Abstract
The biogenesis of plasmalemma glycoproteins of rat small-intestinal villus cells was studied by following the incorporation of l-[1,5,6-3H]fucose, given intraperitoneally with and without chase, into Golgi, lateral basal and microvillus membranes. Each membrane fraction showed distinct kinetics of incorporation of labelled fucose and was differently affected by the chase, which produced a much greater decrease in incorporation of label into Golgi and microvillus than into lateral basal membranes. The kinetic data suggest a redistribution of newly synthesized glycoproteins from the site of fucosylation, the Golgi complex, directly into both lateral basal and microvillus membranes. The observed biphasic pattern of label incorporation into the microvillus membrane fraction may be evidence for a second indirect route of incorporation. The selective effect of the chase suggests the presence of two different pools of radioactive fucose in the Golgi complex that differ in (1) their accessibility to dilution with non-radioactive fucose, and (2) their utilization for the biosynthesis of membrane glycoproteins subsequently destined for either the microvillus or the lateral basal parts of the plasmalemma. The radioactively labelled glycoproteins of the different membrane fractions were separated by sodium dodecyl sulphate/polyacrylamide-slab-gel electrophoresis and identified by fluorography. The patterns of labelled glycoproteins in Golgi and lateral basal membranes were identical at all times. At least 14 bands could be identified shortly after radioactive-fucose injection. Most seemed to disappear at later times, although one of them, which was never observed in microvillus membranes, increased in relative intensity. All but two of the labelled glycoproteins present in the microvillus membrane corresponded to those observed in Golgi and lateral basal membranes shortly after fucose injection. The patterns of labelled glycoproteins in all membrane fractions were little affected by the chase. These data support a flow concept for the insertion of most surface-membrane glycoproteins of the intestinal villus cells.
