Abstract
Triglyceride deposit cardiomyovasculopathy (TGCV) is an emerging rare heart disease with high mortality, characterized by defective intracellular lipolysis of triglycerides (TG). We developed diagnostic criteria for TGCV, in which low washout rate of BMIPP (BMIPP-WR) is a key factor. The working group of the Japan Society of Nuclear Cardiology recently published practice recommendations for measuring BMIPP-WR. We reported that hemodialysis (HD) patients with TGCV exhibited a markedly higher cardiovascular risk than those without TGCV. Secondary carnitine deficiency is common in patients undergoing HD, as carnitine is removed from the circulation. However, clinical evidence linking carnitine levels to BMIPP-WR is limited. Here we report the effect of L-carnitine administration on the BMIPP-WR in 9 chronic HD patients with TGCV in a retrospective cohort. The mean age at TGCV diagnosis was 59 years. Following standard doses of oral L-carnitine administration, plasma free carnitine levels significantly increased. However, BMIPP-WR was not changed. In normal condition, most BMIPP taken up were esterified/incorporated into TG pool, hydrolyzed by intracellular lipases, and then transported by carnitine shuttle to mitochondria. In TGCV, intracellular TG lipolysis is defective. During the intracellular metabolism of BMIPP, carnitine shuttling occurs downstream of TG lipolysis. Therefore, even when carnitine levels were increased, BMIPP-WR did not change in patients with TGCV who underwent chronic HD. A phase IIb/III clinical trial for TGCV, is underway (jRCT2051210177). Increased awareness of the disease concept of TGCV, along with its diagnostic principles and procedures using BMIPP scintigraphy, is warranted.
Keywords: BMIPP, Carnitine, Hemodialysis, Triglyceride deposit cardiomyovasculopathy, Washout rate
Dear Editor,
Triglyceride deposit cardiomyovasculopathy (TGCV) is an emerging rare heart disease characterized by defective intracellular lipolysis of triglycerides (TG) (1, 2), leading to severe heart failure (3), diffuse coronary artery disease with TG deposition (4), and ventricular arrhythmia with high mortality (5). 123I-β-methyl-p-iodophenylpentadecanoic acid (BMIPP) is a useful radiopharmaceutical to evaluate metabolism of long-chain fatty acid (LCFA) and TG in various kinds of heart diseases since its approval in the 1990s (6). The Japan TGCV Study Group developed diagnostic criteria for TGCV, in which low washout rate of BMIPP (BMIPP-WR) is a key factor (7). The working group of the Japan Society of Nuclear Cardiology recently published practice recommendations for measuring BMIPP-WR (8).
Patients undergoing chronic hemodialysis (HD) experience severe cardiovascular mortality, despite recent advances in medical and invasive revascularization therapies, including cholesterol-lowering therapies (9). Therefore, understanding the mechanisms underlying residual risk in these patients is crucial. We recently reported that the prevalence of TGCV was not uncommon in patients undergoing HD and those with TGCV exhibited a markedly higher cardiovascular risk than those without TGCV in a single-centered retrospective study (10).
Carnitine is an essential protein for the LCFA entry into mitochondria. Genetic defects of molecules in the carnitine shuttle lead to severe cardiomyopathy with cellular steatosis in children (11). Secondary carnitine deficiency is common in patients undergoing HD, as carnitine is removed from the circulation. However, clinical evidence linking carnitine levels to BMIPP-WR is limited. Only one previous study (12) reported that carnitine administration increased BMIPP-WR in non-TGCV patients on HD whose BMIPP-WR exceeded the TGCV diagnostic cutoff value (10%). Therefore, we aimed to report the effect of L-carnitine administration on the BMIPP-WR in HD patients with TGCV in a retrospective cohort.
From August 2015 to June 2021, the Japan TGCV Study Group identified 9 patients who met the following inclusion criteria: 1) underwent BMIPP scintigraphy both before and after carnitine administration and 2) underwent carnitine levels measurements both before and after administration. TGCV diagnosis was established based on the latest diagnostic criteria (7). The original BMIPP scintigraphy data were obtained from primary physicians and verified by an independent researcher of the Japan TGCV Study Group. This observational study was approved by the ethics review committee.
