Abstract
In vitro analysis of the catalytic DNA polymerase encoded by vaccinia virus has demonstrated that it is innately distributive, catalyzing the addition of <10 nucleotides per primer-template binding event in the presence of 8 mM MgCl2 or 40 mM NaCl (W. F. McDonald and P. Traktman, J. Biol. Chem. 269:31190–31197, 1994). In contrast, cytoplasmic extracts isolated from vaccinia virus-infected cells contain a highly processive form of DNA polymerase, able to catalyze the replication of a 7-kb template per binding event under similar conditions. To study this holoenzyme, we were interested in purifying and characterizing the vaccinia virus processivity factor (VPF). Our previous studies indicated that VPF is expressed early after infection and has a native molecular mass of ∼48 kDa (W. F. McDonald, N. Klemperer, and P. Traktman, Virology 234:168–175, 1997). Using these criteria, we established a six-step chromatographic purification procedure, in which a prominent ∼45-kDa band was found to copurify with processive polymerase activity. This species was identified as the product of the A20 gene. By use of recombinant viruses that direct the overexpression of A20 and/or the DNA polymerase, we verified the physical interaction between the two proteins in coimmunoprecipitation experiments. We also demonstrated that simultaneous overexpression of A20 and the DNA polymerase leads to a specific and robust increase in levels of processive polymerase activity. Taken together, we conclude that the A20 gene encodes a component of the processive DNA polymerase complex. Genetic data that further support this conclusion are presented in the accompanying report, which documents that temperature-sensitive mutants with lesions in the A20 gene have a DNA− phenotype that correlates with a deficit in processive polymerase activity (A. Punjabi et al, J. Virol. 75:12308–12318, 2001).
Vaccinia virus exhibits a high degree of genetic and physical autonomy from the host cell, possessing a genome that encodes more than 200 genes and directing a replicative cycle in which DNA replication, gene expression, and morphogenesis take place solely within the cytoplasm of the infected cell. It is therefore likely that the trans-acting functions required for DNA replication are virally encoded, and several laboratories have engaged in studies aimed at identifying and characterizing these functions. The catalytic DNA polymerase of vaccinia virus is encoded by the E9 gene (6, 20, 31). The 116-kDa enzyme possesses both polymerase and proofreading exonuclease activity and retains many of the conserved domains that are found within the replicative polymerases of mammalian and yeast cells, as well as those encoded by other large DNA viruses. In addition, the vaccinia virus enzyme, like the catalytic subunit of these other replicative polymerases, is inherently distributive. Our studies on the purified E9 protein indicate that in the presence of 40 mM NaCl or 8 mM MgCl2, the enzyme catalyzes the addition of <10 nucleotides (nt) per primer-template binding event (21). Such distributive behavior would be incompatible with efficient replication of a large genome in vivo, and it is therefore no surprise that a highly processive form of the vaccinia virus enzyme is found in unfractionated cytoplasmic extracts of infected cells (19). This processive enzyme is capable of catalyzing the addition of >7,000 nt per primer-template binding event.
This dichotomy between the distributive behavior of the catalytic subunit of a polymerase and the processive behavior of the replicative holoenzyme is virtually universal in viral, prokaryotic, and eukaryotic systems. Comparative analyses of these systems has revealed several molecular strategies for generating a processive enzyme. The cellular enzymes interact with a toroidal processivity factor that associates topologically, but not directly, with the template DNA. Loading these sliding clamps (a homotrimer of PCNA in eukaryotic cells; a homodimer of the β subunit in Escherichia coli) onto the DNA requires an ATP-hydrolyzing multisubunit clamp loader (replication factor C in eukaryotic cells; the γ complex in E. coli) (27). Bacteriophage T4 also uses a comparable strategy for achieving processivity. Bacteriophage T7 utilizes a novel strategy in which processivity is achieved by interaction of the polymerase with thioredoxin; however, since thioredoxin does not bind to DNA, the mechanism by which processivity is gained remains unclear. A distinct mechanism appears to be used by some eukaryotic viruses. In herpes simplex virus, the UL42 protein binds both to DNA and to the catalytic polymerase, serving to tether the polymerase to the primer-template without inhibiting its ability to translocate along the DNA (1, 15). The UL42 protein is able to confer processivity on the viral DNA polymerase by increasing its rate of association with, and decreasing its rate of disassociation from, the primer-template junction (32).
We have previously shown that the factor(s) responsible for conferring processivity on purified vaccinia virus DNA polymerase is present within cytoplasmic extracts prepared from infected, but not uninfected, cells. Moreover, we have demonstrated that accumulation of the factor required only the early phase of gene expression and that the vaccinia virus processivity factor (VPF) appeared to have a native molecular mass of approximately 48 kDa (19). In this report, we describe the purification of a 49-kDa component of the processive polymerase and its identification as the product of the A20 gene. In an accompanying report (28), we present the utilization of clustered charge-to-alanine mutagenesis and transient dominant selection to generate vaccinia viruses containing altered alleles of the A20 gene, and we show that two such viruses have a temperature-sensitive DNA− phenotype that correlates with a defect in processive polymerase activity.
MATERIALS AND METHODS
Materials.
DEAE-cellulose and phosphocellulose resins were obtained from Whatman Biosystems, Ltd. (Maidstone, Kent, England). Heparin agarose (type I), double-stranded (ds) DNA-cellulose, protein A-Sepharose, hydroxyurea, and 5′-bromo-2′-deoxyuridine (BrdU) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Mono-S HR 5/5 was acquired from Pharmacia LKB Biotechnology (Piscataway, N.J.). Hydroxylapatite, gel filtration standards, and immunoblotting reagents were obtained from Bio-Rad (Richmond, Calif.). Nitrocellulose was obtained from Schleicher & Schuell (Keene, N.H.). Bovine serum albumin was obtained from ICN Biomedicals, Inc. (Costa Mesa, Calif.). DNA modification enzymes were purchased from New England Biolabs, Inc. (Beverly, Mass.) or Roche Molecular Biochemicals (Indianapolis, Ind.) and were used according to the manufacturers' instructions. 32P-labeled deoxynucleoside triphosphates (dNTPs) and [35S]methionine were acquired from Dupont, New England Nuclear Corp. (Boston, Mass.). 14C-labeled high-molecular-weight protein markers were obtained from Gibco/BRL, Inc. (Gaithersburg, Md.). Oligonucleotide primers were synthesized using an Applied Biosystems (Foster City, Calif.) model 391 DNA synthesizer. E. coli SSB was generously provided by M. O'Donnell (Howard Hughes Medical Institute, Rockefeller University, New York, N.Y.).
Cells, virus, and DNA plasmids.
BSC40 monolayers were cultured in Dulbecco's modified Eagle meduim (DMEM) containing 5% fetal calf serum (Gibco/BRL) at 37°C in the presence of 5% CO2. HeLa cells were kindly provided by J. Hurwitz (Sloan Kettering Institute, New York, N.Y.); spinner cultures were grown in Joklik's modified essential medium supplemented with 2.5% horse serum and 2.5% calf serum (Gibco/BRL). The recombinant vaccinia virus vTF7.5, human TK− 143 cells, and the DNA plasmid pTM1 (8) were provided by B. Moss. Viral stocks were prepared by ultracentrifugation of cytoplasmic lysates through 36% sucrose. Virus was titrated on confluent monolayers of BSC40 cells; infected monolayers were fixed and stained with 0.1% crystal violet–3.7% formaldehyde.
