ABSTRACT
Premature ovarian insufficiency (POI, defined as age at menopause < 40 years) affects 1%–3% of postmenopausal women. It is positively associated with an increased risk of diabetes mellitus, arterial hypertension, cardiovascular disease, osteoporosis, fractures, cognitive impairment, and depression. Early menopause (EM, defined as age at menopause < 45 years) is also associated with these adverse health consequences, in most cases to the same degree as in POI. Therefore, a unifying term for EM and POI, such as ‘premature menopause’, may be proposed, using the age threshold of < 45 years. This could provide broader coverage of these women, substantiating the need for prompt administration of menopausal hormone therapy (in this case, ‘hormone replacement therapy’). However, the benefits of this approach, which precludes a higher oestrogen dose up to the normal age of menopause, need to be proven in well‐designed randomized controlled trials.
Keywords: cardiovascular disease, dementia, early menopause, fractures, hormone replacement therapy, premature ovarian insufficiency
1. Introduction
The average age at menopause is 50–51 years. Menopause occurring at an age > 2 standard deviations below this threshold is considered ‘premature’ [1]. This definition refers to the age of 40 years, which has widely, although arbitrarily, been agreed as the set point for defining ‘premature menopause’, also termed ‘premature ovarian insufficiency’ (POI) [1]. A broader limit of 45 years has been adopted to define ‘early menopause’ (EM) to include women with an age at menopause of 40–44 years [1]. Notably, 10% of women enter menopause before the age of 45 years and 1%–3% before the age of 40 [1].
POI predisposes to a 1.5–2.4‐fold increased risk of cardiovascular disease (CVD) compared with women of a normal age at menopause, mainly due to coronary heart disease (CHD). The excessive risk is attributed to the accumulation of CVD risk factors, such as type 2 diabetes mellitus (T2DM), atherogenic dyslipidemia, arterial hypertension and abdominal obesity [1, 2, 3]. Except for CVD, POI adversely affects other aspects of the woman's health, such as the musculoskeletal (osteoporosis, sarcopenia, fractures) and the central nervous system (CNS) (cognitive dysfunction, depression) [1, 4, 5].
Nonetheless, a cumulative body of evidence during the last decade suggests that the hypoestrogenic state seen in EM is also associated with an increased risk of the complications described in POI and, in some cases, to the same degree [1, 2, 3, 6, 7].
This paper aims to provide evidence regarding the association of EM with an increased risk of long‐term health consequences, as seen in POI. It also underscores the need for managing women with EM as those with POI, which may provide a broader coverage of these patients.
2. Diagnostic Criteria
POI is diagnosed in the presence of oligo‐ or amenorrhoea for ≥ 4 weeks and follicle‐stimulating hormone concentrations > 25 IU/l on two occasions, > 4 weeks apart. Further laboratory investigation should exclude other causes of secondary amenorrhoea, such as pregnancy, hyperprolactinemia and thyroid dysfunction [8, 9]. After confirmation of POI diagnosis, medical history taking to identify additional characteristics associated with secondary causes of POI is recommended, although in the majority of cases (39%–67%), POI is considered idiopathic [8, 9]. Genetic causes, which account for 10%–30% of cases, include either chromosomal abnormalities (e.g., Turner's syndrome, fragile X premutation) or specific gene mutations, which are involved in gonadogenesis, oogenesis, folliculogenesis, mitochondrial and immune function, with or without syndromes [1, 8, 9]. The latter include blepharophimosis, ptosis and epicanthus inversus syndrome (due to pathogenic mutations in the gene FOXL2 in chromosome position 3q23), and deficiencies in enzymes, such as galactose‐1‐phosphate uridyltransferase (galactosaemia), cholesterol desmolase, 17‐alpha‐hydroxylase (a form of congenital adrenal hyperplasia) and 17–20‐desmolase [1, 8, 9]. The remainder of secondary causes of POI may be iatrogenic (6%–47% of cases, after cancer treatments or pelvic surgery), autoimmune (either as a part of autoimmune polyglandular syndrome or co‐existing with non‐endocrine autoimmune diseases, such as coeliac disease, vitiligo, rheumatoid arthritis, accounting for 5%–17% of cases), toxic, metabolic or infectious diseases (1% of cases) [1, 8, 9].
