEs), while the hair growth satisfaction scores were calculated as mean ± standard deviation (SD). Data analysis was performed using IBM SPSS statistics for Windows, version 26. The results were compared across different time points with baseline and between groups using repeat measure ANOVA, estimated mean effects, and the confidence interval adjustments via Bonferroni correction. Significant differences were classified at a 95% confidence interval (p-value < 0.05)
Results
Subject Population
Of the 90 male subjects originally enrolled, 9 dropped out for personal reasons unrelated to the treatment. Data analysis was compiled from the remaining 81 subjects who successfully completed the study. The age range of the subjects was 20–57 years old, with respective group means of 41.74, 43.85, and 40.22 years old. The baseline scores for the TAHC in the three groups did not exhibit significant differences (p-value > 0.05) (Table 1).
Table 1.
Subject Demographics Data at Baseline.
| Demographics | Treatment groups | ||
|---|---|---|---|
| 5% minoxidil | HT-teak | Placebo | |
| N | 27 | 27 | 27 |
| Age (years) | |||
| Range | 24–57 | 20–56 | 22–57 |
| Mean | 41.74 | 43.85 | 40.22 |
| SD | 8.64 | 8.27 | 9.09 |
| Hamilton-Norwood | |||
| Type II | 10 (37.04%) | 9 (33.33%) | 7 (25.93%) |
| Type III | 8 (29.63%) | 12 (44.44%) | 11 (40.74%) |
| Type IV | 7 (25.93%) | 3 (11.11%) | 4 (14.81%) |
| Type V | 2 (7.41%) | 3 (11.11%) | 5 (18.52%) |
| Target area hair counts (TAHC) | |||
| Mean (SE) | 126.60 (4.93) | 124.74 (4.38) | 120.73 (5.34) |
Target Area Hair Count (TAHC)
A significant difference in the percentage change of TAHC between the placebo and treatment groups (minoxidil and HT-teak) was observed after 12 weeks of application. Additionally, a significant increase in the percentage change of TAHC in the HT-teak group was identified at 12 weeks when compared with baseline (p-value = 0.023). Minoxidil also showed a significant difference compared to the placebo group at week 24. Although the percentage change in TAHC consistently demonstrated an increasing trend from baseline throughout the study following the application of HT-teak, the increases in TAHC were not statistically significant at weeks 16, 20, and 24. Likewise, minoxidil also exhibited a similar trend of increasing TAHC from baseline, with a significant increase observed at week 24 (p-value = 0.010). In contrast, the placebo group did not display statistically significant differences in TAHC at any of the assessment points throughout the study period (Table 2).
Table 2.
The Percentage Change of Target Area Hair Count (TAHC).
| Evaluation time | % TAHC (mean ± SE) | Pairwise Comparisons (p-value) | |||
|---|---|---|---|---|---|
| Minoxidil | HT-teak | Placebo | Minoxidil versus Placebo | HT-teak versus Placebo | |
| Baseline | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - | - |
| Week 4 | 102.61 ± 1.81 | 102.95 ± 1.52 | 98.23 ± 2.59 | 0.404 | 0.324 |
| Week 8 | 106.11 ± 2.22 | 105.44 ± 2.47 | 100.93 ± 1.54 | 0.272 | 0.420 |
| Week 12 | 105.93 ± 1.83 | 106.45 ± 2.32* | 96.82 ± 1.39 | 0.003 | 0.002 |
| Week 16 | 103.69 ± 2.77 | 105.18 ± 2.14 | 99.08 ± 1.82 | 0.473 | 0.190 |
| Week 20 | 102.40 ± 2.31 | 104.55 ± 1.83 | 97.01 ± 3.03 | 0.382 | 0.103 |
| Week 24 | 107.36 ± 2.25* | 105.04 ± 2.13 | 99.57 ± 1.54 | 0.023 | 0.177 |
; significant difference compared with baseline at p-value < 0.05.
Anagen to Telogen Ratio (A/T Ratio)
The percentage change in the A/T ratio showed a significant difference between HT-teak and placebo at week 24, while the percentage change in the A/T ratio in the minoxidil group was significantly higher than shown in the placebo group, at week 20, and 24. Furthermore, the percentage change in the A/T ratio of HT-teak application exhibited a significant increase from baseline at week 24 week (p-value = 0.002), while the application of minoxidil showed a significant increase in the A/T ratio at week 20 (p-value = 0.026).