Singly primed M13 DNA replication assay.
A primed template was constructed by annealing a 24-mer oligonucleotide primer (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′) to M13mp10 at a molar ratio of 20:1. Unless otherwise indicated, DNA polymerase was assayed in reaction mixtures (25 μl) that contained 10 mM Tris-HCl (pH 7.5), 40 mg of bovine serum albumin/ml, 4% glycerol, 0.1 mM EDTA, 5 mM dithiothreitol (DTT), 8 mM MgCl2, 25 fmol of singly primed M13mp10 single-stranded (ss) DNA, 750 ng of E. coli SSB (10 pmol of tetramer), 60 μM (each) dCTP, dGTP, and dATP, and 20 μM [32P]TTP (2,400 cpm/pmol). Reaction mixtures were preincubated with the enzyme and two of the four dNTPs (dCTP and dGTP) at 30°C for 3 min. Primer extension was initiated by addition of dATP and [32P]TTP, and incubation was continued at 30°C. To visualize primer extension products, reactions were quenched with an equal volume of 1% sodium dodecyl sulfate (SDS)–40 mM EDTA and fractionated on 0.8% agarose gels containing 0.125 μg of ethidium bromide/ml. Gels were cast and run in 1× Tris-borate-EDTA, dried, and subjected to autoradiography. Relative levels of RFII product were quantitated on a phosphorimager.
VPF purification. (i) Infected cell lysate.
HeLa cells grown to a density of 6 × 105/ml were collected by sedimentation, resuspended in medium lacking serum at a density of 1.2 × 107 cells/ml, and infected with wild-type (wt) vaccinia virus at a multiplicity of infection (MOI) of 15. Following 1 h of adsorption at 37°C, infected cells were diluted into their original medium and hydroxyurea was added to a final concentration of 10 mM. The infection was allowed to proceed at 37°C, and cells were harvested at 5 h postinfection (hpi). Cell pellets were stored at −80°C. To prepare cytoplasmic lysates, cell pellets prepared from 35 liters of infected cells were thawed slowly in 80 ml of hypotonic lysis buffer (10 mM Tris [pH 7.8], 10 mM KCl, 5 mM EDTA). A cocktail of protease inhibitors was added (1 mM phenylmethylsulfonyl fluoride, 4 μg of leupeptin/μl, and 0.7 μg of pepstatin/μl) prior to disruption of the cells by Dounce homogenization. Nuclei were removed by centrifugation at 2,000 × g for 10 min at 4°C. The cytoplasmic fraction (supernatant) was saved, and nuclei were resuspended in 80 ml of hypotonic buffer with protease inhibitors and subjected to an additional round of homogenization and centrifugation. Supernatants were pooled and adjusted to 50 mM Tris (pH 7.4)–1 mM EDTA–1 mM DTT–10% glycerol (buffer A) containing 50 mM NaCl. The lysate was clarified by centrifugation at 15,000 × g for 30 min at 4°C to generate fraction I.
(ii) Purification scheme.
To monitor the chromatographic behavior of the VPF, fractions were assayed for the ability to enable limiting amounts of purified DNA polymerase (approximately 37 fmol) to convert a singly primed M13 template (25 fmol) to the ds RFII form under conditions that demand processivity (19, 21). All purification steps were performed on a Pharmacia fast protein liquid chromatography system at 4°C unless otherwise indicated. Fraction I (614 ml) was applied to a 200-ml DEAE column which was equilibrated with buffer A containing 50 mM NaCl. Two column volumes of the starting buffer were added, and the flowthrough fraction was collected. The column was then developed by stepwise application of buffer A solutions containing 250 and then 500 mM NaCl. After reequilibration of the column, the original flowthrough fraction was reapplied to the 200-ml DEAE column and the same protocol was followed. VPF activity (see below) was detected within the pooled 250 mM NaCl eluate (fraction II; 294 ml). A cocktail of protease inhibitors (as described above) was added to all fractions which contained VPF (here and throughout the purification). Fraction II was applied to a 25-ml heparin agarose column that had been equilibrated with buffer A containing 250 mM NaCl; after a wash with 2 column volumes of the equilibration buffer, the column was developed with a 250-ml linear gradient of 250 mM to 1 M NaCl in buffer A. Fractions containing VPF, eluting at approximately 600 mM NaCl, were pooled (fraction III; 63 ml). The next chromatographic step was performed manually using a 7.5-ml-hydroxylapatite column equilibrated with buffer A containing 600 mM NaCl. Fraction III was applied, and the column was then developed stepwise according to the following protocol: 15 ml of 600 mM NaCl in buffer A; 15 ml of 10 mM NaPO4 in buffer B (10% glycerol, 1 mM DTT, 1 mM EDTA); 15 ml of 75 mM NaPO4 in buffer B; 22.5 ml of 150 mM NaPO4 in buffer B; and 15 ml of 285 mM NaPO4 in buffer B. VPF activity eluted with 150 mM NaPO4 (fraction IV; 23 ml). Fraction IV was diluted by addition of 11.25 ml of buffer B to bring the final salt concentration to 100 mM NaPO4, and then, using the fast protein liquid chromatography system, was applied to a 15-ml phosphocellulose column equilibrated with buffer B containing 100 mM NaPO4. The column was washed with the equilibration buffer and then developed with a 150-ml linear gradient of 100 to 300 mM NaPO4 in buffer B. VPF activity eluted with 250 mM NaPO4 (fraction V; 34 ml). Fraction V was dialyzed against buffer A containing 50 mM NaCl and applied to a 10-ml dsDNA-cellulose column equilibrated with the same buffer. The column was washed with the starting buffer and then developed with a 100-ml linear gradient of 50 mM to 1 M NaCl in buffer A. VPF activity eluted with 360 mM NaCl (fraction VI; 10 ml). Finally, fraction VI was diluted with buffer A to bring the final salt concentration to 100 mM NaCl before being applied to a 1-ml Mono-S column equilibrated with buffer A containing 100 mM NaCl. The column was washed with the equilibration buffer and then developed with a 10-ml linear gradient of 100 mM to 1 M NaCl in buffer A. The peak of VPF activity eluted at 580 mM NaCl (fraction VII); fractions within the peak were analyzed individually for activity and protein composition.
(iii) Sequence determination.
The individual fractions comprising fraction VII were resolved by SDS-polyacrylamide gel electrophoresis (PAGE); samples were analyzed by silver staining and, in parallel, transferred electrophoretically {10 mM 3-[(cyclohexylamino)-1-propane-sulfonic acid] [CAPS] in 10% methanol [pH 11.3]} to a polyvinylidene difluoride (PVDF) membrane. The membrane was stained with amido black, and the prominent band at 45 to 49 kDa was excised, as was a blank area of comparable size. These samples were sent to the Rockefeller University Protein Sequencing Facility for N-terminal sequence analysis or for microdigestion and internal sequence analysis.
Polyclonal antiserum preparation. (i) Construction of the PATHA20trpE fusion construct.