It is estimated that 1%–3% of women will become menopausal before the age of 40 and 0.1% below the age of 30 [1, 8, 9]. Vasomotor symptoms may not always be present at the initial diagnosis of POI. It may take months or years to progress from stages of ovarian insufficiency to true menopause [1, 8, 9]. In some cases, such as genetic syndromes (i.e., Turner's syndrome, which is due to the loss of an X chromosome and has a prevalence of 1/2000–2500 births), primary hypogonadism may be evident even earlier. Specific phenotypical abnormalities (e.g., webbed neck, small mandible, high‐arched palate, crowded teeth, Madelung deformity) predominate, and POI is initially presented as primary amenorrhoea [9]. This is also the case for partial loss of an X chromosome, terminal deletions of its long arm or X autosomal translocations [9]. POI also develops in 20% of premutation carriers in the gene encoding fragile X messenger ribonucleoprotein 1 (FMR1), the so‐called ‘fragile X syndrome’ (1/4000 male and 1/8000 female persons) [9].
The age at which ovarian insufficiency occurs cannot be accurately determined and more research is needed for this purpose. Although POI is universally defined as menopause occurring at an age > 2 standard deviations below the mean estimated for the reference population [1], specific attention should be paid to younger ages, in which the likelihood of identifying a secondary cause is high. Except for karyotype and FMR1 gene testing, extensive investigation for rare genetic syndromes is not recommended for every patient presenting with POI [1]. This is particularly indicated in those women with an identifiable family history of early ovarian insufficiency, especially if it has occurred at an age younger than 30 years. It may provide some advantages regarding the therapeutic management of POI, and genetic counselling of the patient's family members [1].
3. Epidemiological Evidence
3.1. CVD
Regarding CVD risk, according to a meta‐analysis published in 2016 (32 studies, n = 310,329), women who experience EM are at increased risk of overall (relative risk [RR] 1.50, 95% confidence interval [CI] 1.28–1.76) and fatal CHD (RR 1.11, 95% CI 1.03–1.20). CVD mortality is also increased (RR 1.19, 95% CI 1.08–1.31) compared with women of a normal age at menopause (> 45 years) [10].
Furthermore, according to the International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events (InterLACE), which constitutes a pooled analysis of individual patient data from 15 observational studies, the adjusted hazard ratio (HR) for non‐fatal CVD in women with POI and EM (defined as age at menopause 40–44 years) was 1.55 (95% CI 1.38–1.73) and 1.30 (95% CI 1.22–1.39), respectively, compared with women with age at menopause 50–51 years [11]. These associations were more evident for women < 60 years (HR 1.88 and 1.40, respectively, for POI and EM), but attenuated after that, with no association for women > 70 years [11]. This increased CVD risk involved both CHD (adjusted HR 1.52 [95% CI 1.34–1.74] and 1.30 [95% CI 1.20–1.41]) and stroke (adjusted HR 1.72 [95% CI 1.43–2.07] and 1.32 [95% CI 1.18–1.48]) [11].
According to a prospective US cohort study not included in the aforementioned meta‐analysis (n = 3474), increased CVD mortality was observed in women who underwent bilateral oophorectomy before the age of 45, compared with those without oophorectomy (HR 1.67, 95% CI 1.16–2.40). However, no association was found with CHD (HR 1.40, 95% CI 0.80–2.44) or cerebrovascular disease (HR 1.22, 95% CI 0.54–2.74) [12].
In a very recent prospective cohort study from South Korea (n = 1,159,405 postmenopausal women), both POI and EM were independent risk factors for CVD, including myocardial infarction (MI) and ischaemic stroke [13]. After adjustment for potential confounders (i.e., age, smoking, alcohol, exercise, income, body mass index, T2DM, hypertension, dyslipidaemia, estimated glomerular filtration rate and hormone replacement therapy [HRT]), the respective HR for MI in women with POI (30–39 years) and EM (40–44 years) was 1.40 (95% CI 1.31–1.50) and 1.18 (95% CI 1.13–1.24), respectively, compared with those with age at menopause ≥ 50 years [13].
Additionally, EM predisposes to an increased risk of heart failure. According to a recent meta‐analysis of nine cohort studies (n = 6,255,783 postmenopausal women), the HR for women with POI and EM for heart failure was 1.39 (95% CI 1.31–1.47) and 1.23 (95% CI 1.10–1.37), respectively, compared with those who had undergone menopause after the age of 45 [14]. This was also the case, although to a lesser extent, for atrial fibrillation (HR 1.15 [95% CI 1.01–1.31] and 1.08 [95% CI 1.04–1.13], respectively) [14].
