Abstract
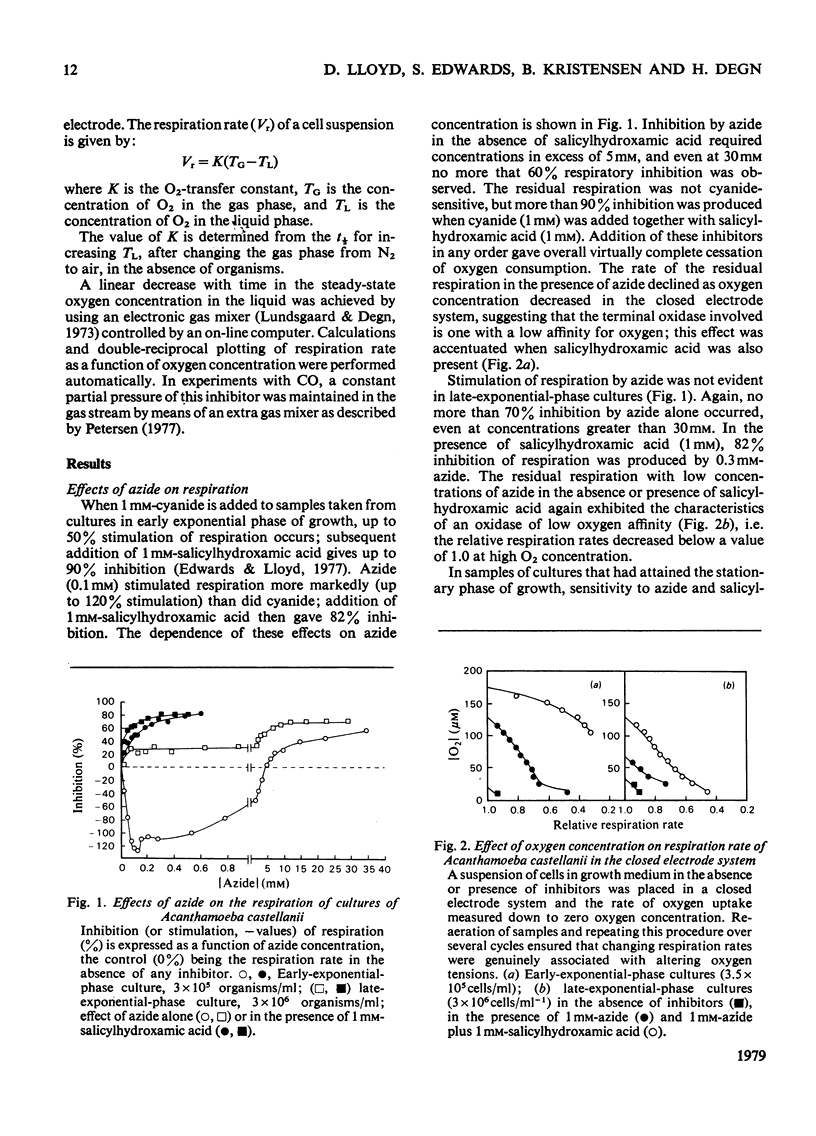
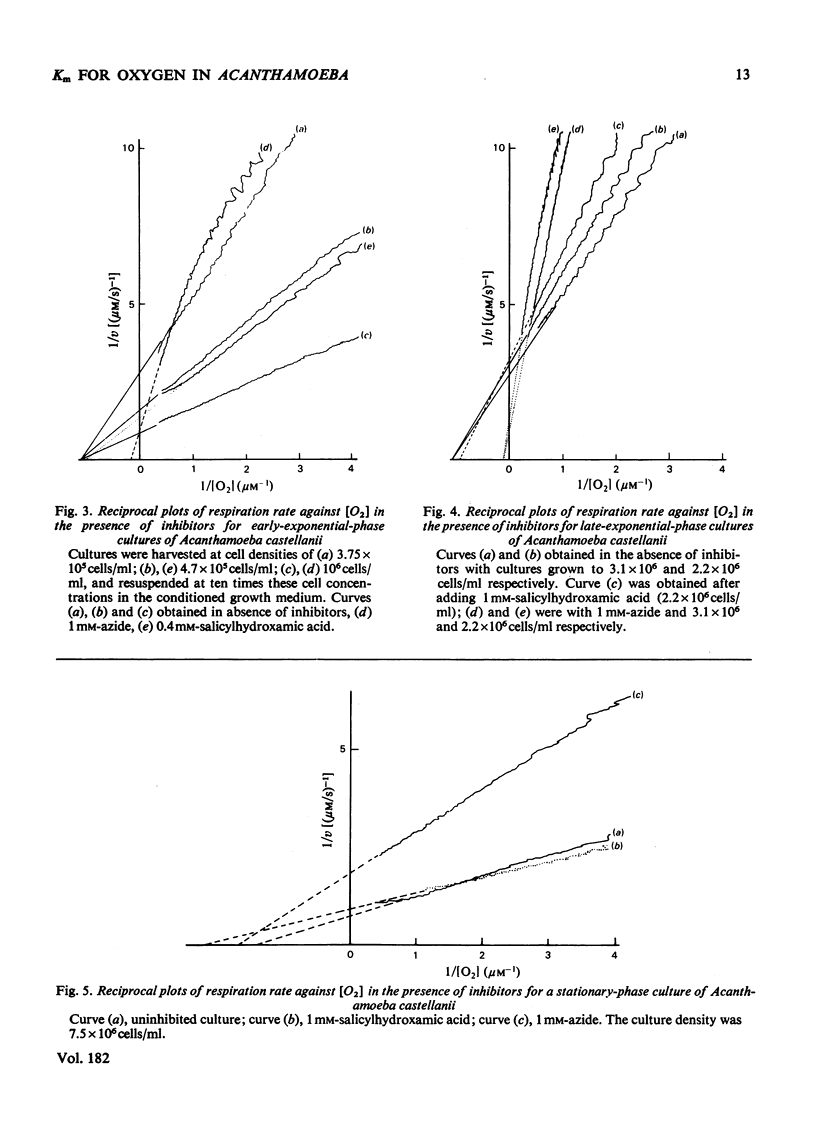
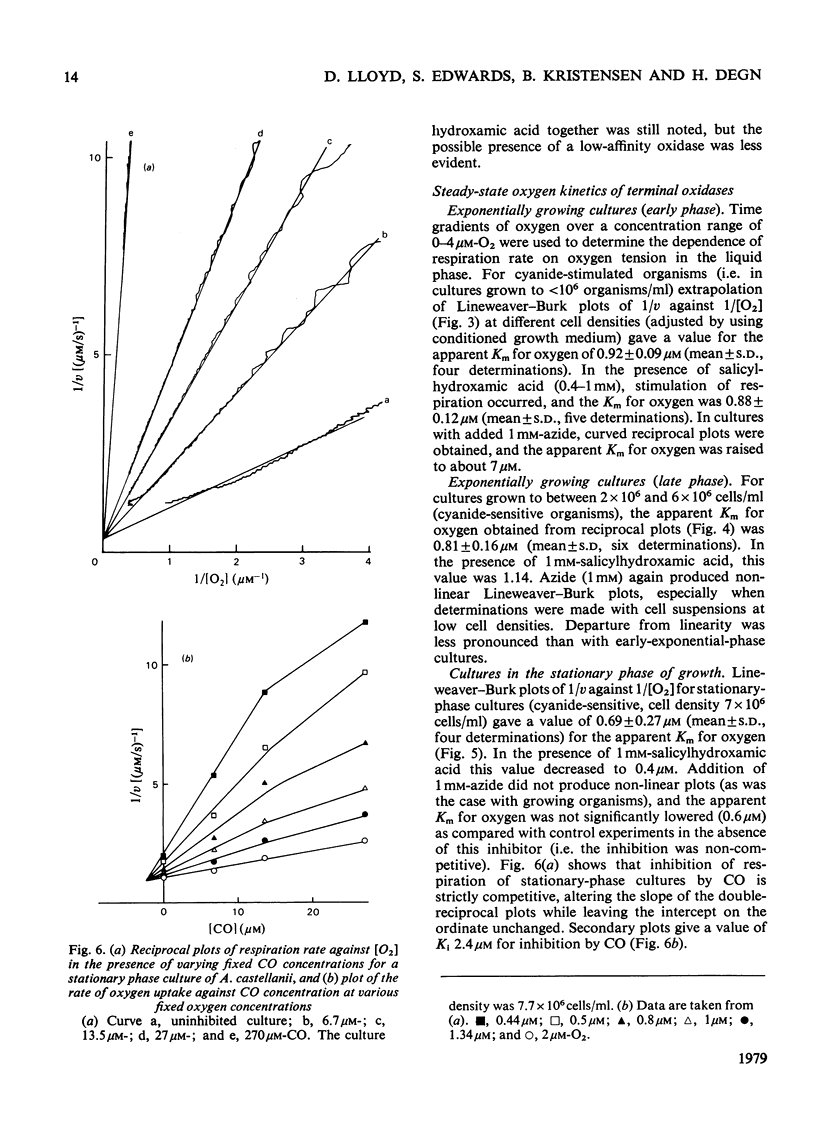
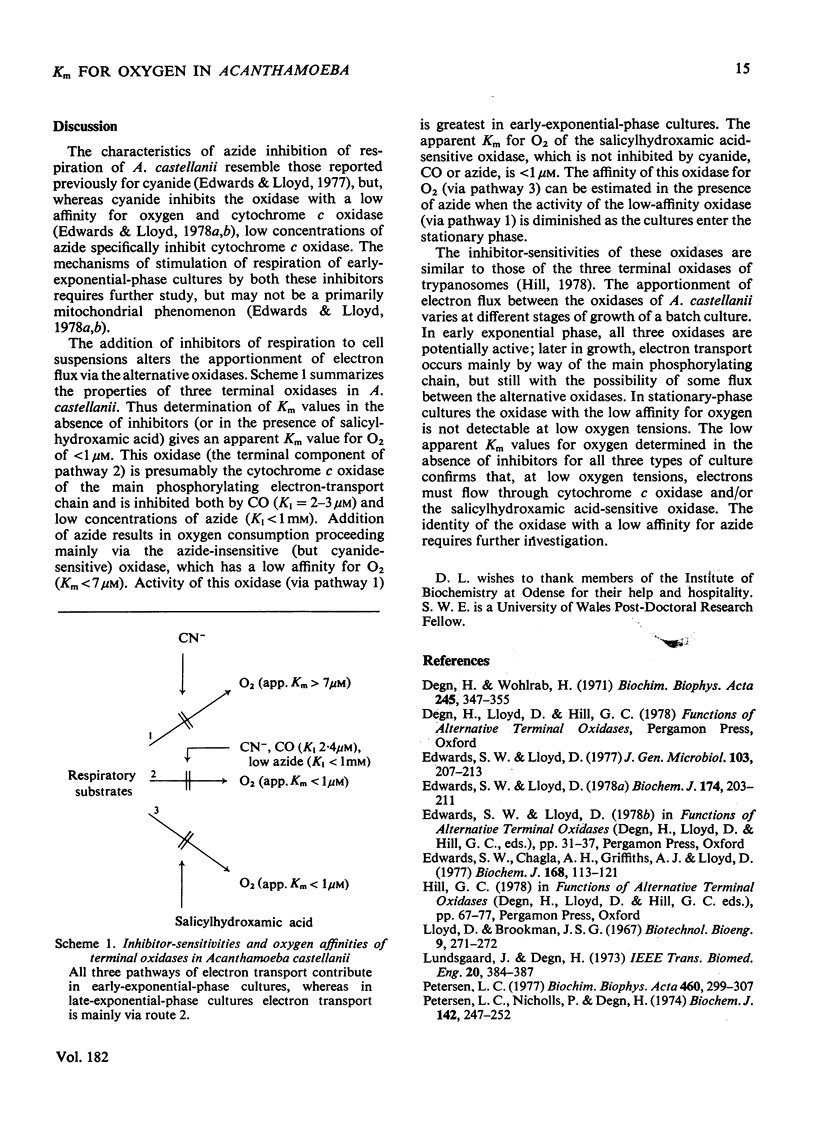
1. Respiration of growing cultures of Acanthamoeba castellanii is inhibited less than 60% by azide (35 mM); the respiration of early-exponential-phase cultures differs from that of late-exponential-phase cultures in being stimulated by up to 120% by low concentrations (less than 1 mM) of this inhibitor. Azide (0.5 mM) plus 1 mM-salicylhydroxamic acid gives 80% inhibition of respiration in early- or late-exponential-phase cultures. 2. Lineweaver-Burk plots of 1/v against 1/[O2] for growing and stationary-phase cultures give values of less than 1 muM for the apparent Km for oxygen. 3. These values are not significantly altered when determined in the presence of 1 mM-salicylhydroxamic acid. 4. Higher values (greater than 7 muM) for apparent Km values for oxygen were obtained in the presence of azide, which gives non-linear Lineweaver-Burk plots. 