The A20 open reading frame (ORF) was amplified using vaccinia virus genomic DNA as a template. The upstream oligonucleotide primer (5′-GGTCTAGACATATGACTTCTAGCGCTGAT-3′) contained a novel XbaI site (underlined), and the downstream primer (5′-CCGGATTCTCACTCGAATAATCTTT-3′) included a BamHI site (underlined). The 1,300-bp PCR product was treated with the indicated restriction enzymes and cloned into the corresponding restriction sites within the pATH23 expression vector (16). This construction places the A20 ORF downstream of, and in frame with, the bacterial trpE ORF.
(ii) Overexpression of TrpE-A20 fusion.
The construct was propagated in E. coli, and expression was induced by tryptophan starvation and the addition of indoleacrylic acid (16). Lysates from induced bacteria were fractionated on SDS–8% acrylamide gels, and the fusion protein was visualized by soaking briefly in cold 0.3 M KCl. The band was excised and used to immunize rabbits following the collection of preimmune serum. The specificity of the serum was determined by immunoblotting and immunoprecipitation analyses.
Recombinant virus construction.
The plasmid pTM-A20 was constructed by subcloning the vaccinia virus A20 ORF into the expression vector pTM1 (8, 25). The A20 ORF was amplified from vaccinia virus genomic DNA using an upstream primer (5′-GCTCTAGATCATGACTTCTAGCGC-3′) containing a unique BspHI site (underlined) and a downstream primer (5′-CCGGATCCTCACTCGAATAATCTTT-3′) containing a BamHI site (underlined). The PCR product was digested with BspHI and BamHI, and the pTM1 vector was digested with NcoI and BamHI. BspHI digestion of the PCR product produces a 5′ overhang that is compatible with the cleaved NcoI site of pTM1, enabling the A20 ORF to be placed in an optimal position for expression from the T7 promoter and the encephalomyocarditis virus internal ribosomal entry site.
The pTM-A20 construct was used to generate the recombinant vaccinia virus vTMA20. Homologous recombination between the vaccinia virus thymidine kinase (TK) gene sequences that flank the A20 ORF in pTMA20 were utilized to insert the inducible copy of A20 into the nonessential viral TK gene. Confluent monolayers of BSC40 cells were infected with wt vaccinia virus at an MOI of 0.03. Following adsorption for 1 h, infected cultures were fed with DMEM containing 5% fetal calf serum. At 3 hpi, pTMA20 DNA that had previously been linearized with ScaI was introduced into the infected cells using standard calcium phosphate transfection procedures. Infections were allowed to proceed for 2 days. Cultures were then harvested, and TK− virus recombinants were isolated by two rounds of plaque purification on TK− 143 cells in the presence of 25 μg of BrdU/ml. Viral isolates that directed the expression of the 49-kDa A20 protein upon coinfection with vTF7.5 (a virus which constitutively expresses the T7 RNA polymerase) (14) were chosen for large-scale overexpression studies.
Overexpression of A20 and DNA polymerase using the vaccinia virus/T7 overexpression system.
Overexpression of either DNA polymerase alone, A20 alone, or both simultaneously was performed by coinfecting confluent monolayers of BSC40 cells with vTMpol (20) and/or vTMA20 (at an MOI of 2) and vTF7.5 (at an MOI of 2). Infections were initiated at 37°C but then shifted to 32°C at 4 hpi to maximize the proportion of the overexpressed protein that was soluble. The protocol used has been described previously (20); briefly, cells were harvested at 24 to 36 hpi, washed in isotonic buffer, swollen in hypotonic buffer (1 ml/107 cells), and subjected to 15 strokes in a tight-fitting Dounce homogenizer. Nuclei were removed by sedimentation, and the cytoplasmic lysate was clarified by centrifugation at 15,000 × g and stored at −80°C in the presence of 50% glycerol.
Immunoprecipitations.
To monitor the time course of expression of the A20 and DNA polymerase proteins, confluent monolayers of BSC40 cells were infected with wt virus at an MOI of 15. At the times indicated, cultures were rinsed with DMEM lacking methionine (DMEM−Met) and then labeled for 45 min in DMEM−Met containing [35S]Met at 100 μCi/ml. Cultures were rinsed with ice-cold phosphate-buffered saline and lysed by addition of 1 ml of 1× phospholysis buffer (0.1 M NaPO4 [pH 7.4], 0.1 M NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate) per 3 × 106 cells. Lysates were clarified by low- and high-speed sedimentation and then subjected to immunoprecipitation with polyclonal antisera directed against the A20 protein or the E9 DNA polymerase (18) and protein A-Sepharose. Immunoprecipitates were washed extensively, resolved by SDS-PAGE, and visualized by fluorography. To monitor the immunoprecipitation and coimmunoprecipitation of A20 and DNA polymerase when overexpressed, cells were infected with vTF7.5 and vTMpol and/or vTMA20 (each at an MOI of 2) at 37°C. Cells were metabolically labeled with [35S]methionine as described above from 6.5 to 8.5 hpi. Lysates were prepared and subjected to immunoprecipitation with either preimmune serum, anti-A20, or anti-DNA polymerase. Immunoprecipitates were resolved by SDS-PAGE and visualized by autoradiography.
Immunoblot assays.
Cell extracts were resolved by SDS-PAGE and transferred electrophoretically to nitrocellulose filters in CAPS buffer (as described above) (for Fig. 2) or Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol) (Fig. 4 and 5). Filters were incubated with polyclonal antisera directed against the DNA polymerase or the A20 protein and then with a horseradish peroxidase-conjugated secondary serum (goat anti-rabbit immunoglobulin G). Filters were then developed with colorimetric (Fig. 2 and 5) or chemiluminescent (Fig. 4) reagents.
FIG. 2.
Vaccinia virus A20 protein: comparison of poxvirus homologs and characterization of temporal profile of synthesis. (A) Alignment of poxvirus A20 homologs. Predicted amino acid sequences of the A20 homologs encoded by vaccinia virus (Copenhagen strain) (VV) (accession no. NP063806), Yaba monkey tumor virus (Yaba) (accession no. BAA88884), myxoma virus (Myx) (accession no. NP051825), molluscum contagiosum virus (MCV) (accession no. NP044077), and fowlpox virus (FPV) (accession no. NP039149) were retrieved from the public databases and aligned using the Clustal program and Lasergene software. Amino acids showing identity in ≥4 of the homologs are highlighted. (B) Temporal profile of A20 synthesis. BSC40 cells were infected with wt vaccinia virus (WR strain) at an MOI of 15. Cultures were radiolabeled with [35S]Met for 45 min prior to being harvested at the times shown. The lane marked C represents a culture that was treated with araC (20 μM) throughout the infection and was harvested at 3 hpi; the lane marked M represents an uninfected culture analyzed in parallel. Lysates were subjected to immunoprecipitation analysis using antisera directed against the DNA polymerase (POL) or the A20 protein. Immunoprecipitates were resolved by SDS-PAGE and visualized by fluorography; the relevant portions of the film are shown. The electrophoretic migration of the 96- and 43-kDa molecular size standards is shown on the left.
FIG. 4.