Regarding individual risk factors, transition to menopause at the age of < 45 years compromises glucose homoeostasis and increases T2DM risk, as shown in a meta‐analysis including 191,762 postmenopausal women (odds ratio [OR] 1.12, 95% CI 1.01–1.20; the respective OR for POI was 1.53 [95% CI 1.03–2.27]) [2]. This is also the case for arterial hypertension. According to another meta‐analysis (n = 273,994 postmenopausal women), women with EM are at increased risk for arterial hypertension compared with those of normal age at menopause (> 45 years) (OR 1.10, 95% CI 1.01–1.19). This risk is marginally non‐significant for women with POI (OR 1.14, 95% CI 0.97–1.37) [3].
In the rare causes of POI, such as Turner's syndrome, cardiovascular complications are more severe since they include not only atherosclerotic CVD but also congenital structural cardiovascular defects, such as valvular and aortic abnormalities (coarctation, dilatation and dissection) [15]. This is also the case for fragile X syndrome, in which CVD disorders may include autonomic dysfunction, arterial hypertension and arrhythmias [9].
3.2. Musculoskeletal Health
Regarding musculoskeletal health, both POI and EM are associated with decreased bone mineral density (BMD), increased risk of sarcopenia, and subsequently, increased risk of fractures. Of note, the exact risk associated with POI may be underestimated due to the more frequent use of HRT in these women compared with EM. The latter is associated with an almost two‐fold increased risk of osteoporosis compared with women with normal age at menopause (RR 1.83 [95% CI 1.22–2.74]) for women entering menopause before 47 years compared with those with a younger age at menopause (i.e., < 47 years) [16].
In a recent meta‐analysis, EM and POI were associated with impairment in sarcopenia indices [5], potentially predisposing to increased fracture risk. These results align with a recent study (n = 1249 community‐dwelling women), which showed a twofold increased risk of frailty in women with POI or EM (OR 1.90, 95% CI 1.28–2.81) [17].
Concerning fracture risk, according to a meta‐analysis published in 2019 (n = 462,393), EM increases fracture risk (OR 1.36, 95% CI 1.11–1.66) compared with normal age at menopause, although chronological age modifies it [7]. No association with POI was found, perhaps indicating the beneficial effect of HRT in this regard (OR 0.54, 95% CI 0.22–1.29) [7]. A recent cross‐sectional study (n = 985 postmenopausal women) confirmed that women entering menopause before the age of 45 are at high risk of hip fracture (OR 1.65, 95% CI 1.14–2.40) and have a higher mean FRAX score (1.60 vs. 1.30, p < .004), compared with women who entered menopause after the age of 45 [18].
In the Study of Women's Health Across the Nation (SWAN), a longitudinal cohort study of the menopause transition, EM was associated with low BMD, independently of chronological age [19]. Moreover, an 1‐year decrease in the age of the final menstrual period was associated with a 5% increase in fractures [19].
3.3. CNS
Regarding CNS, women with EM demonstrate an increased risk of dementia. According to a recent meta‐analysis (n = 4,716,862), the risk of dementia is 37% higher in women with EM compared with those of normal age at menopause (OR 1.37, 95% CI 1.22–1.54) [4]. This was also the case for POI (OR 1.18, 95% CI 1.15–1.21) [4]. Notably, the risk of Parkinsonism may also increase in women with POI, as shown in a prospective cohort study (n = 3443; HR 2.55, 95% CI 1.35–5.15). This was marginally non‐significant in women with EM (38–45 years) (HR 1.54, 95% CI 0.85–2.9) [20]. However, the risk for Alzheimer's disease does not differ between women with surgical POI or EM for benign causes and those without a history of oophorectomy [21].
3.4. All‐Cause Mortality
According to the meta‐analysis mentioned above, which was published in 2016, women who experience EM are at increased all‐cause mortality (RR 1.12, 95% CI 1.03–1.21) compared with those of age at menopause > 45 years (average time of follow‐up among studies ranging from 9.2 to 24 years) [10]. According to a prospective cohort study not included in this meta‐analysis (n = 3474), published in 2006, women who underwent bilateral oophorectomy before the age of 45 years experienced increased overall mortality compared with those without oophorectomy (HR 1.67, 95% CI 1.16–2.40) (median follow‐up time: 25 years; range 0.01–53.8) [22]. Furthermore, according to another prospective cohort study published in 2014 (n = 52,846), higher mortality rates were also observed in women undergoing bilateral salpingo‐oophorectomy by the age of 40 (HR 1.12, 95% CI, 1.04–1.21) or 45 (HR 1.10, 95% CI, 1.03–1.17), compared with those not having had this surgery (mean follow‐up time: 22.1 years) [21]. A very recent study, using data from the National Health and Nutrition Examination Survey (NHANES) (1999–2018) and NHANES III (1988–1994), including 14,161 postmenopausal women, showed increased all‐cause mortality for POI or EM (40–44 years) compared with normal age at natural menopause (45–54 years) (HR 1.48 [95% CI 1.15–1.91] and 1.16 [95% CI 1.00–1.35], respectively) [23]. The independent association between both POI and EM with increased all‐cause mortality was also shown in the recent large Korean study (HR 1.19 [95% CI 1.14–1.24] and 1.13 [95% CI 1.11–1.16], respectively), compared with women with age at menopause ≥ 50 years (average follow‐up time: 10 years) [13].