Notably, the application of both hair growth promoters, minoxidil and HT-teak, resulted in a 27% and 39% increase in the A/T ratio at week 24, respectively, whereas subjects who applied the placebo showed no increase in the A/T ratio. This finding suggests that HT-teak and minoxidil can prolong the anagen hair phase and shorten the telogen hair phase (Table 3). Additionally, Figure 2 showed an example TrichoScans of a volunteer who applied HT-teak which indicated an increase in TAHC and anagen hair, while telogen hair decreased. Furthermore, the observed increase in terminal hair and reduction in vellus hair from 4:1 to 5:1 in terminal to vellus ratio resulting from the elevated A/T ratio. This suggests that prolonged anagen phase is associated with the production of thick pigmented terminal hairs, whereas a shortened anagen phase may result in the production of fine short less pigmented vellus hairs. The trichoScans of minoxidil and placebo were also included in the supplementary data.
Table 3.
The Percentage Change of A/T Ratio.
| Evaluation time | % A/T ratio (mean ± SE) | Pairwise comparisons (p-value) | |||
|---|---|---|---|---|---|
| Minoxidil | HT-teak | Placebo | Minoxidil versus Placebo | HT-teak versus Placebo | |
| Baseline | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - | - |
| Week 4 | 114.30 ± 9.70 | 121.55 ± 9.84 | 97.70 ± 11.81 | 0.800 | 0.336 |
| Week 8 | 131.67 ± 11.66 | 129.60 ± 9.16 | 100.37 ± 13.76 | 0.186 | 0.242 |
| Week 12 | 132.65 ± 10.80 | 133.15 ± 9.76 | 103.44 ± 13.46 | 0.225 | 0.211 |
| Week 16 | 132.18 ± 10.14 | 127.31 ± 11.44 | 95.36 ± 10.48 | 0.052 | 0.114 |
| Week 20 | 135.89 ± 11.51* | 127.16 ± 7.51 | 95.80 ± 12.40 | 0.029 | 0.124 |
| Week 24 | 127.34 ± 8.57 | 139.22 ± 10.23* | 91.00 ± 9.71 | 0.026 | 0.002 |
; significant difference compared with baseline at p-value < 0.05.
Figure 2.

Example trichoScan images taken from the target area of the scalp (20-fold magnification), comparing baseline and week 24 from the HT-teak group. The hair types are characterized by trichoscopy software. The upper images show hair characterized by length: green indicates anagen hair, and red indicates telogen hair. The lower images show hair characterized by thickness: blue indicates terminal hair, and yellow indicates vellus hair.
Hair Shedding (Combing Test)
The results shown in Table 4 indicate a significantly different change in hair shedding between the HT-teak and the placebo at week 4, and 16. When comparing the difference change in hair shedding to baseline, HT-teak application showed a significant decrease within 4 weeks. Additionally, both the minoxidil and HT-teak treatment groups exhibited a decreasing trend after application, in contrast with subjects who applied the placebo. Furthermore, a substantial number of subjects reported a decrease in hair shedding, with percentages of 40.74%, and 48.15% among those who used minoxidil and HT-teak, respectively, whereas only 18.50% of the subjects who used placebo reported a decrease in hair shedding (Table 4).
Table 4.
Difference Changes from Baseline of Hair Shedding.
| Evaluation time | Difference change of hair shed (mean ± SE) | Pairwise comparisons (p-value) | |||
|---|---|---|---|---|---|
| Minoxidil | HT-teak | Placebo | Minoxidil versus Placebo | HT-teak versus Placebo | |
| Baseline | 0.00 ± 0.00 | 0.00 ± 0.00 | 00.00 ± 0.00 | ||
| Week 4 | −2.48 ± 1.81 | −5.15 ± 1.81* | 0.48 ± 0.94 | 0.563 | 0.041 |
| Week 8 | −1.48 ± 1.68 | −3.78 ± 1.73 | 1.00 ± 0.74 | 0.697 | 0.069 |
| Week 12 | −1.22 ± 1.40 | −2.19 ± 1.95 | 1.59 ± 0.82 | 0.534 | 0.216 |
| Week 16 | 1.00 ± 2.28 | −3.26 ± 1.79 | 4.67 ± 1.16 | 0.462 | 0.008 |
| Week 20 | −1.00 ± 1.74 | −1.67 ± 2.15 | 0.22 ± 0.51 | 1.000 | 1.000 |
| Week 24 | −0.96 ± 1.96 | −0.59 ± 2.44 | 2.63 ± 0.79 | 0.531 | 0.676 |
| % subjects who decrease hair shedding (week24) | 40.74% (n = 11) | 48.15% (n = 13) | 18.5% (n = 5) | - | - |
; Significant Difference Compared with Baseline at p-Value < 0.05.
