Abstract
Purpose
Radiation therapy (RT) plays a key role in the management of esophageal cancer (EC). However, toxicities caused by proximity of organs at risk (OAR) and daily target coverage caused by interfractional anatomic changes are of concern. Daily online adaptive RT (oART) addresses these concerns and has the potential to increase OAR sparing and improve target coverage. We present the first clinical experience and dosimetric investigations of cone beam CT-based oART in EC using the ETHOS platform.
Methods and Materials
Treatment fractions of the first 10 EC patients undergoing cone beam CT-based oART at our institution were retrospectively analyzed. The prescription dose was 50.4 Gy in 28 fractions. The same clinical target volume (CTV) and planning target volume (PTV) margins as for nonadaptive treatments were used. For all sessions, the timestamp of each oART workflow step, PTV size, target volume doses, mean heart dose, and lung V20Gy of both the scheduled and the adapted treatment plan were analyzed.
Results
Following automatic propagation, the CTV was adapted by the physician in 164 (59%) fractions. The adapted treatment plan was selected in 276 (99%) sessions. The median time needed for an oART session was 28 minutes (range, 14.8-43.3). Compared to the scheduled plans, a significant relative reduction of 9.5% in mean heart dose (absolute, 1.6 Gy; P = .006) and 16.9% reduction in mean lung V20Gy (absolute, 2.3%; P < .001) was achieved with the adapted treatment plans. Simultaneously, we observed a significant relative improvement in D99%PTV and D99%CTV by 15.3% (P < .001) and 5.0% (P = .008), respectively, along with a significant increase in D95%PTV by 5.1% (P = .003).
Conclusions
Although being resource-intensive, oART for EC is feasible in a reasonable timeframe and results in increased OAR sparing and improved target coverage, even without a reduction of margins. Further studies are planned to evaluate the potential clinical benefits.
Introduction
Esophageal cancer (EC) is the seventh most common cancer and the sixth leading cause of cancer-related mortality worldwide.1 Treatment of EC typically includes preoperative radiochemotherapy (RCHT) or perioperative chemotherapy and surgery in a neoadjuvant setting, as well as definitive RCHT for nonoperable cases. Despite improvement in oncological outcomes using multimodality treatment compared to surgery alone, the 5-year survival rate remains poor.2 Although the mortality rate in patients with EC is primarily cancer-related, cardiovascular, and respiratory causes of death become increasingly significant over time, particularly 5 years after diagnosis.3 Given the large clinical target volumes (CTVs) required in radiation therapy (RT) for EC, improving dose delivery to target volumes and better sparing of organs at risk (OAR) could potentially reduce treatment-related toxicity and improve oncological outcomes in EC. Several studies have demonstrated considerable esophageal motion because of respiration, peristalsis, and varying stomach and bowel filling.4, 5, 6, 7 Additionally, substantial interfractional tumor size reduction during RCHT has been described, which can subsequently cause OARs to shift into the high-dose region.8,9 Reducing radiation dose to OARs is achievable to some extent using modern RT techniques such as intensity modulated RT (IMRT)10 or proton therapy (PT).11 However, neither IMRT nor PT accounts for changes in daily anatomy. Online adaptive radiation therapy (oART) allows for daily optimization of the RT plan, potentially reducing the dose to OARs while achieving better coverage for target volumes. Boekhoff et al12 reported an in-silico assessment of the dosimetric benefits of MR-guided RT (MRgRT) for EC. Their findings suggest that MRgRT has the potential to reduce the dose to the heart and lungs. However, there is currently no data in the literature on cone beam CT (CBCT)-based oART for EC. This study aims to investigate the feasibility of CBCT-based oART for patients with EC using the ETHOS machine (Varian). Additionally, we present dosimetric analyses comparing adapted and nonadapted treatment plans for the first 10 EC patients treated with CBCT-based oART.