Table 1 presents the comparative risks for POI and EM regarding CVD, musculoskeletal system, dementia and all‐cause mortality.
Table 1.
Summary of the risks for long‐term health consequences in women with POI compared with those with EM.
| Outcome | Study | POI | EM (age, years) | Reference group |
|---|---|---|---|---|
| CVD, fatal | Rivera et al. [12] | — | HR 1.44, 95% CI 1.01–2.05 (< 45) | No oophorectomy |
| Muka et al. [10] | — | RR 1.19, 95% CI 1.08–1.31 (< 45) | Age at menopause ≥ 45 years | |
| Xu et al. [25] | HR 2.38, 95% CI 1.64–3.45 | HR 1.41, 95% CI 1.09–1.82 (40–44) | Age at menopause 50–52 years | |
| Xu et al. [25]* | HR 1.40, 95% CI 0.86–2.26 | HR 1.12, 95% CI 0.69–1.80 (40–44) | Age at menopause 50–52 years | |
| CVD, non‐fatal | Zhu et al. [11] | HR 1.55, 95% CI 1.38–1.73 | HR 1.30, 95% CI 1.22–1.39 (40–44) | Age at menopause 50–51 years |
| CHD, overall | Muka et al. [10] | — | RR 1.50, 95% CI 1.28–1.76 (< 45) | Age at menopause ≥ 45 years |
| Lee et al. [13] | HR 1.40, 95% CI 1.31–1.50 | HR 1.18, 95% CI 1.13–1.24 (40–44) | Age at menopause ≥ 50 years | |
| CHD, fatal | Rivera et al. [12] | — | HR 1.40, 95% CI 0.80–2.44 (< 45) | No oophorectomy |
| Gierach et al. [21]* | HR 1.37, 95% CI 1.19–1.58 | HR 1.28, 95% CI 1.14–1.43 (≤ 45) | No oophorectomy | |
| Muka et al. [10] | — | RR 1.11, 95% CI 1.03–1.20 (< 45) | Age at menopause ≥ 45 years | |
| CHD, non‐fatal | Zhu et al. [11] | HR 1.72, 95% CI 1.34–1.74 | HR 1.30, 95% CI 1.20–1.41 (40–44) | Age at menopause 50–51 years |
| Stroke, fatal | Rivera et al. [12] | — | HR 1.22, 95% CI 0.54–2.74 (< 45) | No oophorectomy |
| Gierach et al. [21]* | HR 1.15, 95% CI 0.89–1.48 | HR 1.19, 95% CI 0.98–1.45 (≤ 45) | No oophorectomy | |
| Muka et al. [10] | — | RR 0.99, 95% CI 0.92–1.07 (< 45) | Age at menopause ≥ 45 years | |
| Stroke, non‐fatal | Zhu et al. [11] | HR 1.72, 95% CI 1.43–2.07 | HR 1.32, 95% CI 1.18–1.48 (40–44) | Age at menopause 50–51 years |
| Stroke, overall | Muka et al. [10] | — | RR 1.23, 95% CI 0.98–1.53 (< 45) | Age at menopause ≥ 45 years |
| Lee et al. [13] | HR 1.24, 95% CI 1.17–1.31 | HR 1.18, 95% CI 1.14–1.22 (40–44) | Age at menopause ≥ 50 years | |
| Peripheral artery disease | Honigberg et al. [24] |
HR, 1.34, 95% CI 0.33–5.41 HR, 1.34, 95% CI 0.79–2.26* |
— | Age at menopause > 40 years |
| Heart failure | Liu et al. [14] | HR 1.39, 95% CI 1.31–1.47 | HR 1.23, 95% CI 1.10–1.37 (< 45) | Age at menopause > 45 years |
| Atrial fibrillation | Liu et al. [14] | HR 1.15, 95% CI 1.01–1.31 | HR 1.08, 95% CI: 1.04–1.13 (< 45) | Age at menopause > 45 years |