Overexpression of A20 and/or DNA polymerase (Pol) using the hybrid vaccinia virus/T7 system: coimmunoprecipitation of A20 and DNA polymerase. (A) Overexpression of A20 and/or DNA Pol. BSC40 cells were infected with vTF7.5 alone or in combination with vTMpol and/or vTMA20 (at an MOI of 2 for each virus used) and labeled with [35S]Met from 6.5 to 8.5 hpi. A portion of the total cellular lysate was resolved by SDS-PAGE and visualized by autoradiography. The arrow indicates the ↑A20 seen in lanes 2 and 3; the circle indicates the ↑DNA Pol seen in lanes 3 and 4. (B) A20 and DNA Pol coimmunoprecipitate. The samples shown in panel A, representing extracts with ↑A20 (lanes 1 to 3), ↑A20 plus ↑DNA Pol (lanes 4 to 6), or ↑DNA Pol (lanes 7 to 9) were subjected to immunoprecipitation analysis with preimmune (PI) (lanes 1, 4, and 7), anti-DNA Pol (lanes 2, 5, and 8), or anti-A20 (lanes 3, 6, and 9) serum. The immunoreactive proteins were resolved on the same SDS-PAGE gel shown in panel A and then visualized by autoradiography. The arrow indicates the A20 protein; the circle indicates DNA Pol. Clear coimmunoprecipitation of the DNA Pol is seen with the anti-A20 serum. Dashes between panels A and B indicate the positions to which the 97-, 68-, 43-, and 29-kDa 14C-labeled protein standards migrated (from top to bottom, respectively). (C) The anti-A20 and anti-DNA Pol sera do not cross-react. A portion of the extracts shown in panel A was also resolved by SDS-PAGE and transferred to nitrocellulose filters for immunoblot analysis. Duplicate filters were developed with anti-DNA Pol and anti-A20 sera, as described in Materials and Methods. The circle indicates the immunoreactive DNA Pol visible in lanes 3 and 4 of the left panel, which represent extracts with ↑DNA Pol. The arrow indicates the immunoreactive A20 protein seen in lanes 2 and 3 of the right panel, which represent extracts with ↑A20 protein. Dashes between the two panels show the positions to which the 101-, 70-, 44-, and 28-kDa prestained protein standards migrated (from top to bottom, respectively).
FIG. 5.
Overexpression of A20 and DNA polymerase (Pol) using the hybrid vaccinia virus/T7 system leads to an increase in processive polymerase activity. (A) Equalization of A20 and DNA Pol content in overexpressing extracts. BSC40 cells were infected with vTF7.5 alone or in combination with vTMpol and/or vTMA20 (at an MOI of 5 for each virus used) at 37°C; at 4 hpi, cells were shifted to 32°C and maintained under these conditions until 24 hpi. Cytoplasmic lysates were then prepared, and varying amounts were analyzed by immunoblot analysis, using both the anti-DNA Pol and anti-A20 sera. These titrations revealed that 5 μl of the ↑DNA Pol extract contained the same amount of DNA Pol as did 10 μl of the ↑DNA Pol-plus-↑A20 extract and that 10 μl of the ↑DNA Pol-plus-A20 extract contained the same amount of A20 as did 20 μl of the ↑A20 extract. The circle and arrow indicate the DNA Pol and A20 proteins, respectively. Molecular size standards (in kilodaltons) are shown on the left. (B) RFII formation is increased dramatically when both A20 and DNA Pol are overexpressed. (Top) Using the ratio of 1:2:4 obtained above, extracts with ↑DNA Pol (0.1 and 0.5 μl) (lanes 2 and 3), ↑DNA Pol plus ↑A20 (0.2 and 1.0 μl) (lanes 4 and 5), or ↑A20 (0.4 and 2.0 μl) (lanes 6 and 7) were assayed for the ability to convert a singly primed M13 template to the RFII product. An extract prepared from cells infected with vTF7.5, which resembles a wt infection except for the expression of the T7 RNA polymerase, was included as a baseline (2 μl) (lane 1). Replication products were resolved on a native agarose gel and visualized by autoradiography. The relative (Rel.) stoichiometries of DNA Pol and A20 in the assay reactions are shown above the autoradiograph. The arrowhead indicates the RFII product; the bracket shows the position of the short DNA products formed when the DNA polymerase acts distributively. (Bottom) Data shown in the autoradiograph were also quantitated using a phosphorimager, and the relative levels of RFII formed are shown in the bar graph, with each bar placed below its corresponding lane. Numbers represent arbitrary units.
Sequence analysis.
Sequences were retrieved from the public databases using the National Center for Biotechnology Information interface. Alignments and protein analyses were performed using Lasergene software (DNASTAR, Inc., Madison, Wis.).
RESULTS
Our previous studies had indicated that purified vaccinia virus DNA polymerase, which is intrinsically distributive when assayed in the presence of 8 mM MgCl2 or 40 mM NaCl, can be rendered highly processive by mixing with a polymerase-deficient extract prepared at early times after vaccinia virus infection (19). Moreover, glycerol gradient sedimentation analysis of partially purified VPF enabled us to demonstrate that the native molecular mass of VPF is approximately 48 kDa. These preliminary results prompted further investigation into the proteins that comprise the processive form of the viral DNA polymerase.
Purification of VPF through six chromatographic steps.
Because we knew that synthesis of VPF required only the early phase of viral gene expression, we prepared large quantities of extracts from cells infected with wt vaccinia virus in the presence of hydroxyurea, an inhibitor of the viral ribonucleotide reductase and hence of viral DNA replication and the intermediate and late phases of gene expression. In this way, expression of the late viral proteins, which are in general abundant, did not complicate the purification of VPF. As detailed in Materials and Methods, our assay for VPF was based on testing fractions for the ability to enable a limiting amount of purified DNA polymerase to convert a singly primed M13 template to the ds RFII form under conditions that required high processivity. After trial purifications using diverse chromatographic resins developed with a variety of protocols, the final purification scheme outlined in Table 1 was established. The purification scheme provided a dramatic enrichment of VPF: the specific activity of fraction VI was almost 3,000-fold greater than that of fraction I. We used this protocol twice to purify VPF from 35-liter batches of infected cells, and the chromatographic profile of VPF was highly reproducible. Data are shown for the second purification.
TABLE 1.
Purification of VPF
| Fractiona | Amt of proteinb (mg) | Total vol (ml) | Total activity (U) | Sp actc (U/mg) |
|---|---|---|---|---|
| I | 2,755.6 | 614 | 614 | 0.2 |
| II | 1,226.8 | 294 | 659 | 0.5 |
| III | 133.7 | 63 | 126d | 0.9 |
| IV | 22.8 | 23 | 450 | 19.8 |
| V | 2.7 | 34 | 231 | 86.4 |
| VI | 0.4 | 10 | 231 | 577.5 |
| VII | 2 | 46 |
Fractions I to VII represent the cytosolic lysate (I) and the peak fractions obtained after chromatography on DEAE-cellulose (II), heparin agarose (III), hydroxylapatite (IV), phosphocellulose (V), dsDNA-cellulose (VI), and Mono-S (VII) resins.
Determined by Bradford assay.
Refers to the levels of RFII generated in the standard singly primed M13 assay described in Materials and Methods. For fraction VII, there was insufficient material to determine the total protein concentration and therefore the specific activity.
