Abstract
Alzheimer's disease is a fatal neurodegenerative disorder that causes memory loss and cognitive decline in older people. There is increasing evidence suggesting that gut microbiota alteration is a cause of Alzheimer’s disease pathogenesis. This review explores the link between gut dysbiosis and the development of Alzheimer's disease contributing to neuroinflammation, amyloid β accumulation, and cognitive decline. We examine the recent studies that illustrate the gut-brain axis (GBA) as a bidirectional communication between the gut and brain and how its alteration can influence neurological health. Furthermore, we discuss the potential of probiotic supplementation as a management approach to restore gut microbiota balance, and ultimately improve cognitive function in AD patients. Based on current research findings, this review aims to provide insights into the promising role of probiotics in Alzheimer's disease management and the need for further investigation into microbiota-targeted interventions.
Keywords: Probiotic supplementation, Neuroinflammation, Gut microbiota, Alzheimer’s disease, Gut-brain axis
1. Introduction
One of the most highly lethal neurodegenerative diseases in the world is Alzheimer's disease (Iqbal et al., 2024). According to studies, 50 million people are affected by Alzheimer's disease, with most victims being between 65 and 80 years old. Most individuals with this neurodegenerative illness are older people (Luca et al., 2019). Amyloid Beta (Aβ) plaques and hyperphosphorylated tau neurofibrils slowly accumulate throughout the disease, causing neuroinflammation and a progressive loss of cognitive function (Varesi et al., 2022). Alzheimer's disease usually appears as late-onset, sporadic occurrences at the age 65 years of age and older. In addition to Autosomal dominant genetic mutations in presenilin (PSEN1 & PSEN2) that cause disease, amyloid precursor protein, the primary genetic risk factor has been determined to be the apolipoprotein E4 allele (APOE E4) (Virgilio et al., 2022). Alzheimer's disease is the most common kind of dementia, with a predominance of cognitive abnormalities as symptoms, including memory loss, personality disorders, and judgmental disorders. Dementia is the aggregate term for symptoms including loss of memory, mental decline, and aberrant behavior (Dissanayaka et al., 2024). The network of bidirectional communication that exists between the gastrointestinal tract (gut) and the central nervous system (CNS), which includes the brain, is known as the "gut-brain axis" (GBA). Multiple routes in this intricate system enable the gut to affect brain function and the brain to influence gut function (Ashique et al., 2024a). Investigations on the connection between gut microbiota and brain function could provide significant new insights into the early diagnosis and treatment of neurodegenerative illnesses like Alzheimer's Disease (Cryan et al., 2019, Dinan and Cryan, 2017). Maintaining homeostasis depends on the axis of the brain is the communication connection between the brain and the digestive tract. It functions through hormonal signals, immunological processes, and neurological pathways (Enteric nervous system (ENS) and CNS) (Mayer, 2011). Microbiota and its byproducts have been demonstrated to affect intestinal permeability, intestinal motility, and the operation of the central nervous system and enteric nervous system (Galland, 2014). After birth, an infant's gut microbiota develops, and bacteria start to colonies a fetus's intestines in the lower uterus (Mondot et al., 2013). There are usually two major shifts that occur during infancy that lead to the establishment of a steady gut microbiota. The first shift, which is caused by nursing and occurs soon after birth, results in the preponderance of Bifidobacterium in the gut microbiota. The second transition happens when solid meals are added while breastfeeding is still being done throughout the weaning phase. In this phase, Bacteroidota and Bacillota emerge as the dominant phylum and the microbiota becomes more complex, akin to that of an adult. These modifications last until approximately the age of three, at which point humans develop stable "Enterotypes “a balanced host-microbiota symbiosis in their gut microbiomes (Sarkar et al., 2021). Gut Microbiome refers to the entire collection of genetic material within the microorganisms living in the gut. It encompasses all the genomes of the bacteria, viruses, fungi, and other microbes that inhabit the digestive tract. The microbiome is responsible for producing various metabolites, influencing digestion, immunity, and even mental health.
Gut Microbiota refers specifically to the actual microorganisms themselves—bacteria, archaea, fungi, and viruses—that reside in the gastrointestinal tract. It is the physical community of these microbes that directly interacts with the host's body and is a critical component in various physiological processes, such as nutrient absorption and immune function. The colonization of the infant’s gut microbiota is dominated by the maternal microbiome, and the use of antibiotics by the mother during pregnancy may disrupt this process. Such alterations could have a major effect on the immunological development of newborns (Tanaka and Nakayama, 2017). Pregnancy-related variables, including the use of antibiotics, have been connected to the delayed colonization of several microorganisms, especially Lactobacillus species and Bifidobacterium. Due to the beneficial qualities of these species, there may be long-term effects from this delay (Riverol and López, 2011). Furthermore, evidence has shown that children of smoking mothers are more likely to develop Inflammatory Bowel Disease, possibly because of disturbed microbial colonization. It is noteworthy that there was a correlation between the reduced amount of Bacteroidota and Pseudomonadota and an elevated amount of Bacillota and Actinomycetota following smoking cessation and emerging research suggests several important links between the two, primarily through mechanisms involving chronic inflammation, the gut-brain axis, and microbiome alterations (Nguyen et al., 2023). A wide variety of bacteria can be found in the human colon, although the most prevalent genera include those of Bacteroides, Bifidobacterium, Eubacterium, Clostridium, Lactobacillus, and gram-positive cocci. Each human carries several hundred different types of microbes, each with a specific mix of dominating species. The gastrointestinal microbiota in adults tends to remain stable over months, unlike the highly dynamic and changing microbiota in infants. Furthermore, numerous studies have highlighted the significance of gut microbiota in influencing the development or progression of neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's diseases (Lloyd and Tahon, 2022). Many medical conditions have been linked to disruptions in bacterial populations and decreased variety, which are signs of dysbiosis in the gut microbiota. An elevated amount of evidence suggests that gastrointestinal dysfunction and abnormal microbial composition may play an important role in the development of Alzheimer's disease and other neurodegenerative disorders. This relationship draws attention to the complex link between the gastrointestinal microbiota and human health outside of gastrointestinal functions and underscores the significant impact of gut health on brain disorders (Jemimah et al., 2023). By synthesizing current research findings, this review aims to provide insights into the promising role of probiotics in Alzheimer's disease management and the need for further investigation into microbiota-targeted interventions. Therefore, our study aimed to find whether probiotics could be an alternate approach to modulating gastrointestinal microbiota in the management of Alzheimer's disease.
2. Composition and function of gut microbiota
The brain-gut axis connects the brain's emotional and cognitive centers with peripheral intestinal activities through the bidirectional connection between the central and enteric nerve systems (Wojtuś et al., 2024; J. Liu et al., 2022). Through neurological, hormonal, and immunological pathways, this axis influences various physiological functions, including digestion, mood regulation, immune responses, and even stress levels (McGuinness et al., 2022).The microbiome is an assemblage of diverse microbial communities, comprising bacteria, fungi, and microbes, that are found in different parts of the human body (Li et al., 2020). The gut contains the majority of human microorganisms, which include about 1000 distinct bacterial species and almost 150 times more genes than the human genome (Liu et al., 2035a). The three kingdoms into which life is divided are bacteria, Archaea, and Eukaryota. Numerous phyla (plural of phylum, the main taxonomic division that encompasses one or more classes, exist for bacteria, but the majority of them are represented by just a few phyla, which together account for over 160 species. The majority of bacterial types present in a typical, healthy gut include Firmicutes Bacillota, Bacteroidetes Bacteroidota, Actinobacteria Actinomycetota, Proteobacteria Pseudomonadota, Fusobacteria, and Verrucomicrobia. Firmicutes Bacillota and Bacteroidetes Bacteroidota, combined make up about 90 % of the gut's microbial flora. The two genera that make up Gut Bacteroidetes are Bacteroides Bacteroidota and Prevotella. Within the phylum Firmicutes Bacillota, the principal genera are Roseburia, Ruminococcus, Lactobacillus, Bacillus, Clostridium, Enterococcus, and Faecalibacterium prausnitzii. The Bifidobacterium genus mostly represents the Actinobacteria phylum (Borrego-Ruiz and Borrego, 2024, Martinez et al., 2022). Thus, the equilibrium among these phyla is a biomarker of gut stability and health, and it is linked to several illnesses. Alterations in this ratio have also been linked to neurodegeneration as a function of aging (Ekwudo et al., 2024).
2.1. Role of gutmicribiota in the gastrointestinal tract
Children are also impacted by neurodegenerative illnesses, which cause progressive loss of neurological skills and developmental regression. To provide early diagnosis, appropriate care, and genetic counseling—and to give hope for improved outcomes through the development of new treatment modalities—a systematic clinical strategy based on the child's age and brain involvement is essential (Mishra, 2018). The gastrointestinal (GI) tract is colonized from the moment of childbirth and keeps changing and adapting its entire life. As a result, the adult individual gastrointestinal tract supports a distinct ecology consisting of billions of bacteria. The distal colon is where the density of gut microbiota peaks, with an expected quantity of 1011 bacterium per gram of gastrointestinal contents. The gut microbiota's species profile shows remarkable stability over periods spanning from days to months and even years, despite its composition changing with age and along the GI tract. This suggests that the microbiota is generally resilient to environmental changes (Aziz et al., 2013). Microbiota counts gradually increase throughout the gastrointestinal tract, beginning in the stomach with low levels and rising rapidly in the colon. There are very few microorganisms that can survive the acidic conditions of the gut and proximal duodenum, bile, or pancreatic enzymes. However some bacteria can survive in these harsh environments, and some can even grow(Dieterich et al., 2018). The microorganism has a huge range of impacts on an individual's health, including strengthening immunity, dissolving dietary fibers, enhancing nutrient absorption through improved gastrointestinal motility and function, and inhibiting the growth of pathogens. Furthermore, the gastrointestinal mucosa is maintained and repaired in large part by the gut. The microorganism generates metabolites and short-chain fatty acids which serve as anti-inflammatory and support intestinal balance(Aleman et al., 2023). Foods that the stomach and intestines' enzymes are unable to break down are partially broken down by the microbiota in the gut (Jayachandran et al., 2017). Microbial materials can be absorbed by the tissues of the gastrointestinal tract, potentially going into circulation, and reaching other tissues, and then being expelled through breath or urine. In the large intestine, bacteria digest fiber and protein to produce important byproducts, especially short-chain fatty acids (SCFA). The cellular functions that maintain tissue integrity are supported by these Short chain fatty acids, which are essential energy sources for bacteria and colon tissues (Conlon and Bird, 2015). SCFA like butyrate, propionate, and acetate are produced when bacteria in the colon, such as Bacteroides, Roseburia, Bifidobacterium, and Enterobacteria, break down carbohydrates that have eluded initial digestion and unabsorbable oligosaccharides where the host derives substantial energy from these SCFA (Jandhyala et al., 2015). Endotoxins, bacteria, and compounds generated can pass through the intestinal barrier more easily, getting into the bloodstream and perhaps causing autoimmune diseases. Many cardiovascular illnesses (CVDs) including atherosclerosis, heart attacks (MI), arrhythmias, pericardial disease, cardiomyopathies, and heart failure are caused by inflammation and immune dysfunction (Luqman et al., 2024).
2.2. Role of the gut microbiome in neurodegenerative diseases
Gut microbes and metabolites can influence both brain health and the integrity of the BBB. The structural strength of the BBB may be regulated by the digestive microbiota, according to certain theories. It is believed that the regulating effect begins in the first moments of fetal life and lasts the entirety of an individual's life. The ability to protect the BBB and the number of microorganisms in the human body decreases with age (Cryan and O’Mahony, 2011, Wang et al., 2023). In addition, the gut microorganism and its host interact in several intricate ways that have an impact on metabolism, immunology, and central nervous system functions. Molecular cues originating from gut microbiota may regulate brain cell activity. The microglia, which aid in brain growth and homeostasis and are helpful in protecting the central nervous system, are one of the main natural immune cells in the central nervous system (Damiani et al., 2023, Loh et al., 2024a). However, studies have demonstrated that a robust immune system can protect against a wide range of neurodegenerative diseases. Memory is improved and dementia is prevented with the use of probiotic supplements and other medications in conjunction with gut health management. It is well-established that microbiota makeup and dietary practices can influence neurogenesis. Since the proper operation of Gut microbiota depends on food intake and the metabolites created by the microbiota are mostly dependent on the food and microbial composition in the intestine, required for neurotropism (Mitra et al., 2023).