The Hair-Regrowth Satisfaction
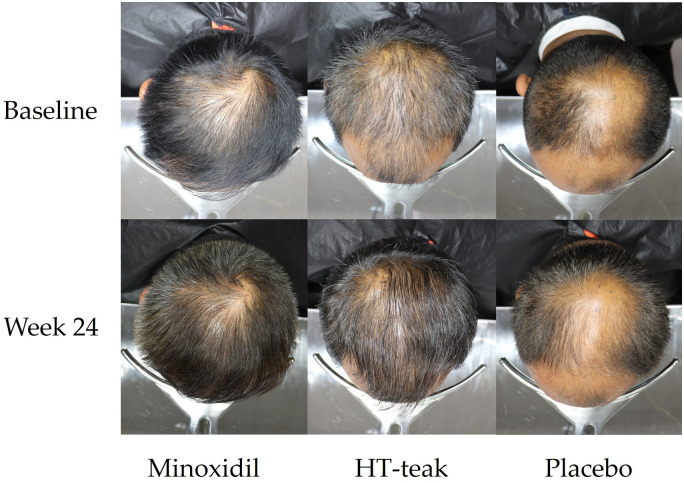
From the subject's hair-regrowth satisfaction assessment (Table 5), all groups (5% minoxidil, HT-teak and placebo) showed either no change (3.70% for minoxidil and 22.22% for placebo) or some improvement (96.30% for minoxidil, 100% for HT-teak and 77.78% for placebo). The highest mean score for overall hair changes was 2.04 ± 0.71 which was for the HT-teak group, followed by minoxidil (1.82 ± 0.72) and placebo (1.43 ± 1.44). Moreover, the assessment from the HT-teak group indicated a significant difference compared to the placebo group at p = 0.037. Furthermore, the visual examination of vertex area photographs for each group, comparing baseline and week 24 (Figure 3), provided additional support for the observed satisfaction with hair regrowth scores.
Table 5.
The Subject's Hair-Regrowth Satisfaction Assessment.
| Assessment of overall changes | 5% minoxidil | HT-teak | Placebo |
|---|---|---|---|
| −3 = significantly worse | 0 | 0 | 0 |
| −2 = moderately worse | 0 | 0 | 0 |
| −1 = minimally worse | 0 | 0 | 0 |
| 0 = no change | 1 (3.70%) | 0 | 6 (22.22%) |
| +1 = minimally improved | 7 (25.93%) | 6 (22.22%) | 9 (33.33%) |
| +2 = moderately improved | 15 (55.56%) | 14 (51.85%) | 5 (18.52%) |
| +3 = significantly improved | 4 (14.81%) | 7 (25.93%) | 7 (25.93%) |
| Mean ± SD | 1.82 ± 0.74ab | 2.04 ± 0.71a | 1.43 ± 1.44b |
: significant difference between groups at p-value < 0.05.
Figure 3.
The representative vertex area photographs of subjects who applied minoxidil, HT-teak, or placebo, at day0 (baseline) and week24. Two additional representative vertex area photographs from each treatment group were provided in the supplementary data.
Skin Irritation and Adverse Effects
No skin irritation was reported among the subjects who applied any of the three hair tonic formulae after 7 days of application, and there was no report of skin irritation after application of HT-teak and placebo for 24 weeks. However, two subjects from the minoxidil group reported minimal dryness and itchiness which did not significantly impact their daily life, and they were able to continue using the assigned products until the end of the study. No sign of any effect on male reproductive and cardiovascular system was observed in any of the groups.