Full text
PDF


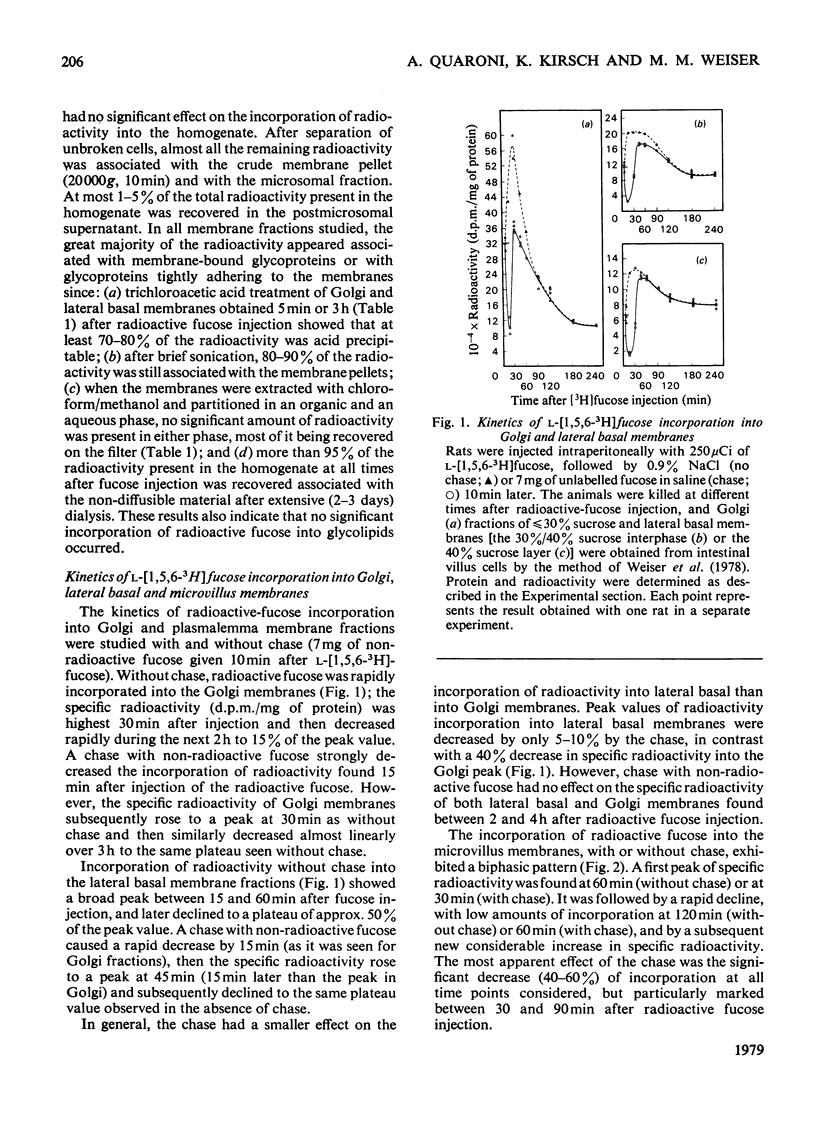
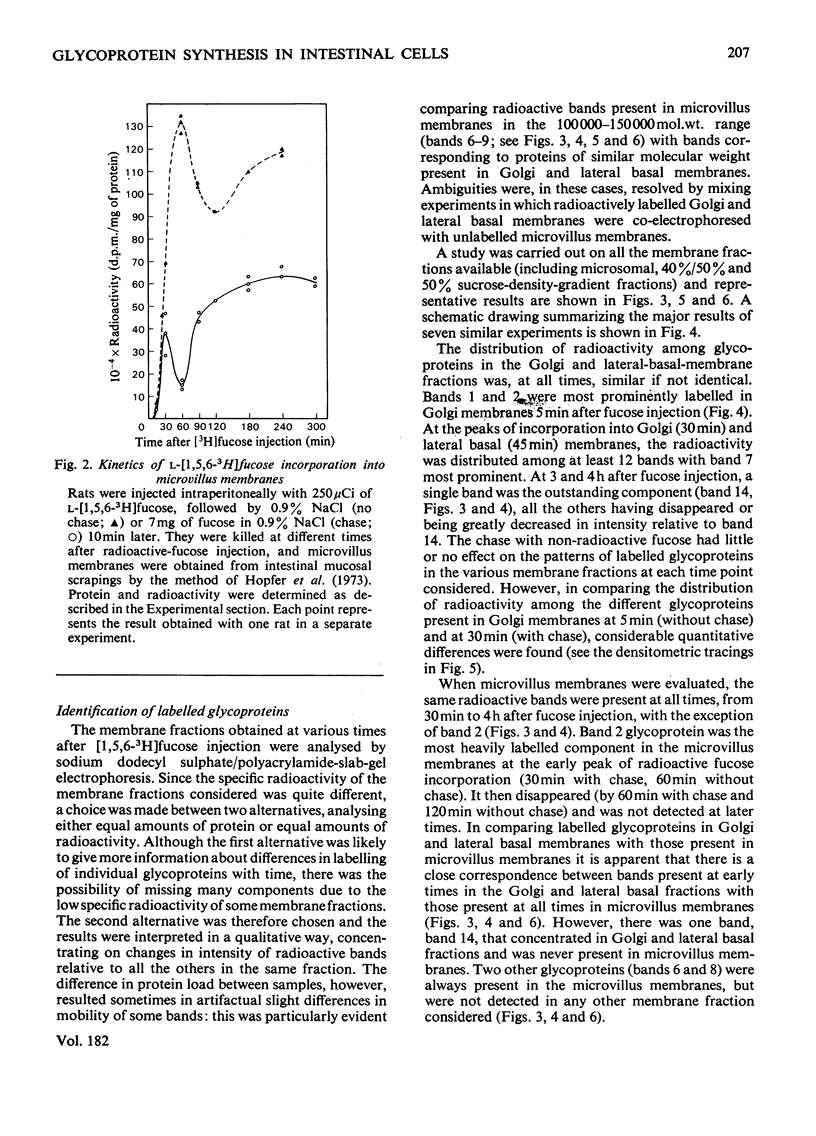

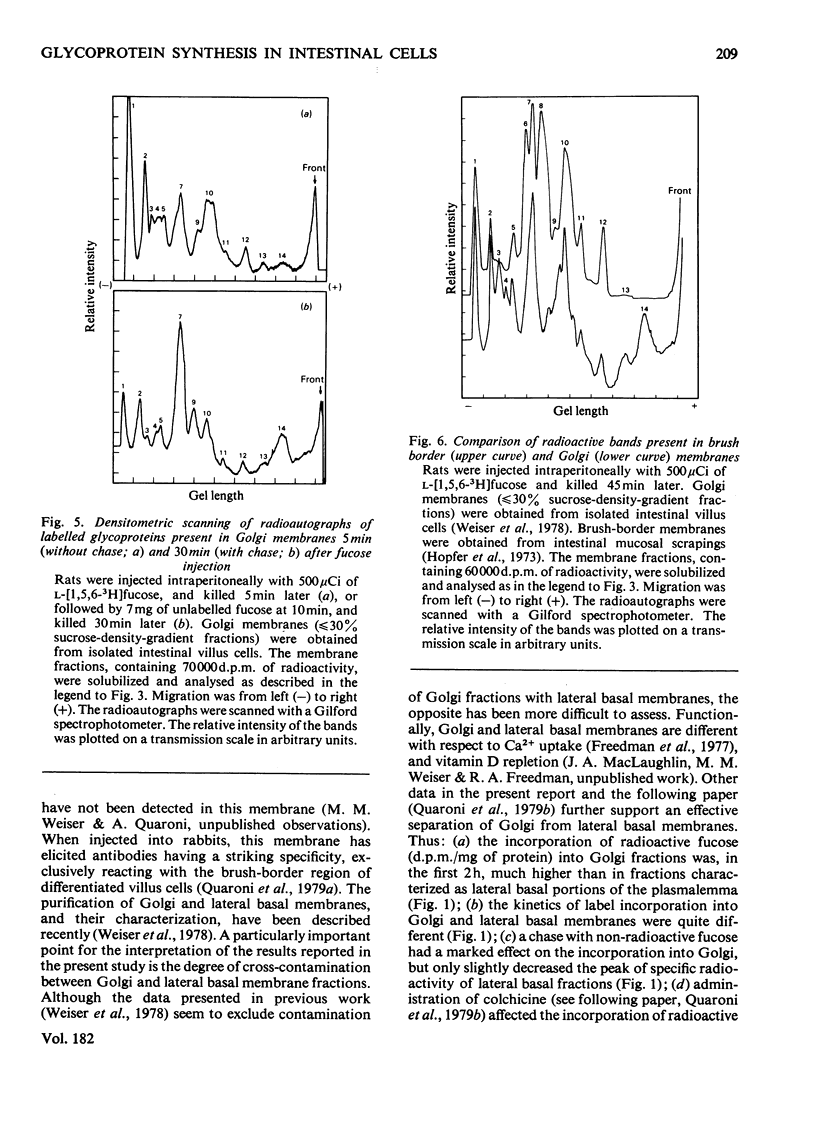



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Protein turnover in intestinal mucosal villus and crypt brush border membranes. Biochem Biophys Res Commun. 1977 Mar 7;75(1):130–135. doi: 10.1016/0006-291x(77)91299-2. [DOI] [PubMed] [Google Scholar]
- Alpers D. H. The relation of size to the relative rates of degradation of intestinal brush border proteins. J Clin Invest. 1972 Oct;51(10):2621–2630. doi: 10.1172/JCI107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. The metabolism of plasma glycoproteins. Studies on the incorporation of L-fucose-1-14-C into tissue and serum in the normal rat. J Biol Chem. 1967 Sep 10;242(17):3873–3879. [PubMed] [Google Scholar]
- Bennett G., Leblond C. P., Haddad A. Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labelled fucose injection into rats. J Cell Biol. 1974 Jan;60(1):258–284. doi: 10.1083/jcb.60.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G., Leblond C. P. Passage of fucose- 3 H label from the Golgi apparatus into dense and multivesicular bodies in the duodenal columnar cells and hepatocytes of the rat. J Cell Biol. 1971 Dec;51(3):875–881. doi: 10.1083/jcb.51.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. Migration of glycoprotein from golgi apparatus to cell coat in the columnar cells of the duodenal epithelium. J Cell Biol. 1970 Jun;45(3):668–673. doi: 10.1083/jcb.45.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Forstner G. G. Goblet cell mucin of rat small intestine. Chemical and physical characterization. Can J Biochem. 1973 Aug;51(8):1154–1166. doi: 10.1139/o73-152. [DOI] [PubMed] [Google Scholar]
- Forstner J., Taichman N., Kalnins V., Forstner G. Intestinal goblet cell mucus: isolation and identification by immunofluorescence of a goblet cell glycoprotein. J Cell Sci. 1973 Mar;12(2):585–602. doi: 10.1242/jcs.12.2.585. [DOI] [PubMed] [Google Scholar]