2.3. Factors modulating gut microbiota balance
Diet: Inter-individual heterogeneity in the human gut microbiota occurs for several reasons, including immunological conditions, age, sex, genotype, and environmental influences. Eating decisions significantly dominate the makeup of the gut microorganism in addition to other factors. The kind, caliber, and source of food ingested influence the composition and activities of the gastrointestinal microorganism as well as the interactions between microorganisms and the hosts that make the composition of gut microorganisms vary from one person to another (Campaniello et al., 2022).Consumers of a Westernized diet that is rich in red meats, processed and refined food, sugary drinks, and little in the way of fruits, vegetables, and fiber are associated with an elevated risk of metabolic diseases such as diabetes mellitus and obesity. These diseases were linked to endotoxemia, a systemic low-grade inflammation (Statovci et al., 2017)..Microorganisms require almost same micronutrients that people do, and many of these micronutrients are acquired through the host's diet (Bear et al., 2020). Prebiotics reduce dysbiosis and the inflammatory conditions that are linked to it, which may reduce cognitive loss. Additionally, they promote the development of bacterium including Lactobacillus and Bifidobacterium (Shabbir et al., 2021). Diversity of good and pathogenic bacterium in the Gastrointestinal tract decreases with a ketogenic diet, and other negative effects include an increase in pathogenic bacteria and altered intestinal metabolism, decline in cognitive function, and an increase in systemic and intestinal inflammation and intestinal barrier disruption (Xiao et al., 2024).
Antibiotics: Microbes, including gut microbiota, and medications like antibiotics can alter the balance of microorganisms in the gut. While antibiotics target disease-causing bacteria, they may also disrupt the beneficial microbiota, potentially leading to gastrointestinal issues and other diseases. Moreover, during drug metabolism, gut bacteria can modify how certain medications function. This interaction may produce metabolic byproducts that disrupt the normal composition of the microbiota, potentially leading to serious adverse effects (Anwar et al., 2021).
Age: As individuals age, the diversity of their microbiota increases, eventually stabilizing into an adult composition predominantly influenced by three major bacterial phyla: Actinomycetota, which includes families such as Bifidobacteriaceae and Coriobacteriaceae; Bacteroidota, which includes families such as Bacteroidaceae, Prevotellaceae, and Rikenellaceae; and Bacillota, which includes families such as Lachnospiraceae and Ruminococcaceae. This stable microbiota plays a crucial role in maintaining health by contributing to immune regulation, digestion, and protection against pathogens (Lloyd and Tahon, 2022). These bacterial phyla result from a combination of factors, including genetics, environment, diet, lifestyle, and digestive physiology. By around age three, a child's gut microbiota reaches a composition and diversity like that of an adult. However, in elderly adults over the age of 70, factors such as weakened immune function, changes in digestion, and reduced nutrient absorption can lead to alterations in the gut microbiota, potentially impacting overall health (Rinninella et al., 2019).
Physical exercise: Exercise is a powerful preventive measure against a wide range of chronic illnesses, and its benefits may be significantly mediated by the gut microbiota. Regular physical activity alters the composition of gut microbes, promoting a balanced microbial environment that supports overall homeostasis and energy regulation. Additionally, frequent exercise fosters an anti-inflammatory, immunoregulatory state by positively influencing the gut-brain axis, contributing to improved mental and physical health (Wegierska et al., 2022). The importance of various forms of long-term exercise training for preventing Alzheimer's disease progression has been highlighted by consistent findings addressing the advantages of Physical exercise for lowering the risk of Alzheimer's disease as well as the positive effects of physical exercise on brain aging and the preservation of cognitive function (Cutuli et al., 2023).
Environmental exposure: The most harmful agents that alter a worker's microbiome include exposure to microbial substances from direct contact with pets and medical personnel, toxic substances such as fabrication fluids, debris, and insecticides, as well as workplace stress, and unhealthy lifestyle choices (Góralczyk-Bińkowska et al., 2022). It is crucial to look beyond merely viewing the changes in the gut microbiota of night shift workers as a risk factor for metabolic disorders, colorectal cancer, and other gastrointestinal illnesses. Although these changes may indeed be causal, this remains a hypothesis that requires further investigation. The exact mechanisms underlying the development of metabolic diseases in night shift workers are still not fully understood. However, current research suggests that disruptions in circadian rhythms, combined with alterations in gut microbiota, could serve as catalysts for the onset of these diseases (Mortaş et al., 2020).
3. Microbiota gut-brain axis
The gut-brain axis is established through bidirectional nerve, endocrine, and immunological connections between the gut and the brain. Changes in one system can significantly impact the other organs involved. Disorders of the microbiota-gut-brain axis can affect both the ENS and CNS. Additionally, these disorders may alter the quantity and composition of gut bacteria, further influencing overall health (Zhu et al., 2017). The ENS is a vital component of the gut-brain axis, positioned at the interface between the gastrointestinal tract and the brain. It plays a key role in regulating nearly all gastrointestinal activities, including motility, secretion, absorption, and blood flow. By communicating with the central nervous system, the ENS influences not only digestive processes but also contributes to the overall health of the brain, highlighting the intricate relationship between gut function and neurological well-being (Chen et al., 2013).
3.1. Gut-microbiota-brain-axis and its signaling pathway
The term Gut-microbiota-brain-axis refers to the intricate web of connections that exist between the brain and the gut. The sympathetic and parasympathetic branches of the autonomic nervous system, the central nervous system, the enteric nervous system, and the broad spectrum of cells present in the digestive atmosphere constitute this network (Bosi et al., 2020). The ENS and CNS systems communicate bidirectionally through both vagal parasympathetic and sympathetic pathways. Vagal afferent signaling from the ENS occurs via mucosal varicose nerve endings, intraganglionic laminar endings, and circular muscle layers. This communication allows the ENS to influence the composition and biology of the gastrointestinal tract independently, modulating functions such as digestion, motility, and local immune responses (Hyland and Cryan, 2016).
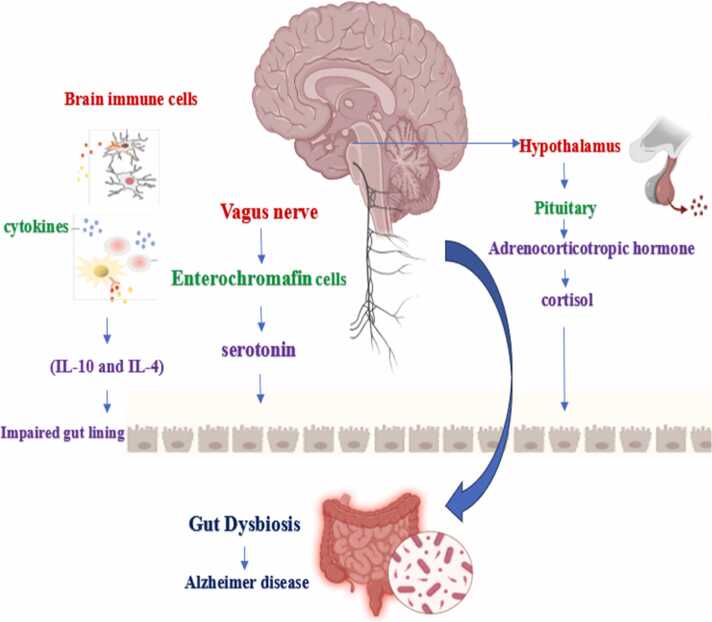
The vagus nerve: Cranial nerve X, also known as the vagus nerve, connects the visceral organs to the brain. It is a paired nerve composed of both sensory and efferent neurons, enabling bidirectional communication between the brain and various internal organs (Kesika et al., 2021). The vagus nerve (VN), located in the brainstem, plays a crucial role in how metabolites stimulate the local gut to influence brain activity via the autonomic nervous system. Cranial nerve X, which consists of distinct and analogous neurons, transmits motor impulses from the brain to various organs, including intestinal cells that are also influenced by the gastrointestinal microbiota. As a key component of the parasympathetic nervous system, the vagus nerve serves as a vital link in the gut-brain axis. It can detect specific signals from the microbiota or enteroendocrine cells, facilitating indirect communication with the gut's luminal microbiome (Chakrabarti et al., 2022). The brain receives signals from the gut microbiota through four main pathways: first, the vagus nerve, which connects the gastrointestinal tract's muscular and mucosal layers to the brainstem; second, the secretion of serotonin from enterochromaffin cells located in the gut's epithelial lining; third, dysfunction of microglia, the immune cells of the central nervous system; and finally, direct chemical signals, such as toxins, short-chain fatty acids, and γ-aminobutyric acid (GABA), which are sent to the brain (Ashique et al., 2024b). Thus, cranial nerve X has been documented to play a crucial role in signal transmission and reciprocal regulation between the gut and the brain. It facilitates communication by transmitting sensory information from the gastrointestinal tract to the brain, while also influencing gut function through its motor and autonomic fibers. This bidirectional communication underscores the importance of the vagus nerve in maintaining gut-brain axis health and may have implications for various neurological and gastrointestinal disorders (Wang et al., 2024).
Immune pathway: The intestinal microbiota, which regulates the immune system, constitutes more than 70 % of the immune system's total capacity. Dysbiosis of the gastrointestinal microbiome significantly impacts human homeostasis and overall health by altering intestinal permeability. The gut microbiota influences inflammation in the gastrointestinal tract, primarily through the immune system’s secretion of cytokines (e.g., IL-10 and IL-4) and other mediators of cellular communication, such as interferon-gamma, during dysbiosis. Disruptions in the gut-brain axis affect intestinal motility, secretion, visceral hypersensitivity, and the cellular functions of the enteroendocrine and immune systems (Qu et al., 2024).
HPA axis: The interactions between the gut and brain are regulated by the hypothalamus-pituitary-adrenal (HPA) axis, which plays a crucial role in controlling the body’s stress response. As one of the primary neuroendocrine systems in humans, the HPA axis is responsible for regulating the body’s reaction to stress. A groundbreaking study has linked gut microbiota to the HPA axis, revealing that plasma levels of adrenocorticotropic hormone (ACTH) and/or corticosterone were significantly elevated in response to restraint stress in adult germ-free (GF) mice compared to specific pathogen-free (SPF) mice, which have a normal composition of microbiota and no specific pathogens (Liu et al., 2035b) Cortisol may influence the gut-brain axis through several different pathways. Many gut cells, including immune cells, enteroendocrine cells, and epithelial cells, express cortisol receptors, indicating a direct relationship between cortisol levels and gut function. Furthermore, glucocorticoids are essential for proper maturation and significantly contribute to the development of the central nervous system. This interplay suggests that cortisol not only impacts gut health but also plays a critical role in neurodevelopment and overall brain function (Rusch et al., 2023).
3.2. Role of neurotransmitter in gut dysbiosis
Chemical messengers known as neurotransmitters carry messages from one neuron to the next; they are vital to neurological processes and have an impact on behavior in humans. Neurotransmitter abnormalities are intimately linked to mental diseases, including mood disorders, anxiety disorders, and depression. Since the intestinal flora and host cells mutually produce neurotransmitters, gut microorganisms also have an impact on the central nervous system through the microbiota(Chen et al., 2022).The four main types of neurotransmitters are excitatory neurotransmitters glutamate, acetylcholine, histamine, Dopamine, Norepinephrine, epinephrine;inhibitoryneurotransmitters GABA, serotonin and dopamine; neuromodulators dopamine,acetylcholine,serotonin, histamine, and norepinephrine; and hormones released from the hypothalamus such as oxytocin and vasopressin, which is also referred to as antidiuretic hormone(Dicks, 2022).Movement, emotion, learning, and memory are just a few of the many brain functions that neurotransmitters actively influence. These neurotransmitter imbalances can result in neurodegenerative disease and psychiatric disease such as depression, anxiety, autism spectrum disorder, Parkinson's disease, and Alzheimer disease(Chen et al., 2021). Fig. 1
Fig. 1.
IMMUNE PATHWAY, VAGUS NERVE PATHWAY, HPA AXIS.