Stability
The stability of cpd.1 and cpd.2 in teak leaf extract had been previously evaluated under the conditions of 50–80°C for 6 months. The shelf life of the extract was determined to be 1.48 years. 17 Additionally, the stability of these markers in HT-teak formulation was confirmed, with results consistent with those obtained from the extract. HT-teak exhibited stability under conditions of 50°C for 4 weeks, suggesting a shelf life of at least 1 year when stored at 25°C according to the Q10 method of self-life estimation.19,20
Discussion
There are several treatment options for AGA, including oral and topical administration of drugs such as finasteride, dutasteride, and minoxidil. Other methods include low-level laser therapy (LLLT), platelet rich plasma (PRP), the use of stem cells, microneedling, and a hair transplant, which are also viable options for treating AGA.21,22 However, natural ingredients are increasingly being utilized for the treatment of AGA due to growing evidence supporting their effectiveness, safety, accessibility, and cost-effectiveness. Topical formulations containing natural extracts have been evaluated for their effectiveness and safety in human subjects such as saw palmetto (Serenoa repens (W.Bartram) Small), 23 rosemary oil, 24 procyanidin from apple juice, 25 Curcuma aeruginosa, 16 and Trifolium pratense. 26 The results have demonstrated promising outcomes for their influence on hair growth. Among these, S. repens, C. aeruginosa, and T. pratense exhibited the mechanism to stimulate hair growth through the inhibition of the S5AR enzyme.16,23,26 Additionally, teak leaf extract has emerged as a prospective active ingredient for promoting hair growth, primarily due to its ability to inhibit the S5AR enzyme and it also exhibits other activities relevant to AGA risk.14,15
Our assessment of HT-teak's effectiveness in promoting hair regrowth yielded promising results comparable to the performance of minoxidil. While the subjects in the placebo group exhibited progressive hair loss, we found a positive relationship between the percentage change in TAHC and the A/T ratio in the HT-teak group. This relationship can be explained by the mechanism through which the decreased level of DHT binding to the ARs in dermal papilla cells, results in an elongation of the anagen phase and a shortening of the telogen phase. This, in turn, helps prevent the miniaturization of hair follicles, resulting in a decrease in the production of telogen hair, which is characterized by thinness and reduced pigmentation. 27 Consequently, the increased proportion of A/T is assumed to result in an increased TAHC. The observed phenomenon must reasonably be attributed to the natural progression of AGA, which aligns with previous research findings and the trend of a decrease in the A/T ratio within the placebo group.26,28 This finding is supported by the fact that there was no statistically significant difference in hair loss in the placebo group.
While the reduction in hair shedding was evident at week 4 of HT-teak application, after that hair shedding unexpectedly increased. When considering this unexpected outcome, the method of evaluation likely used attributed to the result. The assessment of the A/T ratio was confined to a specific target area whereas the physical quantification of hair shedding encompassed the entirety of the scalp. Nonetheless, hair shedding exhibited a consistent and favourable trend within the treatment groups (minoxidil and HT-teak), in contrast to the hair shedding trend shown for the placebo group.
Although these improvements were not statistically significant at every evaluation point, it is important to note the consistent positive trend observed in the HT-teak and minoxidil groups, in contrast to the placebo group in all assessments (THAC, A/T ratio, hair shedding). These critical marker trends of hair growth suggested the HT-teak as a hair growth promoter. The absence of continuous statistical significance may be attributed to several factors such as hormones, nutrition, stress, and environmental factors.29,30 Moreover, the HT-teak can avoid systemic effects associated with long-term oral S5AR inhibitor use and cause less skin irritation. Recent techniques such as LLLT, PRP, stem cell therapy, and microneedling are also promising alternatives for AGA treatment. For instance, treatments with human follicle stem cells (HFSCs) 31 and PRP injections 32 have shown increases in hair count and density by over 20% from baseline evaluations. These alternatives may be particularly appealing to individuals seeking non-pharmaceutical options or who have not responded well to traditional treatments. However, these techniques can be expensive and may have limited accessibility. Additionally, HT-teak can be further developed for more effective results by combining it with other treatments such as PRP, minoxidil, LLLT, and microneedling, leveraging the different targeted mechanisms of each treatment.16,22
In summary, this HT-teak formulation successfully demonstrated its ability to enhance TAHC and A/T ratio, while concurrently reducing hair shedding. The mechanism underpinning the effectiveness of HT-teak appears to be multifaceted, encompassing anti-S5AR activity, anti-testosterone properties, and the inhibition of IL-1β. 14 These findings revealed evidence supporting teak leaf extract as an innovative and alternative source for the development of hair growth promoters intended for the remediation of AGA.