| Diabetes mellitus | Gierach et al. [21]* | HR 1.60, 95% CI 1.01–2.52 | HR 1.45, 95% CI 1.00–2.10 (≤ 45) | No oophorectomy |
| Anagnostis et al. [2] | OR 1.12, 95% CI 1.01–1.20 | OR 1.53, 95% CI 1.03–2.27 (< 45) | Age at menopause ≥ 45 years | |
| Arterial hypertension | Anagnostis et al. [3] | OR 1.14, 95% CI 0.97–1.37 | OR 1.10, 95% CI 1.01–1.19 (< 45) | Age at menopause ≥ 45 years |
| Fracture | Anagnostis et al. [7] | OR 0.54, 95% CI 0.22–1.29 | OR 1.36, 95% CI 1.11–1.66 (< 45) | Age at menopause ≥ 45 years |
| Shieh et al. [19] | HR 1.62, 95% CI 1.17–2.23 |
5% increase in risk for each 1‐year decrement in age at FMP |
Age at menopause ≥ 45 years | |
| Sarcopenia (muscle mass) | Divaris et al. [5] | — | SMD −0.14, 95% CI −0.20 to −0.07 (< 45) | Age at menopause ≥ 45 years |
| Sarcopenia (muscle strength) | Divaris et al. [5] | SMD −0.30, 95% CI −0.58 to −0.01 | SMD −0.15, 95% CI −0.31 to 0.01 (< 45) | Age at menopause ≥ 45 years |
| Sarcopenia (muscle performance) | Divaris et al. [5] | SMD −0.13, 95% CI −0.23 to −0.04 | SMD −0.11, 95% CI −0.29 to 0.05 (< 45) | Age at menopause ≥ 45 years |
| Frailty | Kojima et al. [17] | — | OR 1.90, 95% CI 1.28–2.81 (≤ 45) | Age at menopause 46–55 years |
| Dementia | Karamitrou et al. [4] | OR 1.18, 95% CI 1.15–1.21 | OR 1.37, 95% CI 1.22–1.54 (< 45) | Age at menopause ≥ 45 years |
| Parkinsonism | Rocca et al. [20] | HR 2.55, 95% CI 1.35–5.15 | HR 1.54, 95% CI 0.85–2.9 (38–45) | Median age at menopause 50 years |
| Alzheimer's disease | Gierach et al. [21]* | HR 0.8, 95% CI 0.45–1.43 | HR 0.69, 95% CI 0.43–1.10 (≤ 45) | No oophorectomy |
| All‐cause mortality | Rocca et al. [22]* | — | HR 1.67, 95% CI 1.16–2.40 (< 45) | No oophorectomy |
| Gierach et al. [21]* | HR 1.12, 95% CI 1.04–1.21 | HR 1.10, 95% CI 1.03–1.17 (< 45) | No oophorectomy | |
| Muka et al. [10] | — | RR 1.12, 95% CI 1.03–1.21 (< 45) | Age at menopause ≥ 45 years | |
| Lee et al. [13] | HR 1.19, 95% CI 1.14–1.24 | HR 1.13, 95% CI 1.11–1.16 (40–44) | Age at menopause ≥ 50 years | |
| Xing and Kirby [23] | HR 1.48, 95% CI 1.15–1.91 | HR 1.16, 95% CI 1.00–1.35 (< 45) | Age at menopause 45–54 years | |
| Xing and Kirby [23]* | HR 1.39, 95% CI 1.11–1.75 | HR 1.09, 95% CI 0.86–1.38 (< 45) | Age at menopause 45–54 years |
Note: The bold values indicate statistical significance.
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; EM, early menopause; FMP, final menstrual period; HR, hazard ratio; OR, odds ratio; POI, premature ovarian insufficiency; RR, relative risk; SMD, standardized mean difference.