GABA: Present in different parts of the brain at high (millimolar) concentrations and is released into the synaptic junction when presynaptic neurons depolarize. Throughout the nervous systems of mammals, vertebrates, and invertebrates, GABA serves as the primary inhibitory neurotransmitter. Disruptions in GABA signaling have well-established causal implications for various neurological disorders. In the gastrointestinal tracts of humans and mice, there are two main types of GABA receptors: GABAA and GABAB. These receptors have been shown to play a crucial role in supporting gut health and intestinal motility. Recent research indicates that the microbiota's production of enteric GABA can influence a range of health outcomes, prompting investigations into the precise mechanisms by which microbial GABA may impact human health and vice versa. For example, in a rat model of visceral sensitivity, injection of a GABA-producing species, Bifidobacterium dentium, which is part of the human microbiota, resulted in desensitization of sensory neuron activity. This finding highlights the potential role of gut microbiota in modulating GABAergic signaling and its implications for gut health (Quillin et al., 2021). GABA predominantly influences adult brain function by activating fast hyperpolarizing GABAA receptors, which mediate rapid inhibitory synaptic transmission. In addition, GABAB receptors, which are located both pre- and post-synaptically, modulate brain activity through slower, G-protein-mediated pathways. These GABAB_BB receptors are linked to potassium (K+) and calcium (Ca2+) channels, affecting neuronal excitability and synaptic transmission via membrane-delimited mechanisms (Wu and Sun, 2015). In the human central nervous system, glutamate and its receptors—primarily ligand-gated ionotropic glutamate receptors—mediate most excitatory neurotransmissions. Given their essential roles in this process, these receptors are important therapeutic targets. Disruptions in normal glutamate signaling are implicated in a range of neurodegenerative diseases, highlighting the significance of glutamate in the pathophysiology of conditions such as Alzheimer's, Parkinson's, and Huntington's disease (Chang et al., 2020). Glutamate decarboxylase catalyzes the α-decarboxylation of l-glutamate to produce GABA (gamma-aminobutyric acid). Additionally, putrescine, arginine, and ornithine can also be used as precursors for GABA synthesis. It has been discovered that many human gut microbiota possess similar biosynthetic enzymes for GABA production. Neurotransmitters like GABA serve as a means for gut bacteria to communicate with the central nervous system, releasing chemicals into the bloodstream that regulate physiological activities in the gut wall. Several bacterial species, including Eubacterium, Parabacteroides, Lactobacillus, Bifidobacterium, Bacteroides, and Blautia, particularly Bacteroides fragilis have been identified as GABA producers. Dysregulation of GABA receptors in the brain has been associated with neurodegenerative conditions such as Alzheimer's disease (Vecchio et al., 2021).
Serotonin and tryptophan: Serotonin regulates many physiological functions, including breathing, vasoconstriction, behavior, gastrointestinal secretion and peristalsis, and brain function. Despite its widespread use throughout the body, 90–95 % of serotonin is located in the gastrointestinal tract, where it is primarily produced by enterochromaffin cells in the epithelial lining. This highlights the crucial role of the gut in serotonin production and its impact on both local gastrointestinal functions and systemic processes such as mood and behavior (Strandwitz, 2018). Tryptophan, an essential amino acid found in fruits, dairy products, and meats, plays a vital role in the production of serotonin. After being absorbed and processed by the body, tryptophan crosses the BBB and is converted into serotonin in the raphe nuclei of the brainstem, a key region responsible for regulating mood and behavior (Mittal et al., 2017). Tryptophan hydroxylase is the rate-limiting enzyme in serotonin synthesis, with the gut's enterochromaffin cells (EECs) being the primary source of serotonin production. Maintaining sufficient levels of tryptophan in the digestive system is crucial for regulating serotonin levels, as the concentration of available tryptophan directly influences the rate of serotonin synthesis by EECs. Without adequate tryptophan, serotonin production can be significantly impaired, affecting various physiological functions dependent on this neurotransmitter (Yeo, 2023).
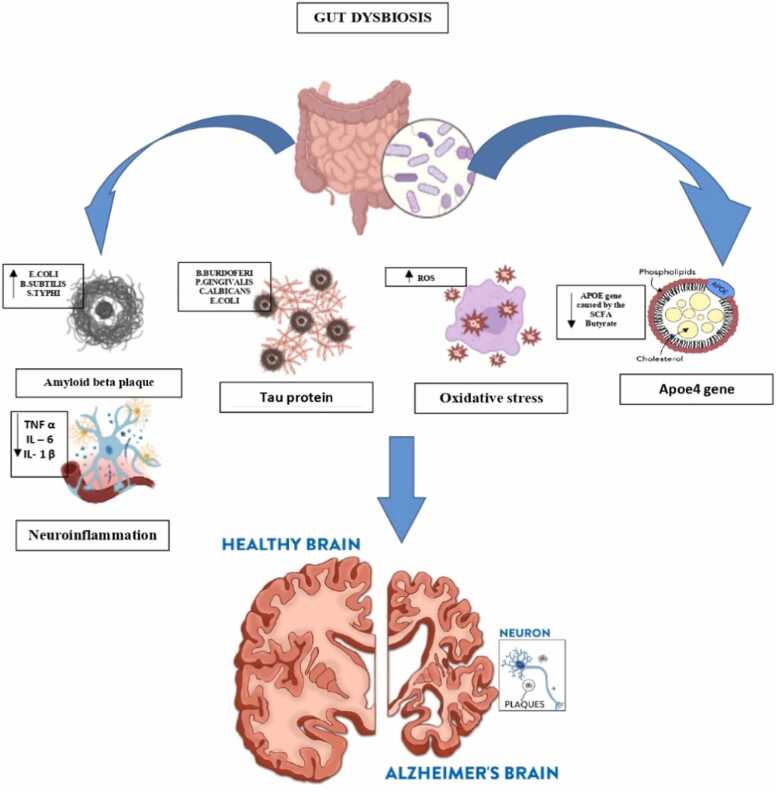
Dopamine: Dopamine, the primary catecholaminergic neurotransmitter, is produced both centrally in the brain and peripherally in various tissues. It plays a crucial role in numerous physiological processes, including regulating emotions, memory, attention, motivation, reward mechanisms, and food intake. Disruptions in dopamine signaling are linked to several neurological and psychiatric disorders, such as Parkinson's disease, schizophrenia, and addiction (Huang and Wu, 2021). Once L-dopa enters the brain, it can follow two primary metabolic pathways in the periphery: one involving catechol-O-methyltransferase (COMT) and the other involving aromatic L-amino acid decarboxylase (AADC). In the periphery, L-dopa is metabolized by AADC into dopamine, but since dopamine cannot cross the blood-brain barrier, it remains in the periphery. This peripheral dopamine can alter intestinal motility and potentially lead to intestinal injury (Xu et al., 2022). Fig. 2
Fig. 2.
Microbiota dysbiosis in Alzheimer’s disease.
3.3. Gut dysbiosis on cognitive decline and neurodegeneration
Dysbiosis, or an imbalance in the gut microbial community, has been connected to several illnesses and diseases that can impair brain illness like Alzheimer's disease (Yang et al., 2024). A decreased amount or variety of the healthy GIT microbiota has been linked to a susceptibility to inflammation and the development of disease (Thornton et al., 2024)..Dysbiosis of the gut microbiota can lead to systemic inflammation and increased intestinal permeability. This disruption in gut health can impact neurological, immunological, endocrine, and metabolic pathways, potentially contributing to the development of Alzheimer's disease (AD) and cognitive impairment. For example, an increase in gram-negative bacteria in the gut raises the levels of lipopolysaccharides (LPS) in the body, which triggers systemic inflammation (Solanki et al., 2023). The movement of lipopolysaccharide (LPS) across the intestinal lining triggers a strong immune response, mediated by TLR4-CD14/TLR2 (Toll-like receptors) through the NF-κB pathway, and activates the hypothalamic-pituitary-adrenal (HPA) axis. In a mouse model, elevated intestinal inflammation (indicated by plasma LPS) and increased apoptosis, particularly in enteric nervous system (ENS) neurons, are linked to gut dysbiosis. LPS also activates mast cells, releasing tryptase, TNF-α, IL-1β, IL-4, IL-13, NF-κB, and pSTAT3, which can worsen mucosal damage through an intensified immune reaction. Moreover, LPS directly affects the blood-brain barrier (BBB), leading to neuroinflammation, microglial activation, and recruitment of immune cells to the central nervous system. This process can contribute to neurodegenerative conditions such as Alzheimer's disease (Bicknell et al., 2023). Disruptions in this axis can increase gut permeability, allowing microbial metabolites and pro-inflammatory molecules to enter the bloodstream. This can exacerbate neuroinflammatory conditions. In turn, systemic inflammatory responses weaken the blood-brain barrier (BBB), promoting neuroinflammation and eventually contributing to neurodegeneration. Alterations in the gut microbiome may be necessary for developing an unstable cerebral endothelial layer and for inflammation-induced breakdown of the BBB (Sochocka et al., 2019). Neurotransmitters play crucial roles in regulating mood and cognition within the brain. The levels of tryptophan in the central nervous system, influenced by peripheral tryptophan availability, significantly affect mood and cognitive functions (Teleanu et al., 2022). Mast cells release corticotropin-releasing hormone (CRH), which increases the permeability of the BBB. This heightened permeability allows circulating cytokines, CRH, and activated immune cells to pass through the BBB, promoting neuroinflammation by stimulating microglia. As a result, bacterial metabolites and toxins can enter the brain, further exacerbating inflammation (Bhuiyan et al., 2021). The gut microbiome plays a crucial role in the production, absorption, and transport of neurotransmitters like serotonin and GABA in the brain. Furthermore, specific bacterial species influence the formation of amyloid plaques, which trigger inflammatory processes and elevate the risk of AD. Thus, an imbalanced gut microbiome is one of the contributing factors to the progression of AD (Loh et al., 2024b). Glutamate activates N-Methyl-D-aspartate (NMDA) receptors, leading to the release of nitric oxide (NO). NO acts as a signaling molecule in the enteric nervous system and the non-adrenergic, non-cholinergic nervous system, helping regulate various physiological functions (Jewett and Thapa, 2024).
Nitrite and nitrate are converted to NO by gut microorganisms, including Lactobacilli and Bifidobacteria. Similarly, other microorganisms like Streptomyces and gut bacilli can utilize the synthesized NO to produce additional NO (Paiva et al., 2024, Sobko et al., 2006). A change in any of these gut bacteria' activity combined with a rise in nitrate consumption might result in an overabundance of nitric oxide, which will cause axonal degeneration, neuroinflammation, and neurological disorder (Khan et al., 2020a).
4. Microbiota dysbiosis in alzheimer disease
4.1. Role of microbiota on amyloid beta cleavage and degradation
Bacterial species that are expected to produce amyloids include Bacillus subtilis, Salmonella Typhimurium, Pseudomonas fluorescens, Staphylococcus aureus, and Escherichia coli. In addition, intestinal microecological dysregulation led to the secretion of amyloid and lipopolysaccharide and increased the permeability of the intestinal and blood-brain barriers (Huang et al., 2023). Certain strains generate amyloids such as curli, TasA, CsgA, FapC, phenol soluble modulins, etc. that encourage the misfolding of Aβ fibrils and oligomers. To form biofilms and withstand being destroyed by immunological or physical forces, bacteria need the creation of amyloid proteins to adhere to each other. Neuronal proteins can promote amyloid production by cross-seeding with bacterial and other amyloid proteins. During self-seeding, a prion-shaped protein induces other molecules of the same protein to misfold into an amyloid structure, enabling the spread of infectious amyloids. The misfolding of several human proteins into amyloid forms is linked to neurodegenerative diseases (Friedland and Chapman, 2017). Aβ peptides found in amyloid plaques are derived from amyloid precursor protein (APP), which is enzymatically cleaved by secretases (α, β, and γ). The formation of Aβ plaques begins with this cleavage process. In particular, β-secretase initiates the cleavage of APP, resulting in C-terminal fragments of 89 or 99 amino acids, which remain attached to the membrane. BACE1 (β-site APP cleaving enzyme), also known as Asp2 or memapsin 2, plays a critical role as the β-secretase responsible for this cleavage (Hampel et al., 2021). Amyloid beta precursor protein is cleaved at β-sites, specifically Asp1 and Glu11, by BACE1. This cleavage produces isoforms such as Aβ1–42 and Aβ1–40 when γ-secretase further processes the remaining 99 amino acid residues attached to the C-terminal membrane. The two primary components of γ-secretase are presenilin 1 and presenilin 2. The most common soluble isoform is Aβ1–40. However, if the cleavage pattern is altered, it can result in Aβ1–42, which tends to aggregate and form plaques due to the presence of two additional amino acids: isoleucine and alanine. These cleavage pattern changes are often caused by mutations in the APOE gene (Khan et al., 2020a, Khan et al., 2020b). The TLRs that detect Aβ and α-synuclein are also activated by curli, a bacterial amyloid. Curli has been shown to compromise the blood-brain barrier by increasing pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. This suggests that bacterial amyloids promote protein aggregation and neuroinflammation, which can lead to neurodegeneration. Additionally, since Aβ, tau, and α-Syn have antibacterial properties, inflammation and gut dysbiosis may trigger their aggregation (Miller et al., 2021).