Study Limitations
The limitations of this study include a small sample size, a short duration of treatment, and the inclusion of a wide age range of subjects, which could introduce the influence of age differences on treatment outcomes. 33 Additionally, information regarding genetic history and the duration of AGA progression was not recorded. Another limitation, which could not have been foreseen, was the impact of the occurrence of COVID-19 infection (29.63% of subjects were infected during enrollment in the experiment), which may have contributed to an increased number of hair fall cases. While this was an unexpected variable in our study, the effect of COVID-19 or other infectious viral diseases on hair loss may be of interest in further studies. This has been previously noted in.34,35
Conclusion
The study's findings indicate that Tectona grandis L.f (Teak) leaf extract is a potential mild hair growth promoter, with positive effects on TAHC, A/T ratio, and hair shedding. The results also indicated that the highest of hair-regrowth satisfaction score was presented in HT-teak group. Furthermore, the absence of adverse effects reinforces its safety profile. These findings underscore the effectiveness and safety of HT-teak as a hair growth treatment, making teak leaf extract as an alternative botanical ingredient for hair growth-promoting products.
Supplemental Material
Supplemental material, sj-docx-1-chp-10.1177_2515690X241291141 for Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia by Nutchaninad Tanuphol, Neti Waranuch, Vanuchawan Wisuitiprot, Wudtichai Wisuitiprot, Kamonlak Insumrong, Prapapan Temkitthawon, Nungruthai Suphrom, Katechan Jampachaisri, Corine Girard and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-2-chp-10.1177_2515690X241291141 for Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia by Nutchaninad Tanuphol, Neti Waranuch, Vanuchawan Wisuitiprot, Wudtichai Wisuitiprot, Kamonlak Insumrong, Prapapan Temkitthawon, Nungruthai Suphrom, Katechan Jampachaisri, Corine Girard and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine
Acknowledgements
We extend our gratitude to Mr Roy I. Morien of the Naresuan University Graduate School for his assistance in editing the grammar, syntax and general English expression in this paper.
Footnotes
Author Contributions (Roles): NT, VW, PT, NS, KKI, and NW were responsible for study design, and result interpretation. KJ was responsible for the appropriate statistical analysis and result interpretation. WW was responsible for product development and investigation of its effectiveness. NT, VW, and KMI were responsible for the investigation of effectiveness and safety, as well as results interpretation. NT wrote the first draft of the manuscript. CG, NW, and KKI checked and edited the final draft of the manuscript. All authors read and approved the final manuscript. KKI, and NW were responsible for funding acquisition.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors are grateful for financial support from the National Science, Research and Innovation Fund (NSRF) via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation (B16F640099), the Ministry of Higher Education, Science, Research and Innovation (MHESI) via Reinventing University Program 2023, Global and Frontier Research University Fund, Naresuan University; Grant number R2566C053, National Research Council of Thailand (NRCT); Grant number N21A640421, Agricultural Research Development Agency (ARDA), Naresuan University and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), and Franco–Thai Scholarship Program.
Ethical Approval: This study was conducted according to the guidelines of the Declaration of Helsinki, The Belmont Report, CIOMS Guidelines, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP). This protocol was approved by the Institutional Review Board of Naresuan University (IRB number P10123/64; approval date 20 July 2021).
Clinical Trial Registration: The clinical registration was retrospectively registered to ISRCTN.com; Registration number ISRCTN 24541842 (Registration date; 08 Jan 2024).
Patient Consent Statement: The subjects signed a consent form before participating in the study.