5. Competitive inhibition of respiration by CO occurs with Ki 2.4 muM. 6. The results are discussed in terms of the presence of three terminal oxidases in this organism, namely two oxidases with high affinities for oxygen (cytochrome c oxidase of the main phosphorylating electron-transport chain and the salicylhydroxamic acid-sensitive oxidase) and a third oxidase with a low affinity for oxygen, sensitive to inhibition by cyanide but not by azide or salicylhydroxamic acid. The relative contributions to oxygen utilization by these oxidases change during the growth of a batch culture.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Degn H., Wohlrab H. Measurement of steady-state values of respiration rate and oxidation levels of respiratory pigments at low oxygen tensions. A new technique. Biochim Biophys Acta. 1971 Sep 7;245(2):347–355. doi: 10.1016/0005-2728(71)90153-8. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Chagla A. H., Griffiths A. J., Lloyd D. The cytochromes of Acanthamoeba castellanii. Biochem J. 1977 Oct 15;168(1):113–121. doi: 10.1042/bj1680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Properties of mitochondria isolated from cyanide-sensitive and cyanide-stimulated cultures of Acanthamoeba castellanii. Biochem J. 1978 Jul 15;174(1):203–211. doi: 10.1042/bj1740203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundsgaard J., Degn H. Digital regulation of gas flow rates and composition of gas mixtures. IEEE Trans Biomed Eng. 1973 Sep;20(5):384–387. doi: 10.1109/TBME.1973.324237. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Nicholls P., Degn H. The effect of energization on the apparent Michaelis-Mentne constant for oxygen in mitochondrial respiration. Biochem J. 1974 Aug;142(2):247–252. doi: 10.1042/bj1420247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta. 1977 May 11;460(2):299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]