When all of the peak fractions were assayed simultaneously, fraction III showed lower total activity than fraction IV. Whether this deficit represents an inaccurate data point or whether fraction III contains an inhibitory component removed in the subsequent purification step was not determined.
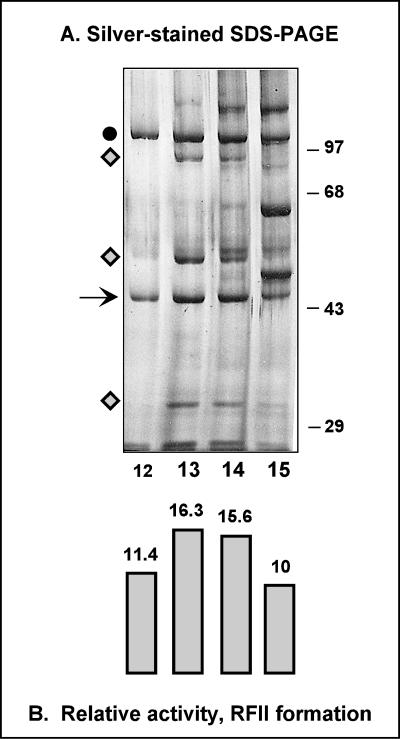
Application of fraction VI to a Mono-S column enabled a final purification which greatly diminished the number of proteins found in the VPF-containing fractions (Fig. 1). The elution profiles of two major proteins of approximately 110 and 45 kDa correlated well with the levels of VPF activity and were present in all four peak fractions (Fig. 1, lanes 12 to 15). A few other proteins (of approximately 90, 52, and 32 kDa) (Fig. 1, lanes 13 and 14) were also present in the center of the Mono-S peak. Because our earlier glycerol gradient analyses had revealed that the native molecular size of VPF was 48 kDa, the 45-kDa protein was selected for amino acid sequencing. Our initial submission was not successful because the N terminus was found to be blocked. After the second purification, the 45-kDa protein in fraction VII was subjected to proteolytic fragmentation and internal amino acid sequencing. The sequence FXILY(D/S)YDGN, which corresponds to residues 272 to 281 of the A20R gene of vaccinia virus, was obtained.
FIG. 1.
Analysis of fraction VII: identification of the putative VPF. (A) Analysis of the protein content of fraction VII. Peak fractions from the Mono-S column (30 μl of fractions 12 to 15) were analyzed by SDS-PAGE and silver staining. Fractions across the Mono-S column (1 μl) were also assayed for the ability to direct processive DNA synthesis using a singly primed M13 template, as described in Materials and Methods. The arrow points to the 45-kDa protein identified as the putative VPF. Fractions 13 (400 μl) and 14 (300 μl) were then applied to a preparative SDS-PAGE gel. The resolved proteins were transferred to a PVDF membrane, and the region of the filter containing the 45-kDa protein was excised and subjected to proteolytic degradation and amino acid sequence analysis. The circle at the left of the gel indicates an abundant 110-kDa protein that copurifies with the 45-kDa protein. Diamonds indicate three other proteins present in the central fractions containing peak VPF activity. The positions of molecular weight standards are shown at the right; molecular masses (in kilodaltons) are given. (B) Quantitation of the RFII-forming activity in the peak fractions of fraction VII. Levels of RFII synthesized were quantitated by phosphorimager analysis and are represented by vertical bars and numbers (arbitrary units [103]) beneath the corresponding gel lanes in panel A.
An A20 homolog is contained in all chordopoxvirus genomes.
The A20R gene is predicted to encode a protein of 426 amino acids with a calculated molecular weight of 49,186. The composition of the protein includes 35% hydrophobic residues (A, I, L, F, W, and V), 27% charged residues (K, R, D, and E), and 29% polar residues (N, C, Q, S, T, and Y). The predicted pI is 6.48. We examined the available chordopoxvirus genomes for A20 orthologs and found that the gene was present. The A20 homologs in vaccinia virus and variola major virus share ≥97% identity. Figure 2A shows an alignment of the predicted sequence of the vaccinia virus A20 protein with those encoded by more distantly related poxviruses: Yaba poxvirus, myxoma virus, molluscum contagiosum virus, and fowlpox virus. These homologs show 44, 38, 26, and 22% identity with the vaccinia virus protein, respectively. Because the identity is moderate, an examination of the profile of conservation may be informative. The N and C termini of the protein appear to exhibit the highest frequency of conserved residues. Moreover, aromatic and hydrophobic residues predominate among those conserved.
The A20 protein is expressed as an early protein of 49 kDa.
We purified the A20 protein from an extract of cells infected in the presence of hydroxyurea, suggesting strongly that A20 is expressed as an early protein. Expression at early times, prior to replication, would be expected for a replicative processivity factor. The regulatory region lying directly upstream of the A20 gene certainly does not resemble that of a late viral gene, but it is also somewhat divergent from the consensus early promoter gene (23, 24). The coding region of the A20 gene does include one early termination signal (T5NT) at nt 695 to 702, although other early genes have been shown to possess silent internal termination signals (18, 22).
To monitor the temporal profile of A20 synthesis more directly, we generated a polyclonal antiserum directed against a TrpE-A20 fusion protein. We used this serum to monitor A20 synthesis in cells metabolically labeled with [35S]methionine at different times postinfection. As shown in Fig. 2B, the serum recognizes a protein of 49 kDa that is expressed with typical early kinetics: the peak of protein synthesis is between 1.5 and 4.5 hpi, and synthesis is not affected by the inclusion of cytosine arabinoside (araC) (20 μM) in the culture medium. araC is an inhibitor of DNA replication and therefore blocks intermediate and late gene expression. The profile of DNA polymerase (E9) synthesis was determined in the same samples. The synthesis of the two proteins followed the same overall pattern, although the signal obtained for the DNA polymerase was more intense and the duration of polymerase synthesis appeared to be longer.
Immunoprecipitation of A20 from cultures labeled with 32PPi failed to yield any evidence that the A20 protein is phosphorylated during the infectious cycle (data not shown). Finally, our immunoblot analyses of purified virions indicated that the A20 protein is not encapsidated (data not shown).
The E9 DNA polymerase copurifies with A20R; fraction VII contains the processive DNA polymerase.
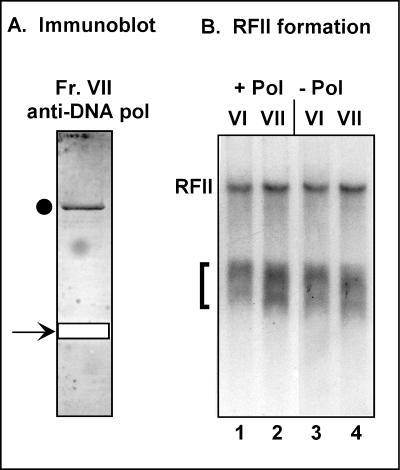
As mentioned above, our assay for VPF was based on its ability to confer processivity on purified DNA polymerase, which was included in all reactions. Therefore, our strategy did not rely on the ability to purify a holoenzyme comprising the catalytic polymerase bound to VPF. Nevertheless, we were struck by the appearance of a prominent 110-kDa protein in Fraction VII (Fig. 1A). The filter from which we excised the 45-kDa protein submitted for amino acid sequencing (subsequently shown to be A20) was utilized in an immunoblot assay using anti-polymerase antiserum. As shown in Fig. 3A, the 100-kDa protein in this fraction is indeed the catalytic DNA polymerase (E9 protein). Moreover, we reassayed the peak fractions from the purification of VPF and compared their abilities to convert ss M13 DNA to the ds RFII form in the absence as well as the presence of exogenous polymerase. As shown in Fig. 3B, both fractions VI and VII were competent to generate RFII in a processive manner in the absence of any added DNA polymerase. The demonstration that the catalytic DNA polymerase copurified with the A20 protein through six chromatographic steps is highly significant, since both the E9 and A20 proteins are present at low levels in these extracts relative to cellular proteins.
