Abstract
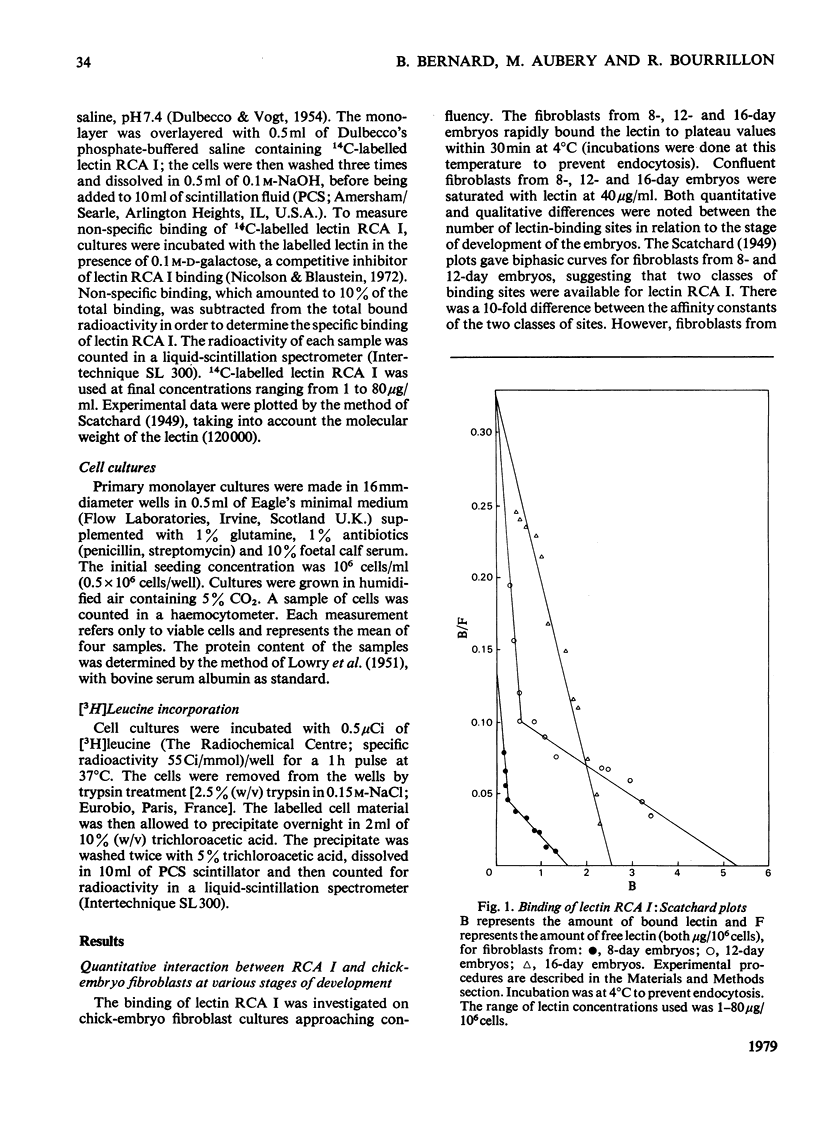
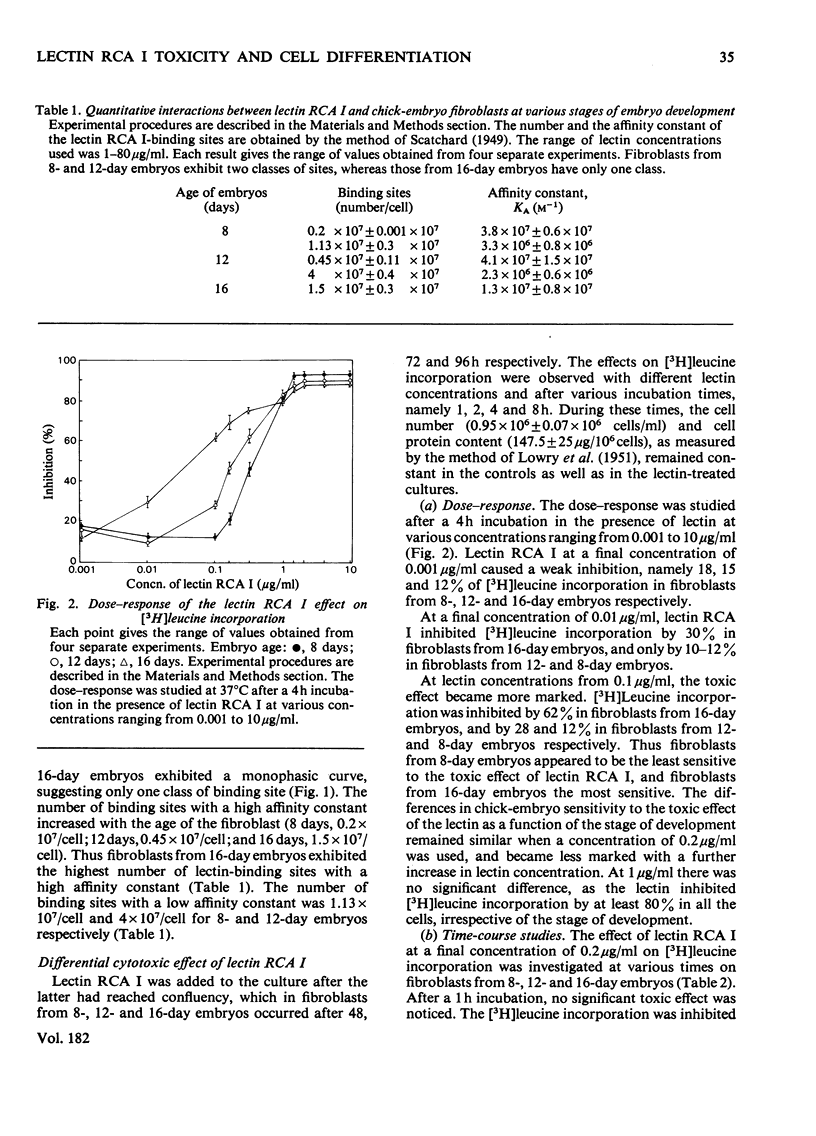
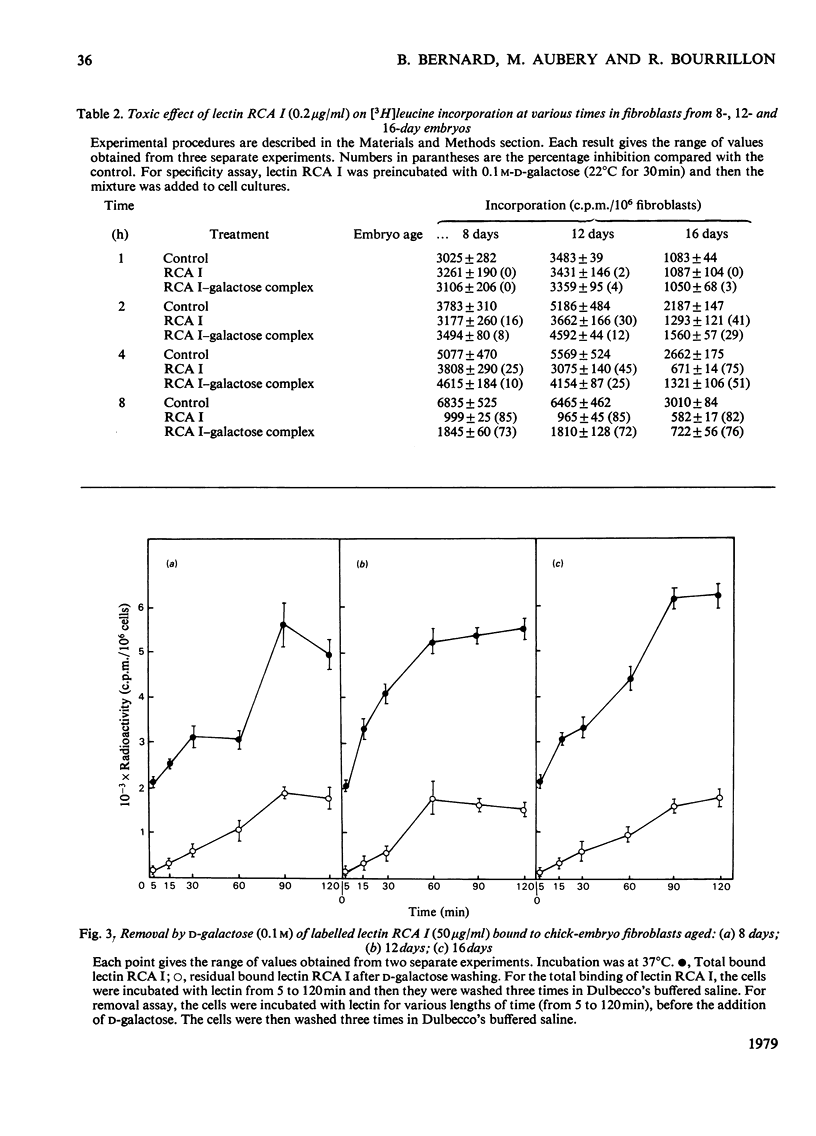
The toxic effect of Ricinus lectin RCA I, as estimated by the inhibition of [3H]leucine incorporation, was investigated on chick-embryo fibroblasts at different stages of development. There appeared to be a differential susceptibility of chick-embryo fibroblasts to lectin RCA I. Fibroblasts from 16-day embryos were the most sensitive to its toxic effect in terms of both concentration and time, and cells from 8-day embryos were the least sensitive. This differential sensitivity to the toxic effect of lectin RCA I was closely related to the binding of the lectin: fibroblasts from 16-day embryos had more binding sites (1.5 x 10(7)/cell) with a high affinity than did 12-day (0.45 x 10(7)/cell) or 8-day embryos (0.2 x 10(7)/cell). Studies on the specificity and the removal of bound lectin RCA I by D-galactose indicated that the lectin binding was necessary but not sufficient in itself to cause the toxic effect and that the lectin needed to enter the cells in order to be toxic. The amount of lectin RCA I needed to induce a 50-60% toxicity enters fibroblasts of 16-day embryos more rapidly than those of 12- and 8-day embryos.