ORCID iDs: Corine Girard https://orcid.org/0000-0002-5116-1395
Kornkanok Ingkaninan https://orcid.org/0000-0002-4415-8489
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Saartok T, Dahlberg E, Gustafsson J-A. Relative binding affinity of anabolic-androgenic steroids: Comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114(6):2100-2106. doi: 10.1210/endo-114-6-2100 [DOI] [PubMed] [Google Scholar]
- 2.Sinclair R. Male pattern androgenetic alopecia. Br Med J. 1998;317(7162):865-869. doi: 10.1136/bmj.317.7162.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.English J, Robert S. A hypothetical pathogenesis model for androgenic alopecia: Clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med Hypotheses. 2018;111:73-81. doi: 10.1016/j.mehy.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 4.Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. Journal of Drugs in Dermatology: JDD. 2010;9(11):1412-1419. [PubMed] [Google Scholar]
- 5.Coskuner ER, Ozkan B, Culha MG. Sexual problems of men with androgenic alopecia treated with 5-alpha reductase inhibitors. Sex Med Rev. 2019;7(2):277-282. doi: 10.1016/j.sxmr.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflammation Allergy Drug Discovery. 2012;6(2):130-136. doi: 10.2174/187221312800166859 [DOI] [PubMed] [Google Scholar]
- 7.Herman A, Herman AP. Topically used herbal products for the treatment of hair loss: Preclinical and clinical studies. Arch Dermatol Res. 2017;309(8):595-610. doi: 10.1007/s00403-017-1759-7 [DOI] [PubMed] [Google Scholar]
- 8.Srivilai J, Rabgay K, Khorana N, et al. Anti-androgenic curcumin analogues as steroid 5-alpha reductase inhibitors. Med Chem Res. 2017;26:1550-1556. doi: 10.1007/s00044-017-1869-y [DOI] [Google Scholar]
- 9.Varma SB, Giri SP. Study of wound healing activity of Tectona grandis linn. Leaf extract on rats. Anc Sci Life. 2013;32(4):241-244. doi: 10.4103/0257-7941.131984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nayeem N, Karvekar M. Comparative phytochemical and pharmacological screening of the methanolic extracts of the frontal and mature leaves of Tectona grandis. Int J Pharma Bio Sci. 2010;1(3):1-7. [Google Scholar]
- 11.Shukla N, Kumar M, Akanksha, et al. Tectone, a new antihyperglycemic anthraquinone from Tectona grandis leaves. Nat Prod Commun. 2010;5(3):417-430. doi: 10.1177/1934578X1000500318 [DOI] [PubMed] [Google Scholar]
- 12.Diallo A, Gbeassor M, Vovor A, et al. Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats. Fitoterapia. 2008;79(5):332-336. doi: 10.1016/j.fitote.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Jaybhaye D, Varma S, Gagne N, Bonde V, Gite A, Bhosle D. Effect of Tectona grandis linn. Seeds on hair growth activity of albino mice. Int J Ayurveda Res. 2010;1(4):211-215. doi: 10.4103/0974-7788.76783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fachrunniza Y, Srivilai J, Wisuitiprot V, et al. Tectona grandis, a potential active ingredient for hair growth promotion. Songklanakarin Journal of Science & Technology. 2020;42(6):1352-1359. doi: 10.14456/sjst-psu.2020.175 [DOI] [Google Scholar]
- 15.Insumrong K, Ingkaninan K, Waranuch N, et al. Isolation and HPLC quantitative determination of 5α-reductase inhibitors from Tectona grandis L.f. Leaf extract. Molecules. 2022;27(9):2893. doi: 10.3390/molecules27092893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pumthong G, Asawanonda P, Varothai S, et al. Curcuma aeruginosa, a novel botanically derived 5α-reductase inhibitor in the treatment of male-pattern baldness: A multicenter, randomized, double-blind, placebo-controlled study. Journal Of Dermatological Treatment. 2012;23(5):385-392. doi: 10.3109/09546634.2011.568470 [DOI] [PubMed] [Google Scholar]
- 17.Davis M, Hylwa SA, Allen EM. Basics of patch testing for allergic contact dermatitis. Semin Cutan Med Surg. 2013;32(3):158-168. doi: 10.12788/j.sder.0029 [DOI] [PubMed] [Google Scholar]
- 18.Richard SB, James PB. A reappraisal of the 21-day cumulative irritation test in man. Journal of Toxicology: Cutaneous and Ocular Toxicology. 1982;1(2):109-115. doi: 10.3109/15569528209051516 [DOI] [Google Scholar]