4.2. Accumulation of tau protein and neurofibrillary tangles
A microtubule-associated protein called tau regulates axonal transit and keeps the microtubules inside the axon stable (Thal and Tomé, 2022). In AD, tau protein loses its ability to bind to microtubules, which impairs its normal role in maintaining the organization of the cytoskeleton within axons. In the brains of individuals with dementia, tau undergoes structural changes and misfolding, leading to abnormal aggregation into fibrillary formations (Kolarova et al., 2012). Studies have shown that AD patients with chronic Helicobacter pylori infections produce elevated levels of inflammatory cytokines (Yu et al., 2018). Furthermore, exposure to H. pylori filtrate has been demonstrated to induce tau hyperphosphorylation in neuroblastoma cells, mimicking the tau pathology characteristic of AD (Wang et al., 2014). Certain bacteria can collaborate to increase the infectious burden in the brains of AD patients. Elevated levels of pro-inflammatory markers, such as IL-1β, CXCL2, and NLRP3, have been linked to the presence of harmful bacteria like Escherichia and Shigella. In contrast, beneficial anti-inflammatory bacteria, such as Eubacterium rectale, are found in reduced quantities in the blood of individuals with brain amyloidosis and cognitive impairment (Cattaneo et al., 2017). The cognitive deficits caused by dysbiosis that encourage AD might take many different forms (Solanki et al., 2023). One important characteristic of AD is the misfolding and aggregation of tau. Studies have demonstrated that bacterial DNA, particularly from specific species linked to AD (B. burgdorferi, P. gingivalis, C. albicans, and E. coli), induces marked tau aggregation (Tetz et al., 2020). These strains, due to their facultative intracellular parasitic nature, facilitate interactions between bacterial DNA and tau proteins within neurons. Bacterial DNA can be secreted across the outer membrane into the cytoplasm of neurons, where it may act as a seed for tau aggregation. This indicates that microbial DNA could play a key role in triggering tau protein misfolding, contributing to the pathogenesis of AD (Liang et al., 2024).
4.3. APOE cholesterol pathway
The gut microbiota can influence gene expression in microglia, which is linked to the regulation of apolipoprotein E (APOE). The APOE ε4 allele, located on chromosome 19q13.2, is the first gene identified to significantly increase the risk of sporadic late-onset AD. While APOE is primarily expressed in astrocytes, it is also produced by microglia under inflammatory conditions, despite being one of the rarer apolipoproteins synthesized in the brain (Murai and Matsuda, 2023). Its primary role in the body is that of a lipid transporter, carrying phospholipids and cholesterol throughout. Hepatocytes and macrophages in the liver are the primary producers of apoE in the periphery. Although apoE is not able to pass through the blood-brain barrier, it is highly produced by astrocytes, activated microglia, vascular mural cells, choroid plexus cells, and to a lesser extent by stressed neurons in the central nervous system. Apolipoprotein E genotypes and the composition of the gut microbiome were found to be related by research on the effects on the physiology and composition of the microorganism in mammals and mice. This work highlights the possibility that the APOE4 allele's effect on neuropathology may be caused by the elimination of bacteria that produce butyrate and short-chain fatty acid in APOE4 carriers (Raulin et al., 2022).
4.4. Acetylcholine production
Acetylcholine, a cholinergic neurotransmitter, serves as a local mediator in both insects and vertebrates by transducing excitatory impulses between neurons in the peripheral and CNS. Its dysregulation is closely associated with Alzheimer's disease. Amyloid-β pathology and cholinergic disorders have a long-standing association. Neuritis plaques were discovered to be more prevalent in the post-mortem brains of AD patients, and this was linked to the enzyme choline acetyltransferase's decreased activity. The correlation between the worsening of dementia and disruption of several cortical cholinergic markers, including reduced acetylcholine synthesis and levels, decreased nicotinic receptor subtypes, and reduced choline-acetyltransferase and choline uptake, suggests a close relationship between cholinergic loss of function and cognitive decline in AD (Giacobini et al., 2022)..Acetylcholine production, storage, transport, and degradation-related proteins are the foundation of cholinergic neurotransmission. About 20 % of choline and active acetate are used to create this in the cytoplasm of cholinergic neurons, with the majority (80 %) synthesizing at the terminal buttons. Axonal termination captures choline by activating a specialized transport pathway. Outside of the cell, choline is produced by the hydrolysis of acetylcholine and the breakdown of lipids, primarily lecithin (Choo et al., 2024). Human acetylcholinesterase a crucial enzyme in neural signaling breaks down acetylcholine and obstructs postsynaptic signal transmission. In the central nervous system, cholinergic neurotransmission is essential for both cell survival and neuronal plasticity. G-protein activity and ion influx are the two ways whereby muscarinic and nicotinic initiate intracellular signaling. The enzyme AchE breaks down the excess Ach in the synaptic cleft into acetate and choline. The presynaptic neurons then recycle and reabsorb choline through choline transporters, primarily CHT1. This reuptake significantly increases the pool of choline that cholinergic neurons will employ for acetylcholine resynthesis. It has been shown that AchE and ChAT, two important markers of cholinergic neuronal function, are expressed at reduced levels in late-stage AD (Stanciu et al., 2020).
4.5. Oxidative stress
Higher levels of reactive oxygen species (ROS) than antioxidants disrupt the redox signaling system in cells, which is known as oxidative stress. In addition to having negative impacts, this imbalance is a major contributor to several neurological disorders. All aerobic metabolic chemical reactions lead to the creation of unstable and short-lived reactive intermediate products reactive oxygen species. It has been shown that the PI3K/AKT pathway plays a role in supporting the proliferation of neural progenitor cells by producing O2 and H2O2 through the action of NADPH oxidase (NOX-2) (Bekdash, 2021). Mitochondrial enzymes are primarily responsible for producing ROS in mitochondria. Superoxide radicals are produced at respiratory complexes I and III of the oxidative phosphorylation pathways by the mitochondrial electron ETC through the single-electron leak. Physiological levels of OS produced by the microbiota may have an impact on the organism's makeup and functioning. The interactions between microorganisms and hosts that may alter the central nervous system redox equilibrium by raising ROS levels, undermining the antioxidant system, or both further complicate the interplay between the gut microbiota and the brain. Consequently, these relationships may lead to higher degrees of oxidative damage in the central nervous system (Shandilya et al., 2022). According to Seungmoon Chooetal., Heat-killed Ruminococcus albus (hkRA) shields neurons from oxidative stress-related damage. To assess how well hkRA protected the human neuroblastoma SH-SY5Y cell from Aβ-induced apoptosis. The SH-SY5Y cells' exposure to HkRA (108 cells/mL) dramatically reduced the cytotoxicity and DNA damage caused by Aβ. Additionally, it demonstrated a remarkable rise in the bax/bcl-2 ratio in the SH-SY5Y cells treated with Aβ. Furthermore, the administration of hkRA enhanced the expression of brain-derived neurotrophic factor (BDNF) and the antioxidant-related genes HO-1, Nrf2, and PKC-δ. In the meantime, it dramatically reduced the cleaved caspase-3 protein expression and caspase-3 activity in the Aβ-treated SH-SY5Y cells. Furthermore, the amounts of mitochondrial and in addition, the cells' levels of the proteins encoding mitochondrial and cytosolic cytochrome c increased and decreased respectively. These findings imply that hkRA guards against oxidative stress and Aβ-induced apoptosis in human neuroblastoma cells (Sharma and Kim, 2021).
5. Epigenetic modifications
5.1. Histone modification
Histone acetylases are a class of enzymes that acetylate the lysine residues of the core histone tails to covalently alter histone proteins (Esposito and Sherr, 2019). Numerous posttranslational modification sites, including methylation, acetylation, phosphorylation, ubiquitylation, and are found in the N-terminal tails of histones. These epigenetic changes to the chromatin structure mediate the accessibility of the genomic information in the nucleus and control gene expression in response to a variety of internal and external stimuli. Histone acetylation has been demonstrated to play a role in the development of excitatory synapses and long-term memory in the hippocampus, which is crucial for the most prevalent types of synaptic plasticity, such as long-term potentiation (LTP) (Santana et al., 2023). In a recent study carried out in germ-free mice, they were treated orally administered with Roseburia hominis and the result suggests that Roseburia hominis act as HDAC inhibitors, and reduce neuroinflammation (Song et al., 2022)
5.2. DNA methylation
Methylation of cytosine residues on a particular DNA molecule's C-5 atom is the most well-studied instance of epigenetics in the process of gene silencing (Qazi et al., 2018). Gene expression is significantly regulated by DNA methylation, and abnormal DNA methylation has been linked to the pathophysiology of Alzheimer's disease (Huo et al., 2019).As neural progenitor cells transition from neurogenesis to astrogliogenesis as neurons proliferate and mature in the adult brain, a methyl group is added to and removed from the promoter gene (Kaur et al., 2022).Lactobacillus rhamnosus GG and Bifidobacterium breve reduced levels of the inflammatory markers IL-2, IL-7, and CD40 by blocking global DNA methylation and histone acetylation (D’Aquila et al., 2020)
5.3. Non-coding RNAs
Non-coding RNAs (ncRNAs) are a broad family of non-protein-coding RNA transcripts that may be used as therapeutic targets and biomarkers for a variety of physiological and pathological conditions because of their crucial regulatory roles in numerous biological processes and disease development (Olufunmilayo and Holsinger, 2023). ncRNAs, which are classified as long non-coding RNA (lncRNA, >200 nt), small ncRNA (sncRNA, < 200 nt), and circRNA, control translation, epigenetic inheritance, and heredity. Certain ncRNAs may be present in a wide range of conditions, and they are essential for regulating inflammation in many conditions. miRNAs support neuronal differentiation, development, and synaptic plasticity (Y. Liu et al., 2022).
6. Diagnostic approches
Diagnosis of a dementia illness is based on clinical indications and symptoms. Neuropsychological testing, laboratory testing (blood and other internal fluids), brain scanning, and genetic testing are some of the procedures used to diagnose dementia after a history of illness, and a neurological exam, which includes a neurologic and psychiatric assessment (Turner et al., 2020).To distinguish behavioral and psychiatric symptoms caused by neurodegenerative illnesses like Alzheimer's disease from those caused by other conditions like mood disorders; clinicians must employ proper measures (Guzman-Martinez et al., 2021). Routine use of these tests in the clinic is still premature due to the potential for overdiagnosis, increased cost, and/or invasiveness of the evaluation method. This is true even though positron emission tomography imaging of amyloid and the levels of tau and amyloid-proteins in the cerebrospinal fluid are increasingly used in clinical studies of AD, improving diagnostic confidence in AD subjects (Porsteinsson et al., 2021). Brain structure can be directly measured via MRI, which may be used as a biomarker to distinguish between the brains of AD patients and normal control brains. Many investigations into the possibility of MRI-based AD biomarkers have been carried out. However, because amyloid-β accumulates early in the course of the illness and reaches a plateau later, MRI scans are unable to visualize the pathophysiological hallmarks of AD, and amyloid PET cannot easily show the progression of AD (Chang et al., 2021). Structural MRI is used not only to evaluate volume decreases but also to identify changes in cortical thickness in specific brain regions, including the temporal, orbitofrontal, and parietal areas. Extensive research has elucidated the impact of AD on cortical thickness and has given rise to the concept of an AD "disease signature," whereby specific brain regions that are known to be impacted by AD exhibit cortical thinning. Because cortical thickness assessment allows for the detection of minute changes in regions known to be impacted by AD, it is thought to be a valuable biomarker in the early identification of the disease (van Oostveen and de Lange, 2021).