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubery M., Bourrillon R. Cell growth and thymidine incorporation changes induced by Dolichos lectin in embryo fibroblasts at various stages of differentiation. Cell Differ. 1975 May;4(2):67–77. doi: 10.1016/0045-6039(75)90019-6. [DOI] [PubMed] [Google Scholar]
- Aubery M., Bourrillon R. Growth response to lectins in chick embryo cells at different stages of development. Cell Differ. 1976 Apr;5(1):27–35. doi: 10.1016/0045-6039(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Humphreys T. Turnover of molecules which maintain the normal surfaces of contact-inhibited cells. Science. 1972 Feb 25;175(4024):905–906. doi: 10.1126/science.175.4024.905. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz P. B., Moscona A. A. Lectin-mediated stimulation of DNA synthesis in cultures of embryonic neural retina cells. Exp Cell Res. 1976 Jun;100(1):177–189. doi: 10.1016/0014-4827(76)90340-2. [DOI] [PubMed] [Google Scholar]
- Kleinschuster S. J., Moscona A. A. Interactions of embryonic and fetal neural retina cells with carbohydrate-binding phytoagglutinins: cell surface changes with dfferentiation. Exp Cell Res. 1972 Feb;70(2):397–410. doi: 10.1016/0014-4827(72)90152-8. [DOI] [PubMed] [Google Scholar]