FIG. 3.
Fraction VII contains the E9 polymerase as well as the A20 protein and possesses processive polymerase activity. (A) Immunoblot analysis of fraction VII (Fr. VII) with anti-DNA polymerase (pol) serum. A portion of the PVDF membrane described above, representing a lane to which 150 μl of fraction 14 of fraction VII had been applied, was then subjected to immunoblot analysis with the anti-DNA pol serum. The circle indicates the immunoreactive polymerase protein; the arrow and open box indicate the region of the filter excised for amino acid sequence analysis of the 45-kDa protein. (B) Fraction VII contains processive polymerase activity. Equivalent amounts (2 μl) of fractions VI and VII were analyzed for processive polymerase activity using the SSB-coated singly primed M13 template and radiolabeled dNTPs. Reactions were performed in duplicate, in the presence and absence of exogenous purified DNA polymerase. Quantitation by phosphorimager analysis showed that equivalent levels of RFII were formed by these fractions in the presence and absence of exogenous polymerase (188,000 versus 271,000 arbitrary units for fraction VI and 263,000 versus 263,000 arbitrary units for fraction VII, respectively). Brackets indicate the short radiolabeled products formed by the polymerase acting in a distributive mode of synthesis.
Generation of vTMA20: overexpression of A20 within vaccinia virus-infected cells.
We wished to prepare purified recombinant A20 protein to facilitate an in-depth biochemical analysis of the VPF. However, our attempts to express and purify recombinant A20 in E. coli and in baculovirus-infected insect cells were frustrated by the insolubility of most preparations and the apparent inaccessibility of N-terminal polyhistidine tags. Therefore, we generated the vTMA20 virus in order to overexpress the A20 protein in mammalian cells using the hybrid vaccinia virus/T7 overexpression system (8, 14, 20). In vTMA20, a second copy of the A20 gene has been inserted into the nonessential TK locus under the regulation of the bacteriophage T7 promoter and the encephalomyocarditis virus internal ribosomal entry site sequence. We utilized this system to overexpress A20 individually or in combination with the DNA polymerase (Fig. 4A). In general, we can obtain approximately 20-fold overexpression of these proteins. Although much of the overexpressed protein is insoluble, the protocol described previously (20) and in Materials and Methods allowed us to optimize the fraction of overexpressed protein that was indeed soluble. We estimate that the level of A20 in these soluble extracts is approximately 5 times greater than that obtained from wt-infected cells.
A20 and DNA polymerase can be coprecipitated with anti-A20 serum.
Because of the copurification of A20 and DNA polymerase through many chromatographic steps, and because it is likely that together they form a holoenzyme, it seemed reasonable to propose that there was a physical interaction between the two proteins. This physical interaction was probed by coimmunoprecipitation studies. Cells were infected with vTF7.5 alone or in combination with vTMA20, vTMpol, or both (each at an MOI of 2). Cells were radiolabeled with [35S]methionine from 6.5 to 8.5 hpi and then harvested and processed for immunoprecipitation analysis. Using this protocol, radiolabeling of the endogenous A20 and polymerase proteins is not significant, because they are early proteins whose synthesis declines after approximately 4.5 hpi. However, the overexpressed proteins synthesized under the regulation of the T7 promoter are strongly radiolabeled.
Precipitations were performed with preimmune rabbit serum (from the rabbit in which the anti-A20 serum was generated), the polyclonal anti-A20 serum, or the polyclonal anti-DNA polymerase serum (Fig. 4B). The preimmune serum did not precipitate any radiolabeled proteins from these extracts (Fig. 4B, lanes 1, 4, and 7). When the anti-DNA polymerase serum was used, a significant amount of the 116-kDa polymerase protein was seen whenever the extract contained overexpressed DNA polymerase (↑DNA Pol) (Fig. 4B, lanes 5 and 8); low levels of the endogenous polymerase were retrieved when only the A20 protein was being overexpressed (lane 2). When the anti-A20 serum was used, a significant amount of the 48-kDa A20 protein was retrieved from extracts in which A20 was overexpressed (Fig. 4B, lanes 3 and 6). Notably, a significant amount of the DNA polymerase protein was also retrieved from those extracts in which the polymerase was overexpressed (Fig. 4B, lanes 6 and 9). This result has been reproduced many times; indeed, in most experiments, the level of DNA polymerase coprecipitated by the anti-A20 serum was equivalent to that retrieved with the anti-DNA polymerase serum. Thus, it is clear that A20 and DNA polymerase coprecipitate when the DNA polymerase is overexpressed. Coprecipitation of ↑DNA Pol in the absence of A20 overexpression (Fig. 4B, lane 9) suggests that the overexpressed polymerase interacts with endogenous A20, which is likely to be present in excess relative to the endogenous DNA polymerase. The anti-DNA polymerase serum is less effective at coprecipitating the A20 protein, but low levels of the A20 protein were retrieved from those extracts in which both proteins were overexpressed (Fig. 4B, lane 5).
To verify that the coprecipitation of the DNA polymerase by the anti-A20 serum was due to the interaction of A20 and DNA polymerase, and not to cross-reactivity in the sera, a portion of the extracts shown in Fig. 4A was also resolved by SDS-PAGE and transferred to nitrocellulose filters for immunoblot analysis (Fig. 4C). Duplicate filters were incubated with anti-DNA polymerase and anti-A20 sera and then developed as described in Materials and Methods. The immunoreactive DNA polymerase is visible in lanes 3 and 4 of the left panel of Fig. 4C, which represent extracts in which the DNA polymerase is overexpressed. The immunoreactive A20 protein is seen in lanes 2 and 3 of the right panel of Fig. 4C, which represent extracts in which the A20 protein is overexpressed. Because of the large amounts of A20 in the overexpressed cells, and because we analyzed a significant amount of protein in order to reveal cross-reactivity if there was any, several species of immunoreactive A20 are seen. The data clearly show that there is no cross-reactivity: the anti-DNA polymerase serum does not recognize the A20 protein, and the anti-A20 serum does not recognize the DNA polymerase.
Overexpression of A20 and DNA polymerase leads to a corresponding increase in processive polymerase activity.
