Abstract
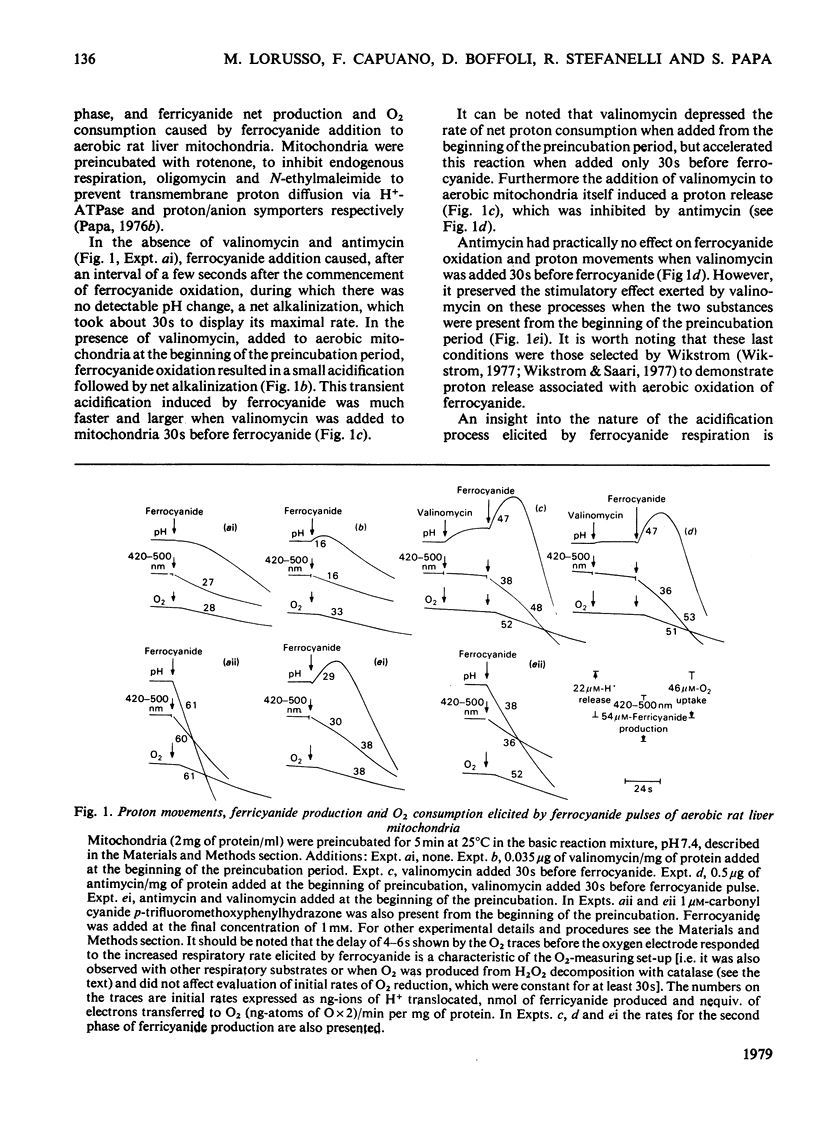
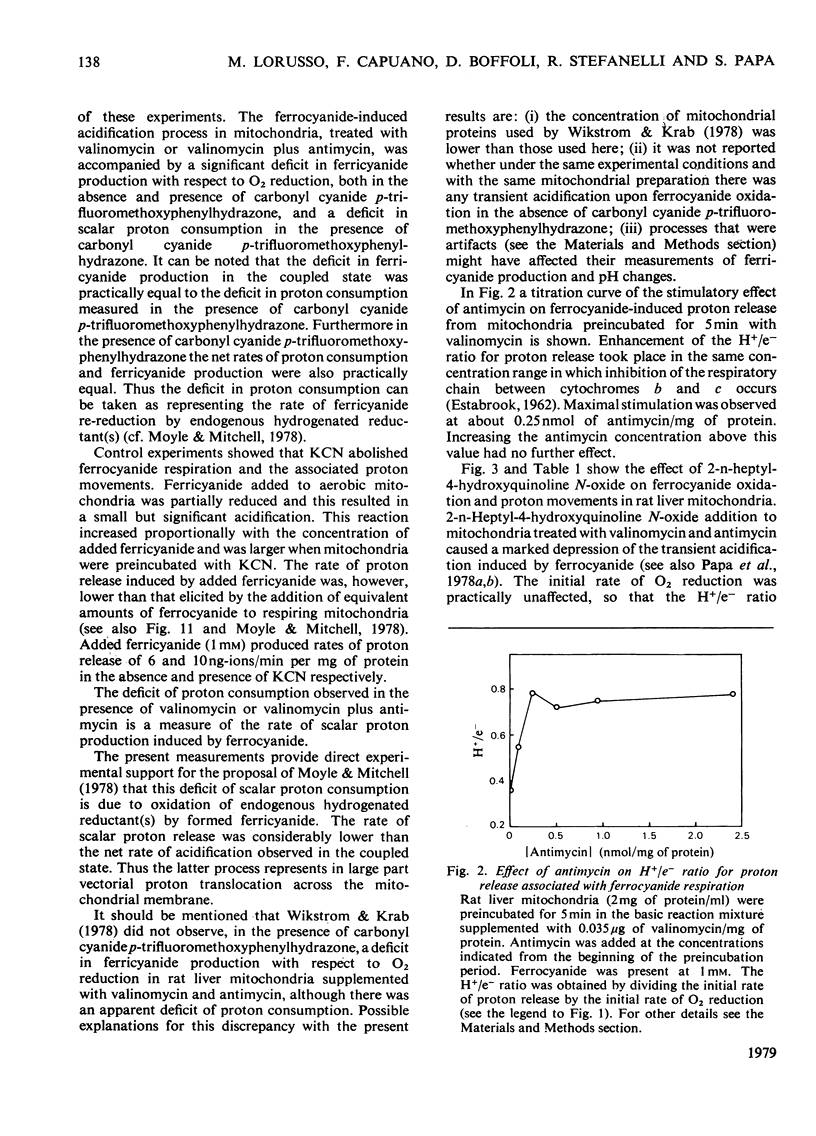
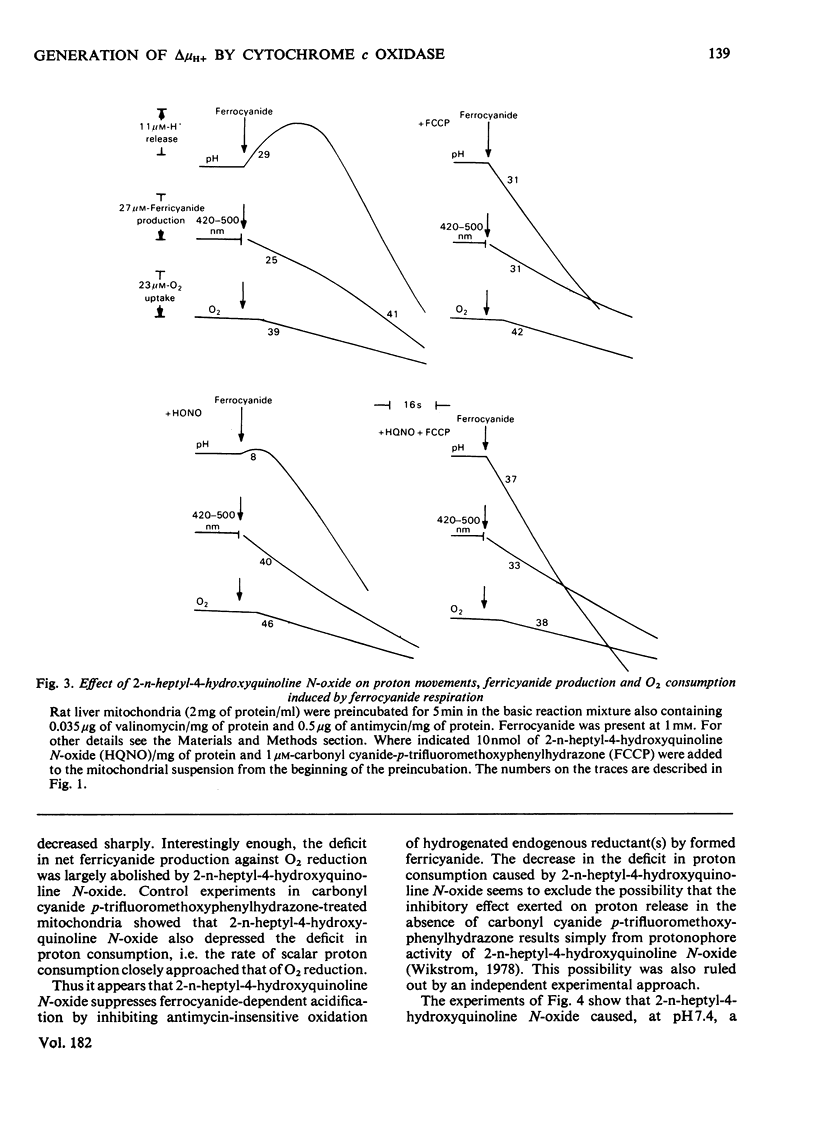
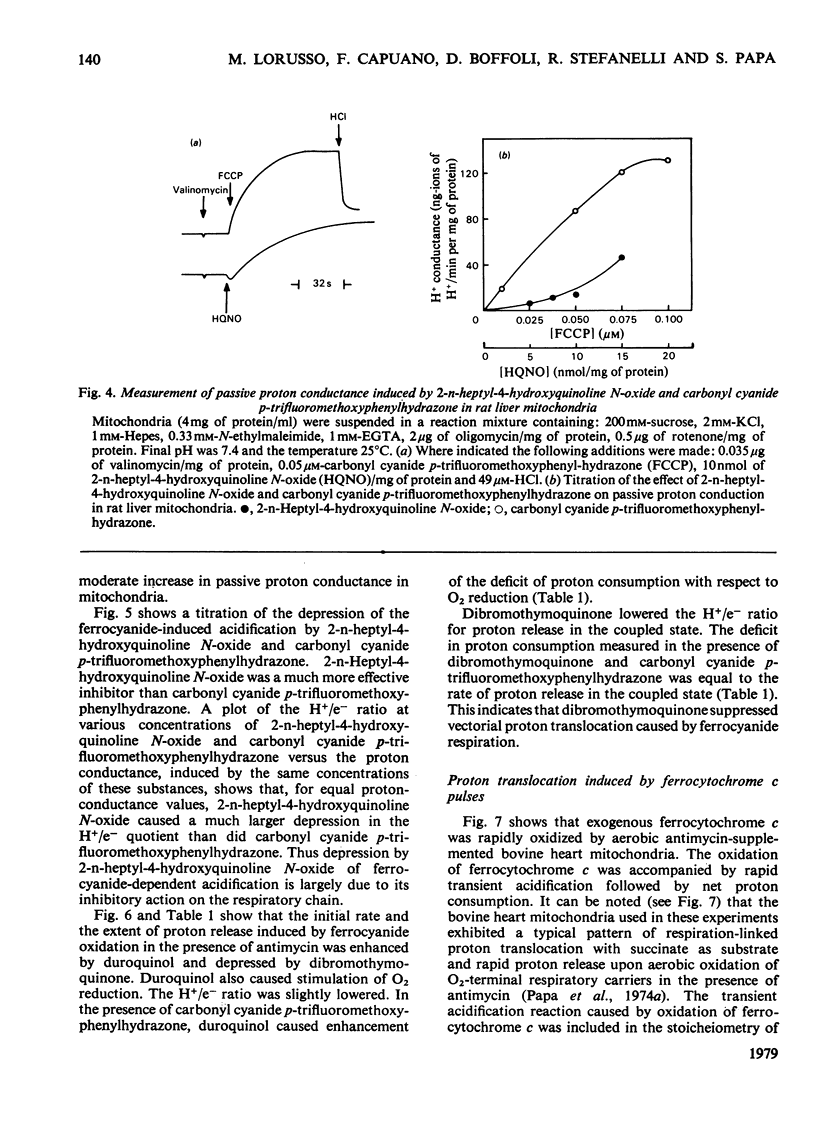
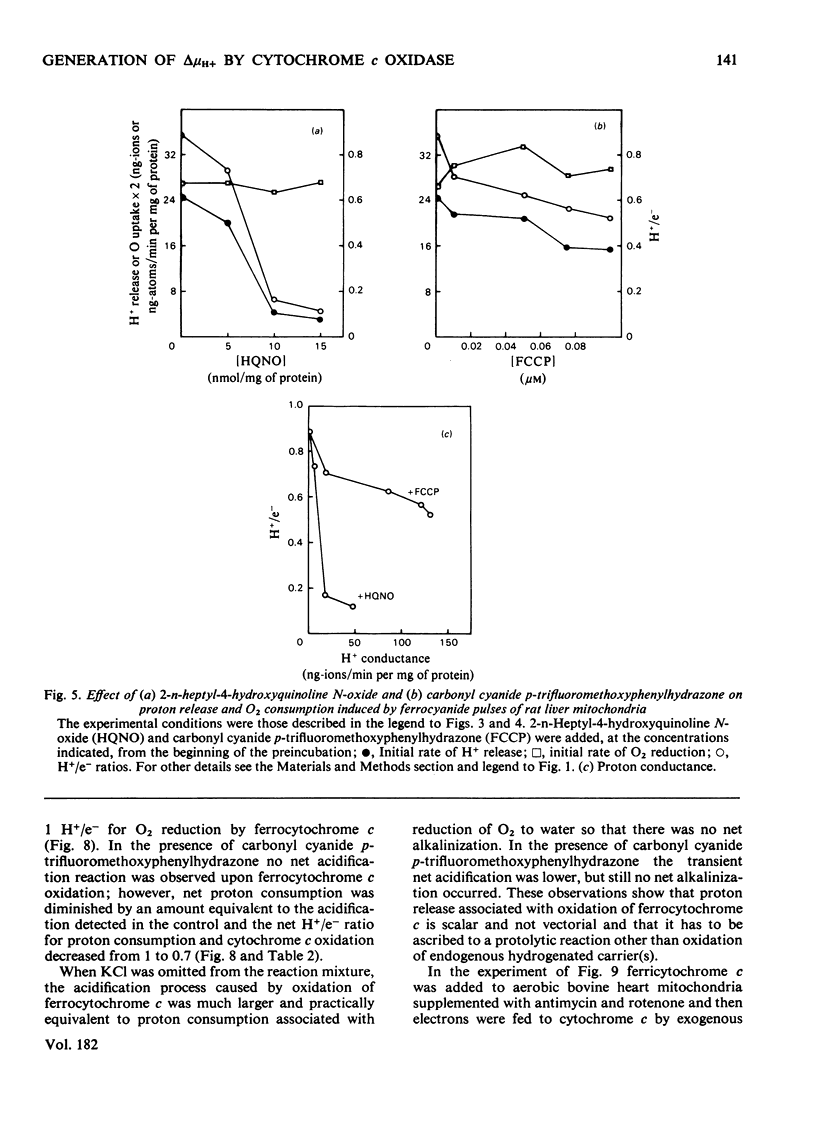
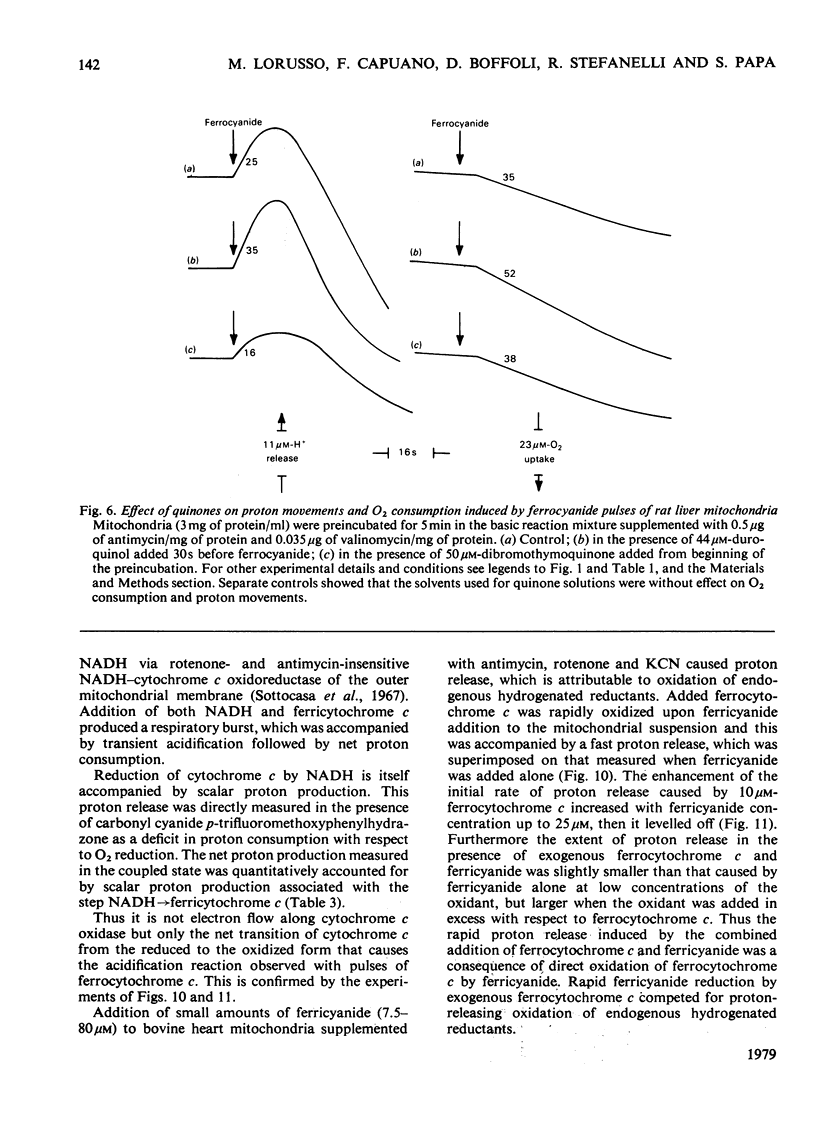
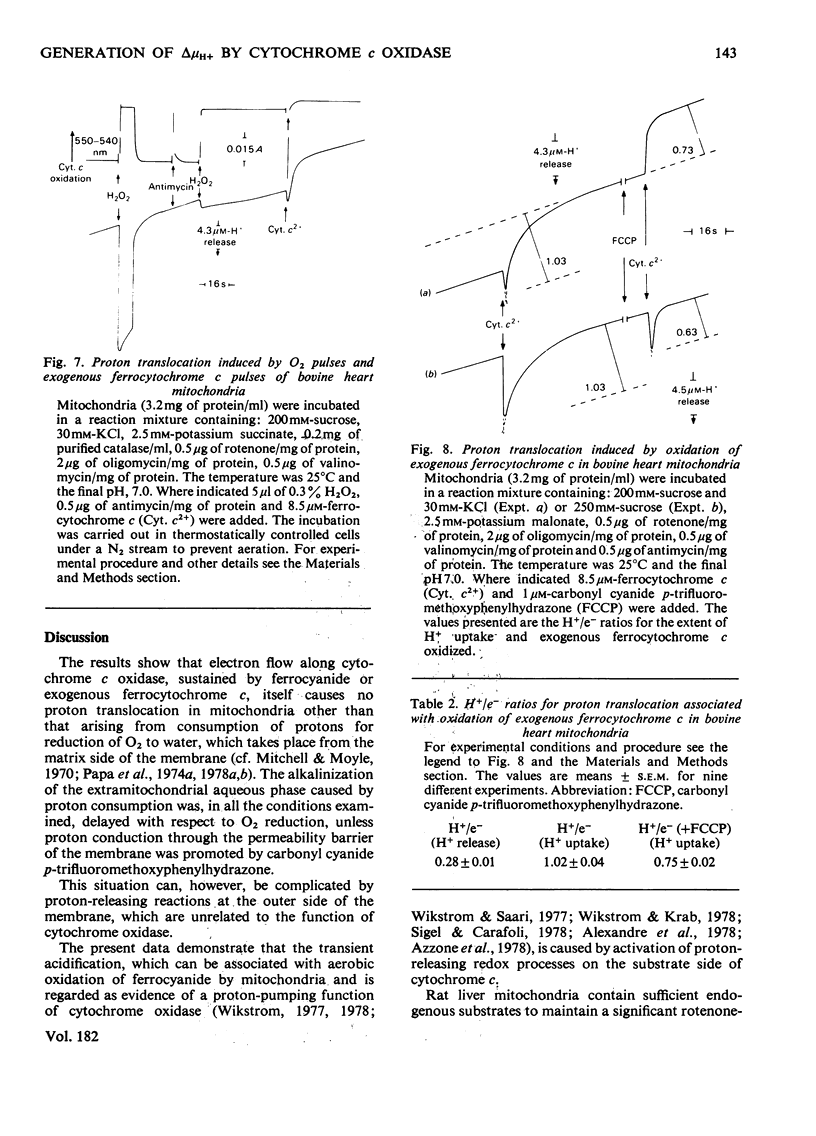
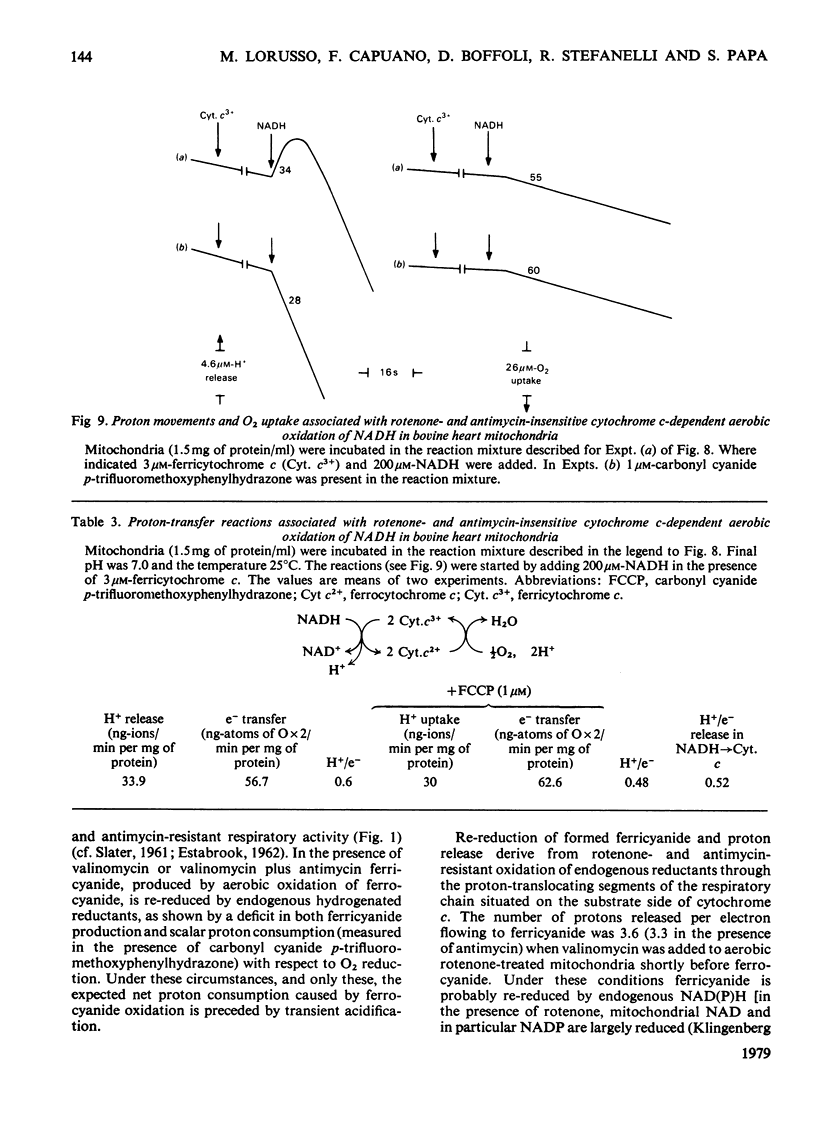
In rat liver mitochondria treated with rotenone, N-ethylmaleimide or oligomycin the expected alkalinization caused by proton consumption for aerobic oxidation of ferrocyanide was delayed with respect to ferrocyanide oxidation, unless carbonyl cyanide p-trifluoromethoxyphenylhydrazone was present. 2. When valinomycin or valinomycin plus antimycin were also present, ferricyanide, produced by oxidation of ferrocyanide, was re-reduced by hydrogenated endogenous reductants. Under these circumstances the expected net proton consumption caused by ferrocyanide oxidation was preceded by transient acidification. It is shown that re-reduction