- Krach S. W., Green A., Nicolson G. L., Oppenheimer S. B. Cell surface changes occurring during sea urchin embryonic development monitored by quantitative agglutination with plant lectins. Exp Cell Res. 1974 Mar 15;84(1):191–198. doi: 10.1016/0014-4827(74)90396-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I. R., Great H. Protein labeling by acetylation. Biopolymers. 1972;11(12):2533–2536. doi: 10.1002/bip.1972.360111212. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Hunter T. R. Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res. 1975 Jan;35(1):144–155. [PubMed] [Google Scholar]
- Obrig T. G., Culp W. J., McKeehan W. L., Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem. 1971 Jan 10;246(1):174–181. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973 Jul 31;12(16):3121–3126. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- Olsnes S. Toxic proteins inhibiting protein synthesis. Naturwissenschaften. 1972 Nov;59(11):497–502. doi: 10.1007/BF00609814. [DOI] [PubMed] [Google Scholar]
- Rein A., Rubin H. Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Exp Cell Res. 1968 Mar;49(3):666–678. doi: 10.1016/0014-4827(68)90213-9. [DOI] [PubMed] [Google Scholar]
- Saltvedt E. Structure and toxicity of pure ricinus agglutinin. Biochim Biophys Acta. 1976 Dec 21;451(2):536–548. doi: 10.1016/0304-4165(76)90149-5. [DOI] [PubMed] [Google Scholar]