Table 1 shows the demographic and clinical characteristics of the patients with TGCV. The mean age at diagnosis was 59 years, and all patients had a long history of HD. Their mean body mass index was 19.1 kg/m2. The etiologies of chronic kidney disease were heterogeneous, including diabetic nephropathy, nephrosclerosis, focal glomerular sclerosis, and polycystic kidney disease. The patients showed mild anemia, normal serum TG levels, and low-density lipoprotein-cholesterol levels under 100 mg/dL. Ultrasonography revealed reduced left ventricular ejection fraction (LVEF) in two patients. Seven demonstrated diffuse coronary atherosclerosis.
Table 1. Demographic and clinical characteristics of patients with TGCV investigated.
| Patients (N=9) | |
| Age of diagnosis | 58.6±11.1* |
| Sex: Male/Female (n) | 6/3 |
| Body mass index (kg/m2) | 19.1±5.5* |
| Hemodialysis duration (days) | 5,244±4,249* |
| Etiologies of end stage renal disease (n) | ADPKD (1), Nephrosclerosis (2), Diabetic nephropathy (3), FSGS (1), Unknown (2) |
| Co-morbidities | |
| Diabetes mellitus (n) | 4 |
| Dyslipidemia (n) | 2 |
| Hypertension (n) | 6 |
| Hemoglobin (g/dL) | 11.4±1.3* |
| Serum lipid levels (mg/dL) | |
| Triglyceride | 111±47* |
| Low-density lipoprotein-cholesterol | 72±25* |
| High-density lipoprotein-cholesterol | 50±11 * |
| Low LVEF (<40%) (n) | 2 |
| Diffuse coronary atherosclerosis (n) | 7 |
| Outcomes | |
| Follow-up period from TGCV diagnosis (days) | 1,713±1,996* |
| All-cause of death (n) | 4 |
Mean±standard deviation
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; FSGS, focal segmental glomerulosclerosis; LVEF, left ventricular ejection fraction; N, number; TGCV, triglyceride cardiomyovasculopathy
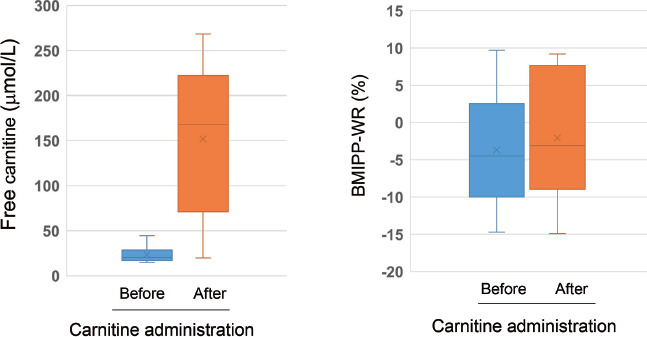
Following standard doses of oral L-carnitine administration (3.0 g/day), plasma free carnitine levels markedly increased (from 23.5±9.0 to 151.8±80.3 µmol/L, p<0.01). However, the changes in BMIPP-WR were not significant (from −3.6±7.5 to −2.0±8.1%, p = 0.78) (Figure 1). Brain natriuretic peptide levels and LVEF did not differ significantly after carnitine administration (data not shown). During the observation period post-TGCV diagnosis (mean duration of 1,713 days), 4patients died of cardiovascular events or suddenly.
Figure 1 Changes in free carnitine levels (left) and BMIPP-WR (right) in patients with TGCV undergoing HD.
Free carnitine levels were markedly increased (p<0.01), whereas BMIPP-WR was not changed after L-carnitine administration.