- 19.Insumrong K, Waranuch N, Ingkaninan K, et al. Effects of solvent system and storage condition on chemical stability of 5α-reductase inhibitor compounds in Tectona grandis L.f. Leaf extracts. Trends in Sciences. 2024;21(4):7339. doi: 10.48048/tis.2024.7339 [DOI] [Google Scholar]
- 20.Al-Haushey L, Moussa N. The shelf life of vitamin C in a w/o emulsion according to the Q10 method. International Journal of Pharmaceutical Sciences Review and Research. 2015;30(2):33-39. [Google Scholar]
- 21.Śliwa K, Synia D, Placek W, Owczarczyk-Saczonek A. The diagnosis and treatment of androgenetic alopecia: A review of the most current management. Forum Dermatologicum. 2023;9(3):99-111. doi: 10.5603/fd.95391 [DOI] [Google Scholar]
- 22.Kaiser M, Abdin R, Gaumond SI, Issa NT, Jimenez JJ. Treatment of androgenetic alopecia: Current guidance and unmet needs. Clin Cosmet Investig Dermatol. 2023;16:1387-1406. doi: 10.2147/CCID.S385861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prager N, Bickett K, French N, Marcovici G. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-α-reductase in the treatment of androgenetic alopecia. Journal of Alternative & Complementary Medicine. 2002;8(2):143-152. doi: 10.1089/acm.2002.8.143 [DOI] [PubMed] [Google Scholar]
- 24.Panahi Y, Taghizadeh M, Marzony ET, Sahebkar A. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: A randomized comparative trial. Skinmed. 2015;13(1):15-21. [PubMed] [Google Scholar]
- 25.Kamimura A, Takahashi T, Watanabe Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine. 2000;7(6):529-536. doi: 10.1016/S0944-7113(00)80040-9 [DOI] [PubMed] [Google Scholar]
- 26.Loing E, Lachance R, Ollier V, Hocquaux M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J Cosmet Sci. 2013;64(1):45-58. [PubMed] [Google Scholar]
- 27.Sinclair R. Androgenetic alopecia. Modelling progression and regrowth. Exp Dermatol. 2016;25(6):424-425. doi: 10.1111/exd.13029 [DOI] [PubMed] [Google Scholar]
- 28.Van Neste D, Fuh V, Sanchez-Pedreno P, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000;143(4):804-810. doi: 10.1046/j.1365-2133.2000.03780.x [DOI] [PubMed] [Google Scholar]
- 29.Lin RL, Garibyan L, Kimball AB, Drake LA. Systemic causes of hair loss. Ann Med. 2016;48(6):393-402. doi: 10.1080/07853890.2016.1180426 [DOI] [PubMed] [Google Scholar]
- 30.Gokce N, Basgoz N, Kenanoglu S, et al. An overview of the genetic aspects of hair loss and its connection with nutrition. J Prev Med Hyg. 2022;63(2 Suppl 3):E228-E238. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investigation. 2017;4(58). doi: 10.21037/sci.2017.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4(11):1317-1323. doi: 10.5966/sctm.2015-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shankar DK, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichology. 2009;1(2):131-133. doi: 10.4103/0974-7753.58556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seyfi S, Alijanpour R, Aryanian Z, Ezoji K, Mahmoudi M. Prevalence of telogen effluvium hair loss in COVID-19 patients and its relationship with disease severity. J Med Life. 2022;15(5):631-634. doi: 10.25122/jml-2021-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarnieri G, Bertagna De Marchi L, Marcon A, et al. Relationship between hair shedding and systemic inflammation in COVID-19 pneumonia. Ann Med. 2022;54(1):869-874. doi: 10.1080/07853890.2022.2054026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-chp-10.1177_2515690X241291141 for Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia by Nutchaninad Tanuphol, Neti Waranuch, Vanuchawan Wisuitiprot, Wudtichai Wisuitiprot, Kamonlak Insumrong, Prapapan Temkitthawon, Nungruthai Suphrom, Katechan Jampachaisri, Corine Girard and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine
Supplemental material, sj-docx-2-chp-10.1177_2515690X241291141 for Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia by Nutchaninad Tanuphol, Neti Waranuch, Vanuchawan Wisuitiprot, Wudtichai Wisuitiprot, Kamonlak Insumrong, Prapapan Temkitthawon, Nungruthai Suphrom, Katechan Jampachaisri, Corine Girard and Kornkanok Ingkaninan in Journal of Evidence-Based Integrative Medicine