Surgical menopause
4. The Effect of the Type on Menopause (Surgical or Natural) on Health Outcomes Associated With POI/EM
The type of menopause (surgical or natural) seems to modify CVD risk. In particular, surgical POI increases the risk of atherosclerotic CVD to a greater extent than natural POI, in comparison with age at menopause > 40 years (HR 1.87 [95% CI 1.36–2.58] and 1.36 [95% CI 1.19–1.56], after multivariable adjustment), according to a large cohort study from the UK Biobank (n = 144,260 postmenopausal women) [24]. This mainly involved CHD (HR 2.52 [95% CI 1.48–4.29] and 1.39 [95% CI 1.06–1.82], for surgical and natural POI, respectively) [24]. In another recent publication from the UK Biobank, the association with increased CVD mortality was evident only for natural (HR 2.38 [95% CI 1.64–3.45] and 1.41 [95% CI 1.09–1.82], respectively), but not for surgical POI and EM (HR 1.40 [95% CI 0.86–2.26] and 1.12 [95% CI 0.69–1.80], respectively), compared with women with age at menopause 50–52 years [25]. Moreover, in the NHANES–NHANES III study, surgical menopause did not significantly modify the association between age at menopause and mortality [23].
Interestingly, peripheral artery disease, mainly that involving the lower extremities, has been less documented in the context of age at menopause. According to the aforementioned study from the UK Biobank, the risk for PAD did not differ between natural (HR 1.34, 95% CI 0.33–5.41) or surgical POI (HR 1.34, 95% CI 0.79–2.26) and menopause at an older age (> 40 years), after adjustment for multiple confounders [24].
Regarding dementia, the risk is elevated for women with natural (OR 1.61, 95% CI 1.30–2.00), but not for surgical EM (OR 0.82, 95% CI 0.16–4.20) compared with women with normal age at menopause (> 45 years) [4].
Therefore, future studies must clarify the exact effect of the type of menopause on long‐term health consequences.
5. The Effect of HRT on POI/EM‐Related Health Outcomes
In general, HRT is safe for women with EM [25]. Data from the UK Biobank study indicated that HRT reduced mortality related to breast cancer both in women with natural (HR 0.59, 95% CI 0.36–0.95) and surgical EM (HR 0.17, 95% CI 0.08–0.36), compared with no use of HRT [25]. However, no effect on CVD mortality and mortality from other causes was shown [25]. In another prospective cohort study (n = 3474), decreased CVD mortality was demonstrated in women with surgical EM who received HRT (HR 0.65, 95% CI 0.30–1.41) compared with those who had not (HR 1.84, 95% CI 1.27–2.68) [12].
In the Korean cohort mentioned above, the association between POI or EM with MI was not affected by HRT (HR for women with POI and EM not receiving HRT: 1.38 [95% CI 1.28–1.48] and 1.17 [95% CI 1.12–1.22], respectively; HR for POI and EM having received HRT for ≥ 5 years: 1.51 [95% CI 1.03–2.22] and 1.39 [95% CI 1.07–1.82], respectively) [13]. However, the risk of ischaemic stroke was not significant in women with a history of HRT use for ≥ 5 years (HR 1.12 [95% CI 0.76–1.67] and 1.06 [95 CI 0.81–1.39] for women with POI and EM, respectively), whereas it was increased for women who had not received HRT (HR 1.23 [95% CI 1.16–1.31] and 1.18 [95 CI 1.14–1.22], respectively) [13]. This was also the case for all‐cause mortality, but only for POI (EM was still associated with increased risk, regardless of HRT use) [13]. However, it must be highlighted that only 18% of women with POI received HRT in this study [13].
It must be underscored that the exact HRT regimen data, as well as adherence to treatment, was unavailable in these studies. Therefore, no safe conclusions can be made regarding the differential effect of HRT according to the dose and route of administration.
In general, current guidelines recommend the use of HRT for all women with POI until the age of natural menopause. On the other hand, women with EM are offered HRT only if they have bothersome menopausal symptoms despite carrying an increased risk for cardiovascular, musculoskeletal and CNS disease [1]. Based on the data presented above, oestrogen therapy should be prescribed to women with EM, not for the sole purpose of vasomotor symptom relief (simply as ‘menopausal hormone therapy’), but as a true ‘HRT’ to potentially reduce the risks mentioned above, covering all women with age at menopause < 45 years (Figure 1). In rare genetic causes of POI, such as Turner's syndrome, initial treatment includes lower oestrogen doses, aiming at the induction of puberty and gradually moving to the conventional HRT regimen used in POI [1, 8, 9].
Figure 1.
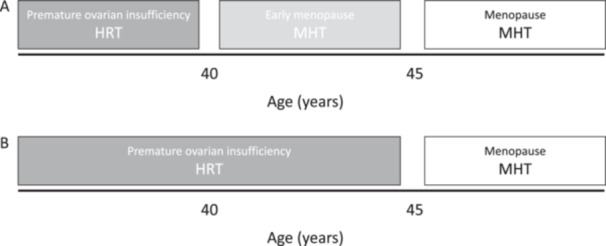
Current (A) and proposed (B) recommendations for menopause treatment approach. HRT, hormone replacement therapy; MHT, menopausal hormone therapy.