of formed ferricyanide and proton release derive from rotenone- and antimycin-resistant oxidation of endogenous reductants through the proton-translocating segments of the respiratory chain on the substrate side of cytochrome c. The number of protons released per electron flowing to ferricyanide varied, depending on the experimental conditions, from 3.6 to 1.5. 3. The antimycin-insensitive re-reduction of ferricyanide and proton release from mitochondria were strongly depressed by 2-n-heptyl-4-hydroxyquinoline N-oxide. This shows that the ferricyanide formed accepts electrons passing through the protonmotive segments of the respiratory chain at the level of cytochrome c and/or redox components of the cytochrome b-c1 complex situated on the oxygen side of the antimycin-inhibition site. Dibromothymoquinone depressed and duroquinol enhanced, in the presence of antimycin, the proton-release process induced by ferrocyanide respiration. Both quinones enhanced the rate of scalar proton production associated with ferrocyanide respiration, but lowered the number of protons released per electron flowing to the ferricyanide formed. 4. Net proton consumption caused by aerobic oxidation of exogenous ferrocytochrome c by antimycin-supplemented bovine heart mitochondria was preceded by scalar proton release, which was included in the stoicheiometry of 1 proton consumed per mol of ferrocytochrome c oxidized. This scalar proton production was associated with transition of cytochrome c from the reduced to the oxidized form and not to electron flow along cytochrome c oxidase. 5. It is concluded that cytochrome c oxidase only mediates vectorial electron flow from cytochrome c at the outer side to protons that enter the oxidase from the matrix side of the membrane. In addition to this consumption of protons the oxidase does not mediate vectorial proton translocation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Massari S., Pozzan T. The generation of the proton electrochemical potential and its role in energy transduction. Mol Cell Biochem. 