The data presented above describe the purification of A20 as a component of the processive form of the DNA polymerase and confirm that A20 and DNA polymerase interact physically. We would predict, therefore, that overexpression of polymerase and A20 would lead to a corresponding increase in the level of processive polymerase activity. Soluble extracts prepared from infected cells overexpressing A20 and/or DNA polymerase were thus prepared and assayed for the ability to convert singly primed M13 to the RFII form in a processive manner. First, various amounts of each extract were subjected to immunoblot analysis with anti-DNA polymerase and anti-A20 sera in order to define the relative amounts of the two proteins in each extract. This titration allowed us to choose appropriate amounts of each extract for further analysis. Figure 5A presents an immunoblot in which we have verified this stoichiometry: comparable amounts of DNA polymerase are present in the ↑DNA Pol and ↑DNA Pol ↑A20 extracts, and comparable amounts of A20 are present in the ↑A20 and ↑DNA Pol ↑ A20 extracts. We then assayed these extracts for the ability to generate RFII in a processive manner. The autoradiograph is shown in Fig. 5B; a quantitation of these data is given in the bar graph at the bottom. With this exposure, RFII formation by cells infected with the parental vTF7.5 virus is almost undetectable (Fig. 5B, lane 1). Overexpression of either DNA polymerase or A20 leads to an increase in RFII formation (Fig. 5B, lanes 2 to 5), and overexpression of DNA polymerase but not A20 leads to a significant increase in the levels of small radiolabeled products formed by distributive synthesis on the primed template (Fig. 5B, lanes 2 and 3). Simultaneous overexpression of both A20 and DNA polymerase leads to a substantial increase in RFII-forming activity: this extract has 6- and 6.7-fold more activity than those prepared from cells overexpressing DNA polymerase or A20 alone, respectively. These data provide compelling evidence that both A20 and DNA polymerase contribute to processive polymerase activity within infected cells.
DISCUSSION
In this report, we address the identity of VPF, the protein that can confer processivity on the intrinsically distributive viral DNA polymerase. The purification of early viral proteins from cytoplasmic extracts of infected cells is not simple, since early proteins are only synthesized transiently from approximately 1.5 to 4.5 hpi and only the genomes introduced in the infecting virions serve as templates for early transcription. Therefore, 35 liters of cells and six chromatographic steps were required to prepare a highly enriched preparation that contained <5 major polypeptides. The elution profile of two of these proteins, one of 110 kDa and one of 45 kDa, corresponded nearly perfectly to the elution profile of the processive polymerase activity. Our previous studies had shown that VPF has a native molecular mass of 48 kDa, and thus we focused our attention on the 45-kDa protein present within fraction VII. Amino acid sequencing of internal peptides generated from the 45-kDa protein indicated that it was the product of the A20R gene. At the time that we obtained these results, there were no reports concerning this protein in the literature. Our immunoblot assays indicated that the 110-kDa protein in the peak fractions was indeed the catalytic component of the DNA polymerase, the product of the E9 gene.
It was intriguing that the E9 polymerase copurified with the A20 protein, since the assay we employed during purification did not demand the purification of a processive holoenzyme but asked only for a VPF activity that could confer processivity on recombinant polymerase, which was added to all reaction mixtures. Several explanations for the copurification of A20 and DNA polymerase seem most likely. First, the two proteins may be found tightly associated as a heterodimer, with little or no monomeric A20 present within cytoplasmic extracts. Alternatively, A20 and DNA polymerase may associate only cotranslationally, such that free A20 was unable to associate with the recombinant DNA polymerase present in the reaction mixtures. This seems unlikely, however, since we have previously shown that processive polymerase activity can be reconstituted by adding purified E9 protein to an E9-deficient extract (19). Nevertheless, in the latter experiment, the association of the exogenous DNA polymerase with A20 might have been enhanced by one or more additional viral proteins present in the extract.
Our data implicate the A20 protein as a major component of the processive form of the vaccinia virus DNA polymerase. Fraction VII contains very few proteins other than the A20 and polymerase proteins, and the first of the Mono-S fractions containing high levels of activity (see Fig. 1, lane 12) does not appear to contain any other proteins. The A20 and E9 proteins can be shown to interact physically, and overexpression of the two leads to a significant increase in the levels of processive polymerase activity. Confirmation that A20 is VPF, however, awaits the reconstitution of processive polymerase from the two individual purified components. This has not been possible to date, because we have been unable to express sufficient levels of soluble recombinant A20. Although A20 and DNA polymerase can be synthesized in coupled in vitro transcription-translation reactions, we have been unable to demonstrate their physical association in vitro. The protein concentrations of A20 and E9 may not be sufficient in these reactions to drive heterodimerization (5), or it may be that optimal association of A20 and DNA polymerase requires another viral protein.
Unfortunately, we were unable to identify the other proteins that were present in fractions 13 and 14 of fraction VII, since we had to use almost all of the small amount of material recovered for determination of the sequence of A20. In work published after this study was completed, the laboratories of B. Moss and S. Fields showed that the A20 protein interacted with the H5 protein (25 kDa; migrating with an electrophoretic mobility of 35 kDa), the D4 uracil DNA glycosylase (20 kDa), and the D5 DNA-independent ATPase (90 kDa) in the yeast two-hybrid system (17). These data are provocative, since genetic studies have shown that both the D4 and D5 proteins play essential roles in viral DNA replication (7, 11–13, 29). Further study of the interaction of A20 and/or DNA polymerase with these proteins is warranted. It is not surprising that the DNA polymerase was not identified as an A20-interacting protein in the two-hybrid analysis, since we have shown that the DNA polymerase gene contains an element that resembles and functions as a yeast transcriptional terminator (W. F. McDonald and P. Traktman, unpublished data).
The identification of A20 as a stoichiometric component of the processive form of the viral DNA polymerase opens new opportunities for dissecting how this complex and autonomous virus directs the rapid and faithful replication of its large DNA genome. To date, the herpesvirus UL42 protein and the cellular sliding clamp have been the only two processivity proteins available for study in mammalian cells. It is clear that vaccinia virus will achieve processivity in a manner distinct from the cellular model, in which a large ATP-hydrolyzing clamp loader opens the clamp ring and loads it onto the DNA, where it is then topologically tethered and so anchors the polymerase molecule (26, 27, 30). These sliding-clamp processivity factors are highly acidic, a property which discourages strong interactions with the DNA they enclose and enables them to slide. The herpesvirus model is more relevant to the vaccinia virus system, since the polymerase associates with a single processivity factor. The availability of the crystal structure of UL42 bound to a peptide derived from the DNA polymerase, in concert with intensive biochemical and genetic analysis of both proteins, has led to a detailed model for processivity (32, 33). UL42 is largely a neutral molecule, except for a basic region that interacts with DNA by virtue of electrostatic attraction. UL42 is thus kept close to the DNA but remains free to slide along it in either direction. By associating with the DNA polymerase, whose catalytic cycle provides an inherent directionality, the polymerase/UL42 complex translocates along the template with the appropriate polarity.
Both biochemical and genetic studies will be required to analyze the structure and function of the vaccinia virus A20 protein. It will be of significant interest to probe the likely interactions of A20 with both DNA and the E9 polymerase and to determine how A20 contributes to polymerase processivity. The predicted amino acid sequence of A20 shows no homology to either the PCNA or UL42 family of processivity factors, and its predicted pI of 6.48 does not conform to the diagnostic pI's of <4.8 or >8 observed for these two families of proteins, respectively. The available collections of vaccinia virus temperature-sensitive mutants have been invaluable to a broad array of investigators pursuing structure and function analyses; however, no mutants with lesions in the A20 gene are present in these collections (2–4, 9,10). The accompanying manuscript describes our successful efforts to construct such mutants using clustered charge-to-alanine mutagenesis and the utility of these mutants in confirming an essential role for A20 in vaccinia virus replication in vivo (28).