Reference values of free carnitine: 36–74 µmol/L
BMIPP-WR of non-TGCV controls: 22.0±6.4% (13)
Abbreviations: BMIPP-WR, washout rate of 123I-β-methyl-p-iodophenylpentadecanoic acid; HD, hemodialysis; TGCV, triglyceride deposit cardiomyovasculopathy
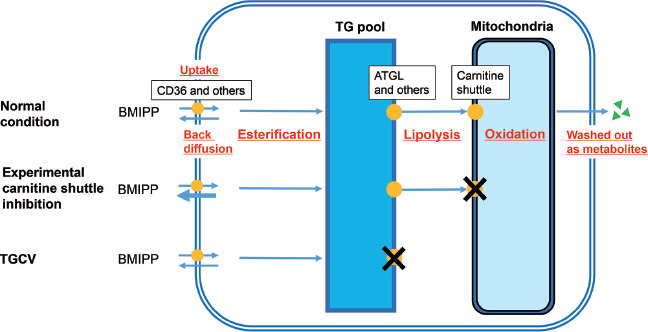
The cellular kinetics and metabolism of BMIPP are illustrated in Figure 2, according to previous and recent literature. Approximately 75% of injected BMIPP enters the cells via LCFA transporters, including CD36 (14, 15). Approximately 90% of cellular BMIPP is esterified and incorporated into the TG pool, whereas the remainder is rapidly back-fluxed from cells following injection (15, 16). TG with BMIPP are hydrolyzed by intracellular lipases, including adipose triglyceride lipase (ATGL) (3, 17). The released BMIPP enters through carnitine shuttle and is oxidized in the mitochondria. After alpha- and beta-oxidations, various metabolites are removed from cells (14, 15). Previous animal studies demonstrated that irreversible carnitine shuttle blockers altered BMIPP kinetics, increasing back diffusion and inhibiting catabolism (18, 19). In TGCV, intracellular TG lipolysis is defective due to genetic deficiencies of ATGL among other causes. During the intracellular metabolism of BMIPP, carnitine shuttling occurs downstream of lipolysis. Therefore, even when carnitine levels were increased, BMIPP-WR did not change in patients with TGCV who underwent chronic HD.
Figure 2 Cellular kinetics and metabolism of BMIPP in various conditions: normal (upper), experimental inhibition of carnitine shuttle (middle), and TGCV (lower).
Because most cellular BMIPP is esterified and incorporated into TG pool, this radiopharmaceutical is useful to evaluate cellular metabolism of TG as well as that of long-chain fatty acid. The defective lipolysis observed in TGCV is upstream of carnitine shuttle following mitochondrial oxidation, therefore carnitine administration did not alter BMIPP-WR in patients with TGCV (please see description in text).
Abbreviations: BMIPP-WR, washout rate of 123I-β -methyl-p-iodophenylpentadecanoic acid; TGCV, triglyceride deposit cardiomyovasculopathy
The limitations of the present study are as follows: 1) it would be of interest to know BMIPP-WR in genetic carnitine deficiency, even a pediatric orphan disease; 2) it remains to be investigated prevalence of TGCV among HD patients in larger multi-centered cohorts; 3) the relationship between defective TG lipolysis and cardiorenal systems needs further investigation, as we recently observed that genetic ATGL deficiency can cause a novel type of podocytopathy, in addition to TGCV (20).
A phase IIb/III clinical trial for TGCV, featuring a first-in-class orphan drug with tricaprin/trisdecanoin as the active ingredient, is currently underway (jRCT2051210177). Increased awareness of the disease concept of TGCV, along with its diagnostic principles and procedures using BMIPP scintigraphy, is warranted.
Acknowledgments
KH, the principal investigator of the Japan TGCV Study Group, analyzed the data and wrote the manuscript. KK collected the data and participated in scientific discussions. HM, YNagasawa, YNakano, MM, and TA participated in scientific discussions and reviewed the manuscript. KN confirmed the TGCV diagnosis using BMIPP-WR and reviewed the manuscript.
Sources of funding
This study was partially supported by rare disease research grants from the Ministry of Health, Labor, and Welfare of Japan (grant No. 20FC1008) and research grants from Nihon Medi-Physics Co. (Tokyo, Japan) to KH.
Conflicts of interest
KH has held the position of Joint Research Chair in collaboration with Toa Eiyo Ltd. (Tokyo, Japan) since February 2021 and has served as a medical advisor for Toa Eiyo Ltd. since December 2021. KH has a patent pending. KN collaborated with Siemens Medical Solutions USA, Inc. (Princeton, NJ, USA), Spectrum Dynamics Medical (Caesarea, Israel), and PDRadiopharma, Inc. (Tokyo, Japan), and conducted research in a department supported by Siemens Healthcare Japan (Tokyo, Japan), PDRadiopharma, Inc. (Tokyo, Japan), and Nihon MediPhysics (Tokyo, Japan). The other authors declare no conflicts of interest.