1977 Sep 9;17(2):101–112. doi: 10.1007/BF01743433. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Harper W. G., Nicholls D. G., Ingledew W. J. Unequal charge separation by different coupling spans of the mitochondrial electron transport chain. FEBS Lett. 1978 Nov 1;95(1):125–129. doi: 10.1016/0014-5793(78)80066-0. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon J. R., Brocklehurst J. R., Lee C. P. Effect of antimycin A and 2-heptyl-4-hydroxyquinoline N-oxide on the respiratory chain of submitochondrial particles of beef heart. Biochemistry. 1972 Mar 28;11(7):1150–1154. doi: 10.1021/bi00757a006. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Schwartz K. J., Blout E. R. Compartmentalization of cyclic AMP-dependent protein kinases in human erythrocytes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5926–5930. doi: 10.1073/pnas.75.12.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. Observations on the antimycin A inhibition of biological oxidations. I. Stoichiometry and pH effects. Biochim Biophys Acta. 1962 Jul 2;60:236–248. doi: 10.1016/0006-3002(62)90399-2. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Harmon H. J., Crane F. L. Topography of the cristae membrane as elucidated by a new inhibitor, trifluorofurylbutanedione. Biochem Biophys Res Commun. 1973 Nov 1;55(1):169–173. doi: 10.1016/s0006-291x(73)80074-9. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C. Electron transfer across membranes and energy coupling. Fed Proc. 1973 Sep;32(9):1988–1992. [PubMed] [Google Scholar]
- Izzo G., Guerrieri F., Papa S. On the mechanism of inhibition of the respiratory chain by 2-heptyl-4-hydroxyquinoline-N-oxide. FEBS Lett. 1978 Sep 15;93(2):320–322. doi: 10.1016/0014-5793(78)81130-2. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., SCHOLLMEYER P. [On the reversibility of oxidative phosphorylation. III. Effect of adenosine triphosphate on the respiratory chain in respiratory inhibited mitochondria]. Biochem Z. 1961;335:243–262. [PubMed] [Google Scholar]
- Krab K., Wikström M. Proton-translocating cytochrome c oxidase in artificial phospholipid vesicles. Biochim Biophys Acta. 1978 Oct 11;504(1):200–214. doi: 10.1016/0005-2728(78)90018-x. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Cytochrome c oxidase is not a proton pump. FEBS Lett. 1978 Apr 15;88(2):268–272. doi: 10.1016/0014-5793(78)80190-2. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. The proton-translocating nicotinamide-adenine dinucleotide (phosphate) transhydrogenase of rat liver mitochondria. Biochem J. 1973 Mar;132(3):571–585. doi: 10.1042/bj1320571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Lorusso M. Mechanism of respiration-driven proton translocation in the inner mitochondrial membrane. Analysis of proton translocation associated to oxido-reductions of the oxygen-terminal respiratory carriers. Biochim Biophys Acta. 1974 Aug 23;357(2):181–192. doi: 10.1016/0005-2728(74)90059-0. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Reynafarje B., Brand M. D., Lehninger A. L. Evaluation of the H+/site ratio of mitochondrial electron transport from rate measurements. J Biol Chem. 1976 Dec 10;251(23):7442–7451. [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Schneider D. L., Kagawa Y., Racker E. Chemical modification of the inner mitochondrial membrane. J Biol Chem. 1972 Jun 25;247(12):4074–4079. [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The proton pump of cytochrome c oxidase and its stoichiometry. Eur J Biochem. 1978 Aug 15;89(1):119–123. doi: 10.1111/j.1432-1033.1978.tb20903.x. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J., Kell D. B., John P. The protonmotive force in bovine heart submitochondrial particles. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):237–256. doi: 10.1042/bj1740237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Measurements of the components of the protonmotive force generated by cytochrome oxidase in submitochondrial particles. FEBS Lett. 1978 Jun 1;90(1):178–182. doi: 10.1016/0014-5793(78)80324-x. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ark G., Berden J. A. Binding of HQNO to beef-heart sub-mitochondrial particles. Biochim Biophys Acta. 1977 Jan 6;459(1):119–127. doi: 10.1016/0005-2728(77)90014-7. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M. K., Saari H. T. The mechanism of energy conservation and transduction by mitochondrial cytochrome c oxidase. Biochim Biophys Acta. 1977 Nov 17;462(2):347–361. doi: 10.1016/0005-2728(77)90133-5. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Cytochrome c oxidase is a proton pump: a rejoinder to recent criticism. FEBS Lett. 1978 Jul 1;91(1):8–14. doi: 10.1016/0014-5793(78)80006-4. [DOI] [PubMed] [Google Scholar]