7. Therapeutic approaches
7.1. Pharmacological approches
7.1.1. Alzheimer disease medication
Acetylcholine esterase inhibitors: Alzheimer's disease drugs approved by the Food and Drug Administration are memantine, galantamine, donepezil, and rivastigmine, which are acetylcholine esterase inhibitors. Acetylcholine esterase inhibitors work by blocking the enzyme acetylcholinesterase in the synaptic cleft, which is responsible for breaking down acetylcholine levels in the brains of AD patients. Therefore, at least in the first year of treatment, AChEIs tend to ameliorate the decrease in cognition by enhancing central cholinergic neurotransmission. Even a brief stop of these medications causes rapid deterioration and is linked to an increased chance of being placed in a nursing home (Yiannopoulou and Papageorgiou, 2020).
7.1.2. Stem cell therapy: Based on stem cells' ability to generate new neurons, stem cell therapy is an appropriate and successful restorative approach for treating AD and other neurodegenerative illnesses. The proposed method replaces lost neurons during the neurodegenerative stages of AD with stem cells. The most often utilized cell types in AD research in recent years have been induced pluripotent stem cells (iPSCs), Mesenchymal stem cells, brain-derived neural stem cells (NSCs), and embryonic stem cells (ESCs) (Liu et al., 2020). The grafted NSCs directly impact the recipient tissue and make up for the lost neurons. In an AD-like rodent paradigm, for example, the transplantation of NSCs expressing growth factor increases neurogenesis and ameliorates cognitive impairment. The transplanted NSCs may, however, potentially differentiate into non-neuronal glia, which is a drawback for their use (Qin et al., 2022).MSCs can be obtained from a variety of sources. Under specific circumstances, MSCs can differentiate into a variety of mesodermal cell types, including osteoblasts, myocytes, adipocytes, chondrocytes, and tendon cells. MSCs possess various modulatory attributes, including a wide spectrum of distinguishing potential, accessibility, simplicity of handling, and availability. MSCs are characterized by their ability to cross the BBB, active homing ability, and efficient migration capacity towards damaged brain regions (Fouad, 2019).
7.2. Non- pharmacological approaches
7.2.1. Probiotics
Probiotics are living bacteria that are important to human health, particularly the digestive tract. It's common to refer to probiotics as "good" or as aiding in gut health maintenance. The definition of "probiotics" as "live microorganisms that modify microbiota towards a beneficial state" was first used in 1974, and it has since conceptually evolved. The probiotic most frequently found species, Lactobacillus or Bifidobacterium genera, of which only a small number of members are thought to be healthy and normally occur in the gut microbiota. The bacteria linked to probiotics can improve cognitive performance and help prevent memory loss in Alzheimer's disease. Furthermore, probiotics have been demonstrated to influence several cognitive, emotional, learning, and memory activities inside the central nervous system by producing NT and Short-chain fatty acids (Dhami et al., 2023). Maintain the integrity of the intestinal lining, control the body's pH level, and increase brain-derived neurotrophic factors. The brain's neurotrophic factors consist of a particular kind of protein that aids in neurons' survival and differentiation. As such, it is essential to the development of the nervous system. Probiotics have the potential to improve mental health and treat memory impairments and psychiatric diseases by altering brain chemicals like dopamine, γ-aminobutyric acid (GABA), and serotonin, in addition to brain neurotropic factors (Naomi et al., 2022). Probiotics use a variety of methods to produce their advantageous effects, including the generation of Saturated fatty acids, the release of bacteriocin, immunomodulation, and effects on the gut-brain axis. Saturated fatty acids are created in the stomach because of eating fiber. Bacteriodes-mediated fermentation metabolites like acetate, butyrate, and propionate are produced by the Clostridium, Lactobacillus, Bifidobacterium, and Eubacterium species (Ale and Binetti, 2021). Probiotics affect the intestinal microbiome of the host which is the population genetic information of bacteria, viruses, and other microbiota together. Fungus grows on and inside human bodies Furthermore the host's microbes use them only selectively and spare them from the host's enzymes (Dobielska et al., 2022). Probiotics show their protective effects by reducing oxidative stress pathways, which also reduce inflammation and apoptosis. A natural method that is both safe and efficient for minimizing the growth of infections and their unwanted side effects is probiotic therapy (Talebi et al., 2022). There are several scientifically proven advantages to regularly consuming probiotic foods and supplements, including improvements to the gut such as relief from diarrhea and other digestive issues. Advantages to a range of disorders from emotional imbalance to autoimmune diseases, as well as symptoms reduction of inflammation. The evaluation of the impact of probiotic intervention on the gut microbiota of the host requires the use of metabolomic and transcriptomic technologies. Despite the public's widespread promotion of probiotics, several probiotic strains and formulations have inconsistent clinical outcomes. The negative consequences of probiotics, which include immune system stimulation, Gastrointestinal side effects, gene transfer from probiotics to normal microbiota, systemic infection, and detrimental metabolic effects, should receive more study. Additionally, strategies to address colonization resistance must be developed for probiotic therapy for AD in the future (Lee et al., 2022).
7.2.2. Diet
It is widely recognized that changing one's diet can impact the microbiota's makeup in the gut. Dietary adjustments are intended to motivate the growth of the good bacterium that is present in the GIT rather than to introduce new beneficial bacteria (Holmes et al., 2020). Nutritious and healthful food ingredients possess anti-inflammatory and antioxidant qualities, which help control the immune system and potentially alter the neuroinflammatory processes that lead to the development of AD and cognitive decline. Numerous dietary elements have been studied for their involvement in health and disease, including omega-3 fatty acids, nutraceuticals, minerals, micronutrients, and vitamins (Bhatti et al., 2020).
7.2.3. Sleep
Sleep is critical for memory consolidation since it facilitates the transfer of memory storage from the hippocampus to the neocortical circuits. It can also accelerate the removal of metabolites from synapses, particularly neurotoxic compounds like β-amyloid, which are linked to Alzheimer's disease… Additionally, sleep might be crucial for maintaining cognitive reserve. Therefore; it is thought that sleep disturbances may be a risk factor for cognitive impairments (Huynh et al., 2022).
7.2.4. Exercise
Aerobic activity is the majority of exercise that is discussed concerning the microbiota. Regular aerobic exercise is linked to improved cognitive function and a lower risk of dementia throughout life. Exercise also results in a decrease in inflammation by raising levels of important antioxidant enzymes, anti-inflammatory cytokines, and antiapoptotic proteins (Koblinsky et al., 2023). About eight main pathways by which Physical exercise can alter AD pathophysiology were identified by the authors of a recent comprehensive analysis of the literature focused on how PE affects the pathways that underlie AD pathogenesis. It's important to keep in mind the functions of the immune system and inflammation among these pathways, as these are linked to pathways of cell senescence or survival, as well as PE's ability to shield cells from oxidative stress and lipid peroxidation and improve insulin sensitivity and energy metabolism (Cutuli et al., 2023).Exercise is also known to protect against Aβ neurotoxicity associated with the disease and to lower the concentration of Aβ in the plasma (Aczel et al., 2022).Exercises has been shown to significantly modify the Gut microbiota in animal models when it comes to the gut microbiota. After five weeks, they discovered that the microbiota composition of the mice given unrestricted access to the exercise wheel (VWR) differed from that of the control group of sedentary mice (SED). Additionally, they discovered that the butyrate concentration had increased by a factor of two due to exercise-induced changes in two different species of butyrate-producing bacteria (SM7/11 and T2–87). This is noteworthy since colon lining epithelial cells prefer butyrate as their energy source. It has also been shown to have several positive impacts on gut function, such as the regulation of inflammation, sensitivity to insulin, and satiety (Cataldi et al., 2022).
8. Preclinical studies
The primary technique employed in the Microbiota-Gut-Brain Axis research has been the genomic or metagenomics analysis of material obtained from human or animal models. There aren't enough in vitro models available yet to replicate the communication between the intestinal epithelium and the Gut microbiome complex or host-microbiome interactions. studied the activity of lactobacillus fermentum LAB9 or l. casei LABPC by inducing the lipopolysaccharide-activated BV2 cells in vitro produces the result of a decrease in neuroinflammation. Probiotics are hypothesized to have antioxidant benefits by secreting compounds with antioxidant characteristics or by modifying endogenous antioxidant defense systems. Although at different degrees, antioxidant activities were found in the conditioned media and viable cells of (Lab4) and (Lab4b), indicating both consortia as possible neuroprotective effects. Mice models can be used to study the human gut microbiota and its function in diseases and inflammatory processes. Human and mouse digestive tracts are anatomically and physiologically comparable, however, there are noticeable changes between them are observed (Carranza-naval et al., 2021). Two primary pathological characteristics of AD are cerebral amyloidosis and severe tauopathy. Alzheimer's disease is significantly influenced by the gut microbiota. In a recent study, they developed the transgenic mice model with amyloid and neurofibrillary tangles, which exhibits amyloid plaques, reactive gliosis, and neurofibrillary tangles in their brains along with memory deficits, and looked at the role of the gut microbiota on AD pathogenesis. The gut microbiota of healthy wild-type (WT) mice was not the same as that of ADLP APT mice. In addition, ADLP APT mice displayed systemic and intestinal persistent inflammation as well as a loss of epithelial barrier integrity. Glial reactivity, cognitive impairment, and the development of amyloid β plaques and neurofibrillary tangles were all lessened in ADLP APT mice when the fecal microbiota from WT mice was transplanted and transferred often. Furthermore, the fecal microbiota transfer repaired the abnormal colonic expression of genes linked to intestinal macrophage activity and the circulating blood inflammatory monocytes in the ADLP APT recipient mice. Changing the microbiota through methods, such as adding beneficial microorganisms or altering one's diet, may be a promising preventative and treatment strategy for AD. Probiotic formulations with one or more strains proved to be effective oral therapies. Age-related memory decline and cognitive impairment have been observed in mouse research. In mice with AD, in particular, these functions are substantially impaired. In vivo research, probiotics have successfully altered gut microbiota, which may improve age-related cognitive abilities in animal models. For instance, an in vivo study conducted on D-Galactose-induced AD mice by, Lactobacillus plantarum MTCC 1325 reveals decreased levels of NFT and amyloid plaques and increased levels of Ach, a neurotransmitter that helps to improve behavioral activity and restore normal conditions in the hippocampus and cerebral cortex of the brain. Simultaneously, the mice's memory and spatial learning have been shown to improve (Nimgampalle and Yellamma, 2017). The study performed using the (Lactobacillus plantarum KY1032 and Lactobacillus curvatus HY7601 probiotic supplement in Transgenic 3xTg-AD mice improves the delaying of neurodegeneration (Medeiros et al., 2024). They performed the study using the male Sprague Dawley rat by inducing the Aβ(1−42) and by administering the Lactobacillus and Bifidobacterium for six weeks they improved the learning but not the memory impairment by the release of neurotransmitters (Rezaeiasl et al., 2019). The study was carried out in the APP/ PS1 transgenic mouse model by administering the Bifidobacterium Lactisprobio-M 8 to improve the Aβ plaque and elevate cognitive impairment. They performed the study using the lipopolysaccharide rat model, by administering a probiotic supplement containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduced the levels of proinflammatory cytokines in the serum and the neuronal pathway (Mohammadi et al., 2019). Probiotic supplements are one of the alternative approaches for AD. Preclinical studies on probiotics are tabulated in Table 1 and a few are listed below.