- Freedman R. A., Weiser M. M., Isselbacher K. J. Calcium translocation by Golgi and lateral-basal membrane vesicles from rat intestine: decrease in vitamin D-deficient rats. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3612–3616. doi: 10.1073/pnas.74.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Hays D. G., Minter D. Cell adhesion and spreading factor. Partial purification and properties. Exp Cell Res. 1977 Nov;110(1):175–190. doi: 10.1016/0014-4827(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Purification and characterization of the retina-specific cell-aggregating factor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):916–920. doi: 10.1073/pnas.72.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Howard C. B., Kelleher P. C. Plasma fucose and sialic acid concentrations during oral glucose tolerance tests in normal and diabetic (mellitus) humans. Clin Chim Acta. 1971 Jan;31(1):75–80. doi: 10.1016/0009-8981(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J. M. Biosynthesis of membrane glycoproteins in the rat small intestine. FEBS Lett. 1974 Aug 30;44(3):309–312. doi: 10.1016/0014-5793(74)81165-8. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Ochoa P., Isaacs R. A. Regional and cellular localization of glycosyltransferases in rat small intestine. Changes in enzymes with differentiation of intestinal epithelial cells. Biochim Biophys Acta. 1975 May 23;391(1):39–50. doi: 10.1016/0005-2744(75)90150-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maestracci D., Preiser H., Hedges T., Schmitz J., Crane R. K. Enzymes of the human intestinal brush border membrane. Identification after gel electrophoretic separation. Biochim Biophys Acta. 1975 Mar 13;382(2):147–156. doi: 10.1016/0005-2736(75)90173-x. [DOI] [PubMed] [Google Scholar]