Another issue is that, except for the case of bilateral oophorectomy, the permanency of POI or EM cannot be predicted. As with natural menopause, POI and EM are characterized by a gradual decrease in ovarian reserve, leading initially to hypogonadism and, afterwards, to vasomotor symptomatology and cardiometabolic complications. Indeed, a residual ovarian function exists at the time of diagnosis of POI, before the permanent cessation of menstruation, which may discriminate it from the diagnosis of natural menopause. Prompt administration of HRT at the initial stage of POI or EM is essential to restore oestrogen concentrations to normal, as in the case of hypothyroidism. However, in the latter situation, levothyroxine administration depends mainly on thyroid‐stimulating hormone concentrations with or without symptoms, and subclinical hypothyroidism still remains in a much milder state compared with clinical hypothyroidism, regarding its cardiovascular complications. This is not the case with POI and EM, since the latter is also associated with the same consequences as the former (although to a lesser extent), necessitating the need for prompt administration of HRT.
6. Conclusions
In conclusion, EM and POI share common risks for CVD, fracture and dementia, leading to increased morbidity and mortality in later life. Therefore, the management of women with EM, as those with POI, may provide broader coverage of these patients, substantiating the need for prompt administration of HRT. The benefits of this approach, which may result in the administration of higher oestrogen doses than usual until natural menopause, need to be proven in appropriately designed randomized controlled trials. Moreover, long‐term adherence to HRT should also be achieved to obtain the maximum benefit. Therefore, there is an imperative need for more research that indicates the best way to offer HRT at different ages of early hypoestrogenism since, even in women with POI, there is high heterogeneity in the way and time during which it is prescribed.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific grant from any public, commercial or not‐for‐profit funding agency.
References
- 1. Hamoda H. and Sharma A., “Premature Ovarian Insufficiency, Early Menopause, and Induced Menopause,” Best Practice & Research Clinical Endocrinology & Metabolism 38 (2023): 101823. [DOI] [PubMed] [Google Scholar]
- 2. Anagnostis P., Christou K., Artzouchaltzi A. M., et al., “Early Menopause and Premature Ovarian Insufficiency Are Associated With Increased Risk of Type 2 Diabetes: A Systematic Review and Meta‐Analysis,” European Journal of Endocrinology 180, no. 1 (2019): 41–50. [DOI] [PubMed] [Google Scholar]
- 3. Anagnostis P., Theocharis P., Lallas K., et al., “Early Menopause Is Associated With Increased Risk of Arterial Hypertension: A Systematic Review and Meta‐Analysis,” Maturitas 135 (2020): 74–79. [DOI] [PubMed] [Google Scholar]
- 4. Karamitrou E. K., Anagnostis P., Vaitsi K., Athanasiadis L., and Goulis D. G., “Early Menopause and Premature Ovarian Insufficiency Are Associated With Increased Risk of Dementia: A Systematic Review and Meta‐Analysis of Observational Studies,” Maturitas 176 (2023): 107792. [DOI] [PubMed] [Google Scholar]
- 5. Divaris E., Anagnostis P., Gkekas N. K., Kouidi E., and Goulis D. G., “Early Menopause and Premature Ovarian Insufficiency May Increase the Risk of Sarcopenia: A Systematic Review and Meta‐Analysis,” Maturitas 175 (2023): 107782. [DOI] [PubMed] [Google Scholar]
- 6. Anagnostis P., Lambrinoudaki I., Stevenson J. C., and Goulis D. G., “Menopause‐Associated Risk of Cardiovascular Disease,” Endocrine Connections 11, no. 4 (2022): e210537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anagnostis P., Siolos P., Gkekas N. K., et al., “Association Between Age at Menopause and Fracture Risk: A Systematic Review and Meta‐Analysis,” Endocrine 63, no. 2 (2019): 213–224. [DOI] [PubMed] [Google Scholar]
- 8. European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI , Webber L., M. Davies, et al., “ESHRE Guideline: Management of Women With Premature Ovarian Insufficiency,” Human Reproduction 31, no. 5 (2016): 926–937. [DOI] [PubMed] [Google Scholar]