Table 1.
preclinical studies on probiotics.
| Probiotic strain | Species/strain | Induction method | Outcomes | References |
|---|---|---|---|---|
| Lactobacillus plantarum MTCC1325 | Albino rat | Alzheimer's disease in albino rats produced by D-galactose. | It enhances cholinergic neurotransmitters in the brain's cerebral cortex and hippocampal regions, which helps to normalize behavior and improves state. | (Nimgampalle and Yellamma, 2017) |
| Lactobacillus plantarum KY1032 and Lactobacillus curvatus HY7601 | Mice | Transgenic 3xTg-AD mice | It is effective in delaying the neurodegeneration | (Medeiros et al., 2024) |
| Lactobacillus and Bifidobacterium | Male Sprague-Dawley rats | Aβ (1−42)-induced Model |
It improves learning but not memory impairment | (Rezaeiasl et al., 2019) |
| (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) | Male Wistar rat | Lipopolysaccharide Rat Model | Improves the neuronal pathways and decreases the proinflammatory cytokines levels (TNF-α and IL1-β) | (Mohammadi et al., 2019) |
| Lactobacillus fermentum LAB9 or L. casei LABPC) | Mice | lipopolysaccharide (LPS)-activated microglia BV2 cells in vitro | The modulation of neuroinflammation was likely mediated through up-regulation of antioxidants, inhibition of AChE, down-regulation of proinflammatory cytokines. | (Mohammadi et al., 2019) |
| Bifidobacterium breve MCC1274 | Human neuroblastoma cells SH-SY5Y | Lipid droplet formation in neuroblastoma cells undergoing oxidative stress | When taken regularly, B. breve MCC1274 may help prevent brain inflammation in patients with MCI and dementia, including Alzheimer's disease. | (Bernier et al., 2023) |
| Bifdobacterium Lactis Probio-M8 | Mice | APP/PS1 Transgenic mice model | The study findings showed that Probio-M8 guarded against gut microbiota dysbiosis and decreased the amount of Aβ plaque in the entire brain. | (Cao et al., 2021) |
| Bifidobacterium bifidum TMC3115, Lactobacillus plantarum 45 (LP45) | Mice | APP/PS1 mice | In APP/PS1 mice, long-term combination administration of TMC3115 and LP45 can reduce spatial memory impairment, indicating that altering the gut microbiota may offer potential advantages for individuals with AD. | (Wang et al., 2020) |
| Clostridium butyricum | Mice | APP/PS1Transgenic mice | By controlling the GM-gut-brain axis, which is mediated by the metabolite butyrate, CB therapy may reduce microglia-driven neuroinflammation. | (Sun et al., 2020) |
| Lactobacillus lactis | Mice | 3xTg-AD mice increased level of p62 protein is a hallmark symptom for the Alzheimer's disease in 3xTg-AD | The study findings suggest that these extremely safe, non-pathogenic, and non-invasive bacteria used as delivery vehicles for the p62 protein represent an innovative and realistic therapeutic approach in AD | (“aging−12–103900,” n.d.) |
9. Human studies
In humans the nutritional interventions that will alter gut microbiota have been reported; these interventions primarily involve healthy volunteers and older adults with memory issues. It is important to keep in mind that AD, insulin resistance, diabetes, obesity, and cardiovascular disease are all closely related and that developing preventative measures is imperative. In particular, it was noted that in elderly patients with memory impairment, long-term supplementation with beta-Lactobacterium breve A1 restored cognitive skills (Bonfili et al., 2021)A recent study found that giving AD patients a continuous milk kefir supplement improved their metabolic and cognitive problems. It was an unmonitored clinical investigation conducted to observe the impact of giving AD patients with cognitive abnormalities a daily dose of milk kefir (2 mL/kg) for 90 days. The study evaluated blood cell damage biomarkers, cytokine expression, systemic oxidative stress levels, and cognitive performance before and during milk kefir consumption, The study found that most AD patients who supplemented with milk kefir experienced a discernible improvement in their memory, executive/language, and visual-spatial/abstraction abilities. The study done by cytometry revealed 100 % increased NO bioavailability along with a ∼30 % drop in oxidative stress and inflammatory indicators Furthermore; improvements were noted in the areas of mitochondrial malfunction, apoptosis, DNA damage/repair, and serum protein oxidation. The study findings suggest that taking supplements of milk kefir helps with cognitive impairments and AD-related conditions such as oxidative stress, systemic inflammation, and blood cell destruction (Kumar et al., 2022). The study carried out by, the randomized, double-blind, and control studies carried out using probiotic supplementation for twelve weeks have improved cognitive function. Human studies on probiotics are tabulated in Table 2 and a few are listed below.
Table 2.
Human studies on probiotics.
| Probiotic supplementation | Study | Observation | References |
|---|---|---|---|
| probiotic-fermented milk supplementation contains s Acetobacter aceti, Acetobacter sp., Lactobacillus delbrueckiidel brueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Enterococcus faecium, Leuconostoc spp., Lactobacillus kefirano faciens, Candida famata, and Candida krusei | During 90 days, probiotic supplementation (2 mL/kg/daily) was given to elderly Alzheimer's patients to evaluate their cytokine expression cognitive function, blood cell damage, systemic oxidative stress levels | Improving memory, language, executive functions, visual-spatial function, and systemic inflammation | (Kumar et al., 2022) |
| Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum | A randomized, double-blind, and controlled clinical trial was conducted among 60 AD patients by administering for 12 weeks to assess the cognitive status and metabolic functions. | probiotics treatment was ineffective on other biomarkers of oxidative stress and inflammation, FPG, and other lipid profile | (Akbari et al., 2016) |
| probiotic containing L. acidophilus, B. bifidum, and Bifidobacterium longum selenium (200 mg/day) plus | A randomized, double-blind, controlled clinical trial was conducted among 79 patients with Alzheimer's disease | Cognitive performance and certain metabolic profiles were enhanced in Alzheimer's disease patients receiving probiotic and selenium co-supplements for 12 weeks. | (Tamtaji et al., 2019) |
| Bifidobacterium breve | Randomized, Double-Blind, Placebo-Controlled Trial was carried out in 80 healthy older adults | The Study results that B. breve A1 is a safe and effective approach for improving memory functions. | (Xiao et al., 2020) |
| Lactobacillus GG (LGG) | A randomized clinical trial was carried out in a 3-month trial in cognitive functioning in middle-aged and older adults. | The current investigation found that using LGG supplements improved mood and cognitive function. | (Sanborn et al., 2018) |
| Lactobacillus rhamnosus | Randomized Clinical Trial carried out in Middle-aged and Older Adults | Probiotic supplements containing Lactobacillus rhamnosus GG have been linked to better cognitive function in middle-aged and older persons with cognitive impairment. Supplementing with probiotics might be an innovative way to preserve cognitive health during old age. | (Sanborn et al., 2020) |
10. Conclusion
In conclusion, this review underscores the significant connection between gut dysbiosis and the pathophysiology of AD. Investigating the gut-brain axis reveals how changes in gut microbiota composition can adversely affect neurological health outcomes in older adults. Furthermore, probiotic supplementation holds promise for restoring the balance of gut microbiota and enhancing cognitive and behavioral functions. These findings highlight the critical need for further research into microbiota-targeted therapies as a potential strategy for managing AD and improving patient outcomes.
CRediT authorship contribution statement
N Harikrishnan: Visualization, Validation. Ankul Singh S: Writing – review & editing, Validation, Supervision. S Sowmiya: Writing – original draft. L.S Dhivya: Investigation, Formal analysis. Rajendran Praveen: Resources, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aczel D., Gyorgy B., Bakonyi P., Bukhari R., Pinho R., Boldogh I., Yaodong G., Radak Z. The systemic effects of exercise on the systemic effects of Alzheimer’s disease. Antioxidants. 2022 doi: 10.3390/ANTIOX11051028. aging-12-103900, n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari E., Asemi Z., Kakhaki R.D., Bahmani F., Kouchaki E., Tamtaji O.R., Hamidi G.A., Salami M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ale E.C., Binetti A.G. Role of probiotics, prebiotics, and synbiotics in the elderly: insights into their applications. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.631254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman R.S., Moncada M., Aryana K.J. Leaky gut and the ingredients that help treat it: a review. Molecules. 2023 doi: 10.3390/molecules28020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H., Iftikhar A., Muzaffar H., Almatroudi A., Allemailem K.S., Navaid S., Saleem S., Khurshid M. Biodiversity of gut microbiota: impact of various host and environmental factors. Biomed. Res Int. 2021;2021 doi: 10.1155/2021/5575245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique S., Mohanto S., Ahmed M.G., Mishra N., Garg A., Chellappan D.K., Omara T., Iqbal S., Kahwa I. Gut-brain axis: a cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e34092. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ashique S., Mohanto S., Ahmed M.G., Mishra N., Garg A., Chellappan D.K., Omara T., Iqbal S., Kahwa I. Gut-brain axis: a cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e34092. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aziz Q., Doré J., Emmanuel A., Guarner F., Quigley E.M.M. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol. Motil. 2013 doi: 10.1111/nmo.12046. [DOI] [PubMed] [Google Scholar]
- Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., Gopal P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020 doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash R.A. The cholinergic system, the adrenergic system and the neuropathology of alzheimer’s disease. Int J. Mol. Sci. 2021 doi: 10.3390/ijms22031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier F., Kuhara T., Xiao J. Probiotic bifidobacterium breve MCC1274 protects against oxidative stress and neuronal lipid droplet formation via PLIN4 gene regulation. Microorganisms. 2023;11 doi: 10.3390/microorganisms11030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti G.K., Reddy A.P., Reddy P.H., Bhatti J.S. Lifestyle modifications and nutritional interventions in aging-associated cognitive decline and Alzheimer’s Disease. Front Aging Neurosci. 2020 doi: 10.3389/fnagi.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan P., Wang Y.-W., Sha H.-H., Dong H.-Q., Qian Y.-N. Neuroimmune connections between corticotropin-releasing hormone and mast cells: novel strategies for the treatment of neurodegenerative diseases. Neural Regen. Res. 2021;16:2184. doi: 10.4103/1673-5374.310608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell B., Liebert A., Borody T., Herkes G., McLachlan C., Kiat H. Neurodegenerative and neurodevelopmental diseases and the gut-brain axis: the potential of therapeutic targeting of the microbiome. Int J. Mol. Sci. 2023 doi: 10.3390/ijms24119577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L., Cecarini V., Gogoi O., Gong C., Cuccioloni M., Angeletti M., Rossi G., Eleuteri A.M. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. FEBS J. 2021 doi: 10.1111/febs.15571. [DOI] [PubMed] [Google Scholar]
- Borrego-Ruiz A., Borrego J.J. Influence of human gut microbiome on the healthy and the neurodegenerative aging. Exp. Gerontol. 2024;194 doi: 10.1016/j.exger.2024.112497. [DOI] [PubMed] [Google Scholar]
- Bosi A., Banfi D., Bistoletti M., Giaroni C., Baj A. Tryptophan metabolites along the microbiota-gut-brain axis: an interkingdom communication system influencing the gut in health and disease. Int. J. Tryptophan Res. 2020 doi: 10.1177/1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campaniello D., Corbo M.R., Sinigaglia M., Speranza B., Racioppo A., Altieri C., Bevilacqua A. How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients. 2022 doi: 10.3390/nu14122456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Amakye W.K., Qi C., Liu X., Ma J., Ren J. Bifidobacterium Lactis Probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur. J. Nutr. 2021;60:3757–3769. doi: 10.1007/s00394-021-02543-x. [DOI] [PubMed] [Google Scholar]