- Maestracci D., Schmitz J., Preiser H., Crane R. K. Proteins and glycoproteins of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):113–124. doi: 10.1016/0005-2736(73)90435-5. [DOI] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Neutra M., Leblond C. P. Radioautographic comparison of the uptake of galactose-H and glucose-H3 in the golgi region of various cells secreting glycoproteins or mucopolysaccharides. J Cell Biol. 1966 Jul;30(1):137–150. doi: 10.1083/jcb.30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Galactosyltransferase activities in human sera: detection of a cancer-associated isoenzyme. Biochem Biophys Res Commun. 1975 Jul 22;65(2):545–551. doi: 10.1016/s0006-291x(75)80181-1. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Effect of colchicine on the redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes. Biochem J. 1979 Jul 15;182(1):213–221. doi: 10.1042/bj1820213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Mitranic M., Slavik M., Moscarello M. A. The incorporation of L-(14C) fucose into glycoprotein fractions of liver plasma membranes. FEBS Lett. 1974 Oct 15;47(2):248–251. doi: 10.1016/0014-5793(74)81022-7. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Tashima Y. Removal of protein interference in the Fiske-Subbarow method by sodium dodecyl sulfate. Anal Biochem. 1975 Dec;69(2):410–414. doi: 10.1016/0003-2697(75)90143-8. [DOI] [PubMed] [Google Scholar]
- Vicker M. G. BHK21 fibroblast aggregation inhibited by glycopeptides from the cell surface. J Cell Sci. 1976 Jun;21(1):161–173. doi: 10.1242/jcs.21.1.161. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Weiser M. M., Neumeier M. M., Quaroni A., Kirsch K. Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells. Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978 Jun;77(3):722–734. doi: 10.1083/jcb.77.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley W. G., Dauwalder M., Kephart J. E. Golgi apparatus: influence on cell surfaces. Science. 1972 Feb 11;175(4022):596–599. doi: 10.1126/science.175.4022.596. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Atkinson P. H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry. 1977 Mar 8;16(5):944–953. doi: 10.1021/bi00624a021. [DOI] [PubMed] [Google Scholar]