ACKNOWLEDGMENTS
This work was supported by a grant to P.T. (AI 21758) from the National Institutes of Health; N.K. was supported by a fellowship program sponsored by the Norman and Rosita Winston Foundation, and K.B. is supported by an NRSA from the Public Health Service (AI 10428).
We thank R. Tether and B. Tchizhed for technical assistance and members of the O'Donnell laboratory for generously providing reagents and for helpful discussions. We also thank Mira Punjabi for help with antiserum development and for sharing her expertise regarding the coimmunoprecipitation assays.
REFERENCES
- 1.Challberg M. Herpesvirus DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 721–750. [Google Scholar]
- 2.Condit R C, Motyczka A. Isolation and preliminary characterization of temperature sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- 3.Condit R C, Motyczka A, Spizz G. Isolation, characterization and physical mapping of temperature sensitive mutants of vaccinia virus. Virology. 1983;128:429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- 4.Condit R C, Niles E G. Orthopoxvirus genetics. Curr Top Microbiol Immunol. 1990;163:1–39. doi: 10.1007/978-3-642-75605-4_1. [DOI] [PubMed] [Google Scholar]
- 5.Digard P, Bebrin W R, Coen D M. Mutational analysis of DNA polymerase substrate recognition and subunit interactions using herpes simplex virus as prototype. Methods Enzymol. 1995;262:303–322. doi: 10.1016/0076-6879(95)62026-5. [DOI] [PubMed] [Google Scholar]
- 6.Earl P L, Jones E V, Moss B. Homology between DNA polymerase of poxviruses, herpesviruses and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci USA. 1986;83:3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison K S, Peng W, McFadden G. Mutations in active-site residues of the uracil-DNA glycosylase encoded by vaccinia virus are incompatible with virus viability. J Virol. 1996;70:7965–7973. doi: 10.1128/jvi.70.11.7965-7973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elroy-Stein O, Fuerst T R, Moss B. Cap-independent translation of mRNA conferred by EMC virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci USA. 1989;86:6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensinger M. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus. J Virol. 1982;43:778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensinger M, Rovinsky M. Marker rescue of temperature sensitive mutations of vaccinia virus WR: correlation of genetic and physical maps. J Virol. 1983;48:419–428. doi: 10.1128/jvi.48.2.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans E, Klemperer N, Ghosh R, Traktman P. The vaccinia virus D5 protein, which is required for DNA replication, is a nucleic acid-independent nucleoside triphosphatase. J Virol. 1995;69:5353–5361. doi: 10.1128/jvi.69.9.5353-5361.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans E, Traktman P. Molecular genetic analysis of a vaccinia virus gene with an essential role in DNA replication. J Virol. 1987;61:3152–3162. doi: 10.1128/jvi.61.10.3152-3162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans E, Traktman P. Characterization of vaccinia virus DNA replication mutants with lesions in the D5 gene. Chromosoma. 1992;102:S72–S82. doi: 10.1007/BF02451789. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst T R, Fernandez M P, Moss B. Transfer of the inducible lac repressor/operator system from Escherichia colito a vaccinia virus expression vector. Proc Natl Acad Sci USA. 1989;86:2549–2552. doi: 10.1073/pnas.86.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb J, Marcy A I, Coen D M, Challberg M D. The herpes simplex virus type I UL42 gene product: a subunit of DNA polymerase that functions to increase productivity. J Virol. 1990;64:5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koerner T J, Hill J E, Myers A M, Tzagaloff A. High-expression vectors with multiple cloning sites for construction of trpEfusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- 17.McCraith S, Holtzman T, Moss B, Fields S. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:4879–4884. doi: 10.1073/pnas.080078197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald W F, Crozel-Goudot V, Traktman P. Transient expression of the vaccinia virus DNA polymerase is an intrinsic feature of the early phase of infection and is unlinked to DNA replication and late gene expression. J Virol. 1992;66:534–547. doi: 10.1128/jvi.66.1.534-547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald W F, Klemperer N, Traktman P. Characterization of a processive form of the vaccinia virus DNA polymerase. Virology. 1997;234:168–175. doi: 10.1006/viro.1997.8639. [DOI] [PubMed] [Google Scholar]
- 20.McDonald W F, Traktman P. Overexpression and purification of the vaccinia virus DNA polymerase. Protein Expr Purif. 1994;5:409–421. doi: 10.1006/prep.1994.1059. [DOI] [PubMed] [Google Scholar]
- 21.McDonald W F, Traktman P. Vaccinia virus DNA polymerase: in vitro analysis of parameters affecting processivity. J Biol Chem. 1994;269:31190–31197. [PubMed] [Google Scholar]
- 22.Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- 23.Moss B. Vaccinia virus transcription. In: Conaway R C, Conaway J W, editors. Transcription: mechanisms and regulation. New York, N.Y: Raven Press Ltd.; 1994. pp. 185–206. [Google Scholar]
- 24.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2637–2671. [Google Scholar]
- 25.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell M, Kuriyan J, Kong X-P, Stukenberg P T, Onrust R. The sliding clamp of DNA polymerase III holoenzyme encircles DNA. Mol Biol Cell. 1992;3:953–957. doi: 10.1091/mbc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell M, Onrust R, Dean F B, Chen M, Hurwitz J. Homology in accessory proteins of replicative polymerases—E. colito humans. Nucleic Acids Res. 1993;21:1–3. doi: 10.1093/nar/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punjabi A, Boyle K, DeMasi J, Grubisha O, Unger B, Khanna M, Traktman P. Clustered charge-to-alanine mutagenesis of the vaccinia virus A20 gene: temperature-sensitive mutants have a DNA-negative phenotype and are defective in the production of processive DNA polymerase activity. J Virol. 2001;75:12308–12318. doi: 10.1128/JVI.75.24.12308-12318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart D T, Upton C, Higman M A, Niles E G, McFadden G. A poxvirus-encoded uracil DNA glycosylase is essential for virus viability. J Virol. 1993;67:2503–2512. doi: 10.1128/jvi.67.5.2503-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stukenberg P T, Studwell-Vaughan P S, O'Donnell M. Mechanisms of the sliding beta-clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991;266:11328–11334. [PubMed] [Google Scholar]
- 31.Traktman P, Sridhar P, Condit R C, Roberts B E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984;49:125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisshart K, Chow C S, Coen D M. Herpes simplex virus processivity factor UL42 imparts increased DNA-binding specificity to the viral DNA polymerase and decreased dissociation from primer-template without reducing the elongation rate. J Virol. 1999;73:55–66. doi: 10.1128/jvi.73.1.55-66.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuccola H J, Filman D J, Coen D M, Hogle J M. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C terminus of its cognate polymerase. Mol Cell. 2000;5:267–278. doi: 10.1016/s1097-2765(00)80422-0. [DOI] [PubMed] [Google Scholar]