- Carranza-naval M.J., Vargas-soria M., Hierro-bujalance C., Baena-nieto G., Garcia-alloza M., Infante-garcia C., Del Marco A. Alzheimer’s disease and diabetes: Role of diet, microbiota and inflammation in preclinical models. Biomolecules. 2021 doi: 10.3390/biom11020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi S., Poli L., Şahin F.N., Patti A., Santacroce L., Bianco A., Greco G., Ghinassi B., Di Baldassarre A., Fischetti F. The effects of physical activity on the gut microbiota and the gut–brain axis in preclinical and human models: a narrative review. Nutrients. 2022 doi: 10.3390/nu14163293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., Bianchetti A., Volta G.D., Turla M., Cotelli M.S., Gennuso M., Prelle A., Zanetti O., Lussignoli G., Mirabile D., Bellandi D., Gentile S., Belotti G., Villani D., Harach T., Bolmont T., Padovani A., Boccardi M., Frisoni G.B. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Geurts L., Hoyles L., Iozzo P., Kraneveld A.D., La Fata G., Miani M., Patterson E., Pot B., Shortt C., Vauzour D. The microbiota–gut–brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022 doi: 10.1007/s00018-021-04060-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Lin C.H., Lane H.Y. Machine learning and novel biomarkers for the diagnosis of alzheimer’s disease. Int J. Mol. Sci. 2021 doi: 10.3390/ijms22052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Lin C.H., Lane H.Y. D-glutamate and gut microbiota in Alzheimer’s disease. Int J. Mol. Sci. 2020 doi: 10.3390/ijms21082676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Ruan G., Chen L., Ying S., Li G., Xu F., Xiao Z., Tian Y., Lv L., Ping Y., Cheng Y., Wei Y. Neurotransmitter and intestinal interactions: focus on the microbiota-gut-brain axis in irritable bowel syndrome. Front Endocrinol. (Lausanne) 2022 doi: 10.3389/fendo.2022.817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., D’Souza R., Hong S.T. The role of gut microbiota in the gut-brain axis: current challenges and perspectives. Protein Cell. 2013 doi: 10.1007/s13238-013-3017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yijing, Xu J., Chen Yu. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021 doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo S., An M., Lim Y.H. Protective effects of heat-killed ruminococcus albus against β-amyloidinduced apoptosis on SH-SY5Y cells. J. Microbiol Biotechnol. 2024;34:85–93. doi: 10.4014/jmb.2308.08045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015 doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., O’Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol. Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long-Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O’Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., van de Wouw M., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Cutuli D., Decandia D., Giacovazzo G., Coccurello R. Physical exercise as disease-modifying alternative against Alzheimer’s disease: a gut–muscle–brain partnership. Int J. Mol. Sci. 2023 doi: 10.3390/ijms241914686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani F., Cornuti S., Tognini P. The gut-brain connection: Exploring the influence of the gut microbiota on neuroplasticity and neurodevelopmental disorders. Neuropharmacology. 2023 doi: 10.1016/j.neuropharm.2023.109491. [DOI] [PubMed] [Google Scholar]
- D’Aquila P., Carelli L.L., De Rango F., Passarino G., Bellizzi D. Gut microbiota as important mediator between diet and DNA methylation and histone modifications in the host. Nutrients. 2020 doi: 10.3390/nu12030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami M., Raj K., Singh S. Relevance of gut microbiota to Alzheimer’s Disease (AD): potential effects of probiotic in management of AD. Aging Health Res. 2023 doi: 10.1016/j.ahr.2023.100128. [DOI] [Google Scholar]
- Dicks L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms. 2022 doi: 10.3390/microorganisms10091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich W., Schink M., Zopf Y. Microbiota in the gastrointestinal tract. Med Sci. 2018 doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka D.M.S., Jayasena V., Rainey-Smith S.R., Martins R.N., Binosha Fernando W.M.A.D. The role of diet and gut microbiota in Alzheimer’s disease. Nutrients. 2024;16(3):412. doi: 10.3390/nu16030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobielska M., Bartosik N.K., Zyzik K.A., Kowalczyk E., Karbownik M.S. Mechanisms of cognitive impairment in depression. May probiotics help? Front Psychiatry. 2022 doi: 10.3389/fpsyt.2022.904426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwudo M.N., Gubert C., Hannan A.J. The microbiota–gut–brain axis in Huntington’s disease: pathogenic mechanisms and therapeutic targets. FEBS J. 2024 doi: 10.1111/febs.17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M., Sherr G.L. Epigenetic modifications in Alzheimer’s neuropathology and therapeutics. Front Neurosci. 2019;13 doi: 10.3389/fnins.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad G.I. Stem cells as a promising therapeutic approach for Alzheimer’s disease: a review. Bull. Natl. Res Cent. 2019;43 doi: 10.1186/s42269-019-0078-x. [DOI] [Google Scholar]
- Friedland R.P., Chapman M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017 doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. The gut microbiome and the brain. J. Med Food. 2014 doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E., Cuello A.C., Fisher A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain. 2022 doi: 10.1093/brain/awac096. [DOI] [PubMed] [Google Scholar]
- Góralczyk-Bińkowska A., Szmajda-Krygier D., Kozłowska E. The microbiota–gut–brain axis in psychiatric disorders. Int J. Mol. Sci. 2022 doi: 10.3390/ijms231911245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Martinez L., Calfío C., Farias G.A., Vilches C., Prieto R., MacCioni R.B. New frontiers in the prevention, diagnosis, and treatment of Alzheimer’s disease. J. Alzheimer’S. Dis. 2021 doi: 10.3233/JAD-201059. [DOI] [PubMed] [Google Scholar]
- Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., Villemagne V.L., Aisen P., Vendruscolo M., Iwatsubo T., Masters C.L., Cho M., Lannfelt L., Cummings J.L., Vergallo A. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry. 2021;26:5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Finger C., Morales-Scheihing D., Lee J., McCullough L.D. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Wu X. Brain neurotransmitter modulation by gut microbiota in anxiety and depression. Front Cell Dev. Biol. 2021 doi: 10.3389/fcell.2021.649103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Lu Z., Zhang H., Wen H., Li Z., Liu Q., Wang R. A novel strategy for Alzheimer’s disease based on the regulatory effect of amyloid-β on gut flora. J. Alzheimer’S. Dis. 2023 doi: 10.3233/JAD-220651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Z., Zhu Y., Yu L., Yang J., De Jager P., Bennett D.A., Zhao J. DNA methylation variability in Alzheimer’s disease. Neurobiol. Aging. 2019;76:35–44. doi: 10.1016/j.neurobiolaging.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh Q.S., Elangovan S., Holsinger R.M.D. Non-pharmacological therapeutic options for the treatment of Alzheimer’s disease. Int J. Mol. Sci. 2022 doi: 10.3390/ijms231911037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland N.P., Cryan J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016 doi: 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Iqbal I., Saqib F., Mubarak Z., Latif M.F., Wahid M., Nasir B., Shahzad H., Sharifi-Rad J., Mubarak M.S. Alzheimer’s disease and drug delivery across the blood-brain barrier: approaches and challenges. Eur. J. Med Res. 2024 doi: 10.1186/s40001-024-01915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8836–8847. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M., Xiao J., Xu B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int J. Mol. Sci. 2017 doi: 10.3390/ijms18091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemimah S., Chabib C.M.M., Hadjileontiadis L., AlShehhi A. Gut microbiome dysbiosis in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. PLoS One. 2023;18 doi: 10.1371/journal.pone.0285346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett, B.E., Thapa, B., 2024. Physiology, NMDA Receptor. [PubMed]
- Kaur G., Rathod S.S.S., Ghoneim M.M., Alshehri S., Ahmad J., Mishra A., Alhakamy N.A. DNA methylation: a promising approach in management of Alzheimer’s disease and other neurodegenerative disorders. Biology. 2022 doi: 10.3390/biology11010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesika P., Suganthy N., Sivamaruthi B.S., Chaiyasut C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021 doi: 10.1016/j.lfs.2020.118627. [DOI] [PubMed] [Google Scholar]
- Khan S., Barve K.H., Kumar M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020;18:1106–1125. doi: 10.2174/1570159x18666200528142429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Barve K.H., Kumar M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020;18:1106–1125. doi: 10.2174/1570159x18666200528142429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinsky N.D., Power K.A., Middleton L., Ferland G., Anderson N.D. The role of the gut microbiome in diet and exercise effects on cognition: a review of the intervention literature. J. Gerontol. - Ser. A Biol. Sci. Med. Sci. 2023 doi: 10.1093/gerona/glac166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarova M., García-Sierra F., Bartos A., Ricny J., Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J. Alzheimers Dis. 2012 doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.R., Azizi N.F., Yeap S.K., Abdullah J.O., Khalid M., Omar A.R., Osman M.A., Leow A.T.C., Mortadza S.A.S., Alitheen N.B. Clinical and preclinical studies of fermented foods and their effects on Alzheimer’s disease. Antioxidants. 2022 doi: 10.3390/antiox11050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H.F., Ahmad S.R., Lim Y.C., Zulkipli I.N. The use of probiotic therapy in metabolic and neurological diseases. Front Nutr. 2022 doi: 10.3389/fnut.2022.887019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Liu L., Cao Z., Li W., Li H., Lu C., Yang X., Liu Y. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2019.109653. [DOI] [PubMed] [Google Scholar]
- Liang Y., Liu C., Cheng M., Geng L., Li J., Du W., Song M., Chen N., Yeleen T.A.N., Song L., Wang X., Han Y., Sheng C. The link between gut microbiome and Alzheimer’s disease: From the perspective of new revised criteria for diagnosis and staging of Alzheimer’s disease. Alzheimer’s Dementia. 2024 doi: 10.1002/alz.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Tan Y., Cheng H., Zhang D., Feng W., Peng C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022 doi: 10.14336/AD.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Gao, J., Zhu, M., Liu, K., Zhang, H.-L., 2035a. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. 10.1007/s12035-020-02073-3/Published. [DOI] [PMC free article] [PubMed]
- Liu, S., Gao, J., Zhu, M., Liu, K., Zhang, H.-L., 2035b. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. 10.1007/s12035-020-02073-3/Published. [DOI] [PMC free article] [PubMed]
- Liu X.Y., Yang L.P., Zhao L. Stem cell therapy for alzheimer’s disease. World J. Stem Cells. 2020;12:787–802. doi: 10.4252/WJSC.V12.I8.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Cheng X., Li H., Hui S., Zhang Z., Xiao Y., Peng W. Non-coding RNAs as novel regulators of neuroinflammation in Alzheimer’s disease. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.908076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K.G., Tahon G. Science depends on nomenclature, but nomenclature is not science. Nat. Rev. Microbiol. 2022 doi: 10.1038/s41579-022-00684-2. [DOI] [PubMed] [Google Scholar]
- Loh J.S., Mak W.Q., Tan L.K.S., Ng C.X., Chan H.H., Yeow S.H., Foo J.B., Ong Y.S., How C.W., Khaw K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target Ther. 2024;9:37. doi: 10.1038/s41392-024-01743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh J.S., Mak W.Q., Tan L.K.S., Ng C.X., Chan H.H., Yeow S.H., Foo J.B., Ong Y.S., How C.W., Khaw K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target Ther. 2024;9:37. doi: 10.1038/s41392-024-01743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M., Mauro Maurizio, Di, Mauro Marco, Di, Luca A. Gut microbiota in Alzheimer’s disease, depression, and type 2 diabetes mellitus: the role of oxidative stress. Oxid. Med Cell Longev. 2019 doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman A., Hassan A., Ullah Mehtab, Naseem S., Ullah Mehraj, Zhang L., Din A.U., Ullah K., Ahmad W., Wang G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front Immunol. 2024 doi: 10.3389/fimmu.2024.1321395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Taminiau B., Rodriguez C., Daube G. Gut microbiota composition associated with clostridioides difficile colonization and infection. Pathogens. 2022 doi: 10.3390/pathogens11070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A. Gut feelings: the emerging biology of gut-"brain communication. Nat. Rev. Neurosci. 2011 doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness A.J., Davis J.A., Dawson S.L., Loughman A., Collier F., O’Hely M., Simpson C.A., Green J., Marx W., Hair C., Guest G., Mohebbi M., Berk M., Stupart D., Watters D., Jacka F.N. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry. 2022 doi: 10.1038/s41380-022-01456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros D., McMurry K., Pfeiffer M., Newsome K., Testerman T., Graf J., Silver A.C., Sacchetti P. Slowing Alzheimer’s disease progression through probiotic supplementation. Front Neurosci. 2024;18 doi: 10.3389/fnins.2024.1309075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Bessho S., Grando K., Tükel Ç. Microbiome or infections: amyloid-containing biofilms as a trigger for complex human diseases. Front Immunol. 2021 doi: 10.3389/fimmu.2021.638867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. Approach to neurodegenerative disease in children: a short review. Progress. Asp. Pediatr. Neonatol. 2018;1 doi: 10.32474/PAPN.2018.01.000121. [DOI] [Google Scholar]
- Mitra S., Dash R., Nishan A.Al, Habiba S.U., Moon I.S. Brain modulation by the gut microbiota: from disease to therapy. J. Adv. Res. 2023 doi: 10.1016/j.jare.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Debs L.H., Patel A.P., Nguyen D., Patel K., O’Connor G., Grati M., Mittal J., Yan D., Eshraghi A.A., Deo S.K., Daunert S., Liu X.Z. Neurotransmitters: the critical modulators regulating gut–brain axis. J. Cell Physiol. 2017 doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi G., Dargahi L., Peymani A., Mirzanejad Y., Alizadeh S.A., Naserpour T., Nassiri-Asl M. The effects of probiotic formulation pretreatment (Lactobacillus helveticus R0052 and bifidobacterium longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019;38:209–217. doi: 10.1080/07315724.2018.1487346. [DOI] [PubMed] [Google Scholar]