References
- 1.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–8. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Hirano K, Ikeda Y, et al. Triglyceride deposit cardiomyovasculopathy: A rare cardiovascular disorder. Orphanet J Rare Dis 2019; 14: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano K, Ikeda Y, Sugimura K, Sakata Y. Cardiomyocyte steatosis and defective washout of iodine-123-β-methyl iodophenyl-pentadecanoic acid in genetic deficiency of adipose triglyceride lipase. Eur Heart J 2015; 36: 580. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda Y, Hirano K, Fukushima N, Sawa Y. A novel type of human spontaneous coronary atherosclerosis with triglycerid deposition. Eur Heart J 2014; 35: 875. [DOI] [PubMed] [Google Scholar]
- 5.Hirano K, Miyauchi H, Nakano Y, et al. Overall survival rate of patients with triglyceride deposit cardiomyovasculopathy. JACC Adv 2023; 2: 100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamaki N, Morita K, Tsukamoto E, Kawai Y. Future aspects of BMIPP. Int J Card Imaging 1999; 15: 79–89. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Sakata Y, Miyauchi H, et al. The diagnostic criteria 2020 for triglyceride deposit cardiomyovasculopathy. Ann Nucl Cardiol 2020; 6: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima K, Miyauchi H, Hirano K, et al. Practice recommendation for measuring washout rates in 123I-BMIPP fatty acid images. Ann Nucl Cardiol 2023; 9: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet 2016; 388: 276–84. [DOI] [PubMed] [Google Scholar]
- 10.Onishi T, Nakano Y, Hirano K, et al. Prevalence and clinical outcomes of triglyceride deposit cardiomyovasculopathy among haemodialysis patients. Heart 2021; 107: 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohlschütter A, Hausdorf G. Primary (genetic) cardiomyopathies in infancy. A survey of possible disorders and guidelines for diagnosis. Eur J Pediatr 1986; 145: 454–9. [DOI] [PubMed] [Google Scholar]
- 12.Sakurabayashi T, Takaesu Y, Haginoshita S, et al. Improvement of myocardial fatty acid metabolism through L-carnitine administration to chronic hemodialysis patients. Am J Nephrol 1999; 19: 480–4. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Nakajima K, Hirano K, et al. Methods of calculating 123I-β-methyl-p-iodophenyl-pentadecanoic acid washout rates in triglyceride deposit cardiomyovasculopathy. Ann Nucl Med 2022; 36: 986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujibayashi Y, Nohara R, Hosokawa R, et al. Metabolism and kinetics of iodine-123-BMIPP in canine myocardium. J Nucl Med 1996; 37: 757–61. [PubMed] [Google Scholar]
- 15.Yoshizumi T, Nozaki S, Fukuchi K, et al. Pharmacokinetics and metabolism of 123I-BMIPP fatty acid analog in healthy and CD36-deficient subjects. J Nucl Med 2000; 41: 1134–8. [PubMed] [Google Scholar]
- 16.Hosokawa R, Nohara R, Fujibayashi Y, et al. Myocardial metabolism of 123I-BMIPP in a canine model with ischemia: Implications of perfusion-metabolism mismatch on SPECT images in patients with ischemic heart disease. J Nucl Med 1999; 40: 471–8. [PubMed] [Google Scholar]
- 17.Suzuki A, Yamaguchi S, Li M, et al. Tricaprin rescues myocardial abnormality in a mouse model of triglyceride deposit cardiomyovasculopathy. J Oleo Sci 2018; 67: 983–9. [DOI] [PubMed] [Google Scholar]
- 18.Fujibayashi Y, Yonekura Y, Kawai K, et al. Basic studies on I-123-beta-methyl-p-iodophenylpentadecanoic acid (BMIPP) for myocardial functional diagnosis: Effect of beta-oxidation inhibitor. Kaku Igaku 1988; 25: 1131–1135 (in Japanese). [PubMed] [Google Scholar]
- 19.Hosokawa R, Nohara R, Fujibayashi Y, et al. Metabolic fate of iodine-123-BMIPP in canine myocardium after administration of etomoxir. J Nucl Med 1996; 37: 1836–40. [PubMed] [Google Scholar]
- 20.Nagasawa Y, Okumura T, Hara Y, et al. Genetic deficiency of adipose triglyceride lipase is associated with a novel type of podocytopathy. Kidney Int Rep 2021; 6: 2722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