- 9. Stuenkel C. A. and Gompel A., “Primary Ovarian Insufficiency,” New England Journal of Medicine 388, no. 2 (2023): 154–163. [DOI] [PubMed] [Google Scholar]
- 10. Muka T., Oliver‐Williams C., Kunutsor S., et al., “Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All‐Cause Mortality: A Systematic Review and Meta‐Analysis,” JAMA Cardiology 1, no. 7 (2016): 767–776. [DOI] [PubMed] [Google Scholar]
- 11. Zhu D., Chung H. F., Dobson A. J., et al., “Age at Natural Menopause and Risk of Incident Cardiovascular Disease: A Pooled Analysis of Individual Patient Data,” Lancet Public Health 4, no. 11 (2019): e553–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rivera C. M., Grossardt B. R., Rhodes D. J., et al., “Increased Cardiovascular Mortality After Early Bilateral Oophorectomy,” Menopause 16, no. 1 (2009): 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee G. B., Nam G. E., Kim W., et al., “Association Between Premature Menopause and Cardiovascular Diseases and All‐Cause Mortality in Korean Women,” Journal of the American Heart Association 12, no. 22 (2023): e030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J., Jin X., Chen W., Wang L., Feng Z., and Huang J., “Early Menopause Is Associated With Increased Risk of Heart Failure and Atrial Fibrillation: A Systematic Review and Meta‐Analysis,” Maturitas 176 (2023): 107784. [DOI] [PubMed] [Google Scholar]
- 15. Kostopoulou E., Bosdou J. K., Anagnostis P., Stevenson J. C., and Goulis D. G., “Cardiovascular Complications in Patients With Turner's Syndrome,” Current Pharmaceutical Design 26, no. 43 (2020): 5650–5659. [DOI] [PubMed] [Google Scholar]
- 16. Svejme O., Ahlborg H., Nilsson J. Å., and Karlsson M., “Early Menopause and Risk of Osteoporosis, Fracture and Mortality: A 34‐Year Prospective Observational Study in 390 Women,” BJOG: An International Journal of Obstetrics & Gynaecology 119, no. 7 (2012): 810–816. [DOI] [PubMed] [Google Scholar]
- 17. Kojima G., Taniguchi Y., Aoyama R., and Urano T., “Earlier Menopause Is Associated With Higher Risk of Incident Frailty in Community‐Dwelling Older Women in England,” Journal of the American Geriatrics Society 70, no. 9 (2022): 2602–2609. [DOI] [PubMed] [Google Scholar]
- 18. Minakovic I., Zvekic‐Svorcan J., Jankovic T., Vuksanovic M., Mikic D., and Boskovic K., “Early Menopause and Risk of Fractures—A Preventable Gap,” Iranian Journal of Public Health 52, no. 3 (2023): 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shieh A., Ruppert K. M., Greendale G. A., et al., “Associations of Age at Menopause With Postmenopausal Bone Mineral Density and Fracture Risk in Women,” Journal of Clinical Endocrinology & Metabolism 107, no. 2 (2022): e561–e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rocca W. A., Grossardt B. R., and Maraganore D. M., “The Long‐Term Effects of Oophorectomy on Cognitive and Motor Aging Are Age Dependent,” Neurodegenerative Diseases 5, no. 3–4 (2008): 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gierach G. L., Pfeiffer R. M., Patel D. A., et al., “Long‐Term Overall and Disease‐Specific Mortality Associated With Benign Gynecologic Surgery Performed at Different Ages,” Menopause 21, no. 6 (2014): 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rocca W. A., Grossardt B. R., de Andrade M., Malkasian G. D., and L. J. Melton, 3rd , “Survival Patterns After Oophorectomy in Premenopausal Women: A Population‐Based Cohort Study,” Lancet Oncology 7, no. 10 (2006): 821–828. [DOI] [PubMed] [Google Scholar]
- 23. Xing Z. and Kirby R. S., “Age at Natural or Surgical Menopause, All‐Cause Mortality, and Lifespan Among Postmenopausal Women in the United States,” Menopause 31, no. 3 (2024): 176–185. [DOI] [PubMed] [Google Scholar]
- 24. Honigberg M. C., Zekavat S. M., Aragam K., et al., “Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease,” JAMA 322, no. 24 (2019): 2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z., Chung H. F., Dobson A. J., Wilson L. F., Hickey M., and Mishra G. D., “Menopause, Hysterectomy, Menopausal Hormone Therapy and Cause‐Specific Mortality: Cohort Study of UK Biobank Participants,” Human Reproduction 37, no. 9 (2022): 2175–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]