- Mondot S., De Wouters T., Doré J., Lepage P. The human gut microbiome and its dysfunctions : Dig. Dis. S. Karger AG. 2013:278–285. doi: 10.1159/000354678. [DOI] [PubMed] [Google Scholar]
- Mortaş H., Bilici S., Karakan T. The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chrono-.-. Int. 2020;37:1067–1081. doi: 10.1080/07420528.2020.1778717. [DOI] [PubMed] [Google Scholar]
- Murai T., Matsuda S. Therapeutic implications of probiotics in the gut microbe-modulated neuroinflammation and progression of Alzheimer’s disease. Life. 2023 doi: 10.3390/life13071466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naomi R., Embong H., Othman F., Ghazi H.F., Maruthey N., Bahari H. Probiotics for alzheimer’s disease: a systematic review. Nutrients. 2022 doi: 10.3390/nu14010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N.M., Cho J., Lee C. Gut microbiota and Alzheimer’s disease: how to study and apply their relationship. Int J. Mol. Sci. 2023 doi: 10.3390/ijms24044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgampalle M., Yellamma K. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn. Res. 11, KC01–KC05. 2017 doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olufunmilayo E.O., Holsinger R.M.D. Roles of non-coding RNA in Alzheimer’s disease pathophysiology. Int J. Mol. Sci. 2023 doi: 10.3390/ijms241512498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva B., Laranjinha J., Rocha B.S. Do oral and gut microbiota communicate through redox pathways? A novel asset of the <scp>nitrate-nitrite-NO</scp> pathway. FEBS Lett. 2024 doi: 10.1002/1873-3468.14859. [DOI] [PubMed] [Google Scholar]
- Porsteinsson A.P., Isaacson R.S., Knox S., Sabbagh M.N., Rubino I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J. Prev. Alzheimer’S. Dis. 2021 doi: 10.14283/jpad.2021.23. [DOI] [PubMed] [Google Scholar]
- Qazi T.J., Quan Z., Mir A., Qing H. Epigenetics in Alzheimer’s disease: perspective of DNA methylation. Mol. Neurobiol. 2018 doi: 10.1007/s12035-016-0357-6. [DOI] [PubMed] [Google Scholar]
- Qin C., Wang K., Zhang L., Bai L. Stem cell therapy for Alzheimer’s disease: an overview of experimental models and reality. Anim. Model Exp. Med. 2022 doi: 10.1002/ame2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Li Y., Liu F., Fang Y., He J., Ma J., Xu T., Wang L., Lei P., Dong H., Jin L., Yang Q., Wu W., Sun D. Microbiota-gut-brain axis dysregulation in Alzheimer’s disease: multi-pathway effects and therapeutic potential. Aging Dis. 2024 doi: 10.14336/AD.2023.0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillin S.J., Tran P., Prindle A. Potential roles for gamma-aminobutyric acid signaling in bacterial communities. Bioelectricity. 2021 doi: 10.1089/bioe.2021.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulin A.C., Doss S.V., Trottier Z.A., Ikezu T.C., Bu G., Liu C.C. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022 doi: 10.1186/s13024-022-00574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeiasl Z., Salami M., Sepehri G. The effects of probiotic Lactobacillus and Bifidobacterium strains on memory and learning behavior, long-term potentiation (LTP), and some biochemical parameters in β-amyloid-induced rat’s model of Alzheimer’s disease. Prev. Nutr. Food Sci. 2019;24:265–273. doi: 10.3746/pnf.2019.24.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7 doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riverol M., López O.L. Biomarkers in Alzheimer’s disease. Front Neurol. JUL. 2011 doi: 10.3389/fneur.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch J.A., Layden B.T., Dugas L.R. Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol. (Lausanne) 2023 doi: 10.3389/fendo.2023.1130689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn V., Azcarate-Peril M.A., Updegraff J., Manderino L., Gunstad J. Randomized clinical trial examining the impact of Lactobacillus rhamnosus GG probiotic supplementation on cognitive functioning in middle-aged and older adults. Neuropsychiatr. Dis. Treat. 2020;16:2765–2777. doi: 10.2147/NDT.S270035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn V., Azcarate-Peril M.A., Updegraff J., Manderino L.M., Gunstad J. A randomized clinical trial examining the impact of LGG probiotic supplementation on psychological status in middle-aged and older adults. Conte Clin. Trials Commun. 2018;12:192–197. doi: 10.1016/j.conctc.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana D.A., Smith M. de A.C., Chen E.S. Histone modifications in Alzheimer’s disease. Genes. 2023 doi: 10.3390/genes14020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Yoo J.Y., Dutra S.V.O., Morgan K.H., Groer M. The association between early-life gut microbiota and long-term health and diseases. J. Clin. Med. 2021;10:1–24. doi: 10.3390/jcm10030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir U., Arshad M.S., Sameen A., Oh D.H. Crosstalk between gut and brain in alzheimer’s disease: the role of gut microbiota modulation strategies. Nutrients. 2021 doi: 10.3390/nu13020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya S., Kumar S., Kumar Jha N., Kumar Kesari K., Ruokolainen J. Interplay of gut microbiota and oxidative stress: perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022 doi: 10.1016/j.jare.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C., Kim S.R. Linking oxidative stress and proteinopathy in alzheimer’s disease. Antioxidants. 2021 doi: 10.3390/antiox10081231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobko T., Huang L., Midtvedt T., Norin E., Gustafsson L.E., Norman M., Jansson E.Å., Lundberg J.O. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic. Biol. Med. 2006;41:985–991. doi: 10.1016/j.freeradbiomed.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Sochocka M., Donskow-Łysoniewska K., Diniz B.S., Kurpas D., Brzozowska E., Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol. Neurobiol. 2019 doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki R., Karande A., Ranganathan P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front Neurol. 2023 doi: 10.3389/fneur.2023.1149618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Sun Q., Zheng H., Zhang Y., Wang Y., Liu S., Duan L. Roseburia hominis alleviates neuroinflammation via short-chain fatty acids through histone deacetylase inhibition. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu G.D., Luca A., Rusu R.N., Bild V., Chiriac S.I.B., Solcan C., Bild W., Ababei D.C. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules. 2020 doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statovci D., Aguilera M., MacSharry J., Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz, P., 2018. TITLE Neurotransmitter modulation by the gut microbiota AUTHORS AND AFFILIATIONS. [DOI] [PMC free article] [PubMed]
- Sun J., Xu J., Yang B., Chen K., Kong Y., Fang N., Gong T., Wang F., Ling Z., Liu J. Effect of clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 2020;64 doi: 10.1002/mnfr.201900636. [DOI] [PubMed] [Google Scholar]
- Talebi M., Ebrahimi V., Rasouli A., Farjami A., Soofiyani S.R., Soleimanian A., Forouhandeh H., Tarhriz V. A new insight on feasibility of pre-, pro-, and synbiotics-based therapies in Alzheimer’s disease. J. Rep. Pharm. Sci. 2022 doi: 10.4103/jrptps.JRPTPS_170_21. [DOI] [Google Scholar]
- Tamtaji O.R., Heidari-soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E., Tajabadi-Ebrahimi M., Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin. Nutr. 2019;38:2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017 doi: 10.1016/j.alit.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Teleanu R.I., Niculescu A.-G., Roza E., Vladâcenco O., Grumezescu A.M., Teleanu D.M. Neurotransmitters—key factors in neurological and neurodegenerative disorders of the central nervous system. Int J. Mol. Sci. 2022;23:5954. doi: 10.3390/ijms23115954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G., Pinho M., Pritzkow S., Mendez N., Soto C., Tetz V. Bacterial DNA promotes Tau aggregation. Sci. Rep. 2020;10:2369. doi: 10.1038/s41598-020-59364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D.R., Tomé S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res Bull. 2022 doi: 10.1016/j.brainresbull.2022.10.006. [DOI] [PubMed] [Google Scholar]
- Thornton T., Mills D., Bliss E. The impact of lipopolysaccharide on cerebrovascular function and cognition resulting from obesity-induced gut dysbiosis. Life Sci. 2024 doi: 10.1016/j.lfs.2023.122337. [DOI] [PubMed] [Google Scholar]
- Turner R.S., Stubbs T., Davies D.A., Albensi B.C. Potential new approaches for diagnosis of Alzheimer’s disease and related dementias. Front Neurol. 2020 doi: 10.3389/fneur.2020.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostveen W.M., de Lange E.C.M. Imaging techniques in alzheimer’s disease: a review of applications in early diagnosis and longitudinal monitoring. Int J. Mol. Sci. 2021 doi: 10.3390/ijms22042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesi A., Pierella E., Romeo M., Piccini G.B., Alfano C., Bjørklund G., Oppong A., Ricevuti G., Esposito C., Chirumbolo S., Pascale A. The Potential Role Of Gut Microbiota in Alzheimer’s disease: from diagnosis to treatment. Nutrients. 2022 doi: 10.3390/nu14030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio I., Sorrentino L., Paoletti A., Marra R., Arbitrio M. The State Of The Art On Acetylcholinesterase Inhibitors In The Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021 doi: 10.1177/11795735211029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgilio, E., De Marchi, F., Contaldi, E., Dianzani, U., Cantello, R., Mazzini, L., Comi, C., 2022. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. 10.3390/biomedicines10040c. [DOI] [PMC free article] [PubMed]
- Wang F., Xu T., Zhang Y., Zheng T., He Y., Jiang Y. Long-term combined administration of Bifidobacterium bifidum TMC3115 and Lactobacillus plantarum 45 alleviates spatial memory impairment and gut dysbiosis in APP/PS1 mice. FEMS Microbiol Lett. 2020 doi: 10.1093/femsle/fnaa048/5815078. [DOI] [PubMed] [Google Scholar]
- Wang X., Wen X., Yuan S., Zhang J. Gut-brain axis in the pathogenesis of sepsis-associated encephalopathy. Neurobiol. Dis. 2024 doi: 10.1016/j.nbd.2024.106499. [DOI] [PubMed] [Google Scholar]
- Wang X.-L., Zeng J., Yang Y., Xiong Y., Zhang Z.-H., Qiu M., Yan X., Sun X.-Y., Tuo Q.-Z., Liu R., Wang J.-Z. Helicobacter pylori filtrate induces alzheimer-like tau hyperphosphorylation by activating glycogen synthase kinase-3β. J. Alzheimer’S. Dis. 2014;43:153–165. doi: 10.3233/JAD-140198. [DOI] [PubMed] [Google Scholar]
- Wang Y., Du W., Hu X., Yu X., Guo C., Jin X., Wang W. Targeting the blood–brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B. 2023 doi: 10.1016/j.apsb.2023.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegierska A.E., Charitos I.A., Topi S., Potenza M.A., Montagnani M., Santacroce L. The Connection Between Physical Exercise And Gut Microbiota: Implications For Competitive Sports Athletes. Sports Med. 2022 doi: 10.1007/s40279-022-01696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtuś M., Tomaszuk S., Wąsik K. The role of the gut microbiota in the pathogenesis and treatment of Alzheimer disease – review. J. Educ., Health Sport. 2024;51:11–20. doi: 10.12775/jehs.2024.51.001. [DOI] [Google Scholar]
- Wu C., Sun D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015;30:367–379. doi: 10.1007/s11011-014-9560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Katsumata N., Bernier F., Ohno K., Yamauchi Y., Odamaki T., Yoshikawa K., Ito K., Kaneko T. ProbioTic Bifidobacterium Breve In Improving Cognitive Functions Of Older Adults With Suspected Mild Cognitive Impairment: A Randomized, Double-blind, Placebo-controlled Trial. J. Alzheimer’S. Dis. 2020;77:139–147. doi: 10.3233/JAD-200488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Feng Y., Zhao J., Chen W., Lu W. Achieving healthy aging through gut microbiota-directed dietary interventiOn: Focusing on microbial biomarkers and host mechanisms. J. Adv. Res. 2024 doi: 10.1016/j.jare.2024.03.005. [DOI] [PubMed] [Google Scholar]
- Xu K., Sheng S., Zhang F. RelAtionship Between Gut Bacteria And Levodopa Metabolism. Curr. Neuropharmacol. 2022;21:1536–1547. doi: 10.2174/1570159x21666221019115716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xu Z., Guo J., Xiong Z., Hu B. ExploriNg The Gut Microbiome-postoperative Cognitive Dysfunction Connection: Mechanisms, Clinical Implications, And Future Directions. Brain Behav. Immun. Health. 2024 doi: 10.1016/j.bbih.2024.100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo J.D. Influence of food-derived bioactives on gut microbiota compositions and their metabolites by focusing on neurotransmitters. Food Sci. Biotechnol. 2023 doi: 10.1007/s10068-023-01293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannopoulou K.G., Papageorgiou S.G. Current and future treatments in alzheimer disease: an update. J. Cent. Nerv. Syst. Dis. 2020 doi: 10.1177/1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhu S., Li P., Min L., Zhang S. Helicobacter pylori infection and inflammatory bowel disease: a crosstalk between upper and lower digestive tract. Cell Death Dis. 2018;9:961. doi: 10.1038/s41419-018-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017 doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]