Materials and Methods
Patients and treatment
RT sessions of the first 10 patients with EC treated at our institution with CBCT-based oART between January 2022 and December 2022 were analyzed. RT was delivered concomitantly with chemotherapy either as a definitive treatment or in the neoadjuvant setting. Planning CT (PL-CT) with a 3 mm slice thickness was performed 7 to 10 days before the start of oART in free breathing and without contrast medium. Patients were instructed to fast for at least 4 hours before both the PL-CT and the treatment sessions to minimize stomach variations. Positron emission tomography (PET)-CT images were registered to the PL-CT and target delineation was performed according to expert consensus contouring guidelines for IMRT in EC.13 Diagnostic CT images, PET-CT, and gastroscopy findings were used to delineate the gross primary tumor volume (GTV_PT) and gross lymph node disease (GTV_LN). The CTV was defined as an expansion of GTV_PT superiorly by 3 cm, radially by 1 cm in all directions (with a maximum of 0.5 cm into the heart and liver), and inferiorly to encompass the lesser gastric curvature and coeliac lymph nodes. The planning target volume (PTV) was generated as a derived structure by expanding the CTV 0.5 cm in all directions. As we planned to test the feasibility of oART for EC, the same margin concept as in regular nonadaptive treatment plans for EC was applied. We adhered to the aforementioned contouring guidelines in the reference situation and every oART session, which means that no margin reduction was employed for the CTV or PTV (see patient examples in Fig. 1A, B). Treatments were delivered to a prescription dose of 50.4 Gy in 28 fractions using either an IMRT plan (12 fields) or a volumetric intensity modulated arc therapy (VMAT, 3 full arcs) plan. RT plans were normalized such that 100% of the prescribed dose covered 50% of the PTV. All patients gave informed consent prior to initiation of treatment. A waiver from the competent ethics committee was obtained for this study (Kantonale Ethikkommission Bern).
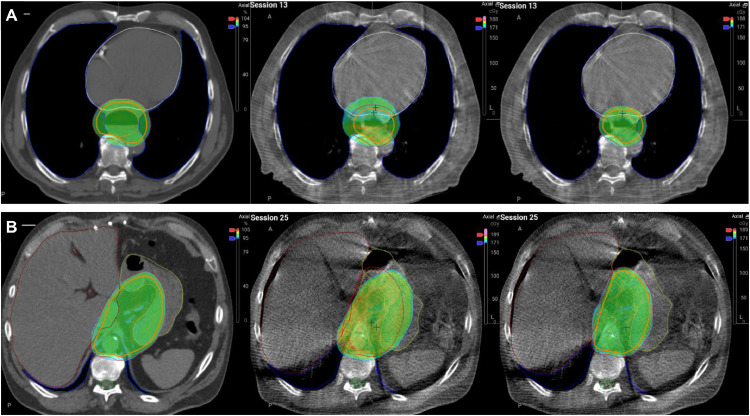
Figure 1.
Mediastinal and abdominal patient examples showing in color wash the distribution of ≥95% of the prescribed dose in the reference situation (left), scheduled plan (middle), and adapted plan (right) under treatment. In the mediastinal example (panel A), reduced esophageal dilatation under treatment (session 13) results in smaller target volumes and therefore less dose to organs at risk in the adapted plan compared to the scheduled plan. Mean heart dose was 17.4, 20.2, and 17.4 Gy, and mean lung V20Gy was 16.8%, 17.3%, and 12.6% for the reference, scheduled, and adapted plan, respectively. In the abdominal example (panel B, session 25), changes in stomach anatomy under treatment lead to suboptimal coverage of target volumes with the scheduled plan. D99%PTV was 96.1%, 78.6%, and 95.5%, and D95%PTV was 98.3%, 94.2%, and 98.1% for the reference, scheduled, and adapted plans, respectively. Note that, according to contouring guidelines, the clinical target volume (CTV) (orange outline) extends up to 1 cm into the lungs (blue), and 0.5 cm into the heart (white) and liver (brown) in both the reference and adapted situations.
Online adaptive workflow
Initially, we planned 40-minute time slots for oART on the ETHOS machine, which could be reduced to 30 minutes over time. The online adaptive workflow was as follows.
-
(1)
CBCT acquisition: First, a CBCT of the treatment area was performed by radiation therapists (RTTs). We used the thorax-fast protocol, which resulted in fewer artifacts and improved image quality. After the CBCT acquisition, the responsible MD was called to the ETHOS machine by the RTTs.
-
(2)
Influencer review: The contours of certain OARs, the so-called "influencers,” were propagated from the PL-CT onto the daily CBCT. For EC the influencers include the heart, both lungs, the esophagus, and, depending on the tumor's location, also the stomach (lower EC) or trachea (middle/upper EC). RTTs began reviewing and correcting the influencer structures. MDs then reviewed the influencer contours in each session. These influencer structures assist the AI-based system in shaping and positioning the daily target volumes appropriately. At the end of this step, the responsible medical physicist (MP) was called to the treatment machine.
-
(3)
Target volumes review: Next, the ETHOS system propagated the target volumes from the reference PL-CT onto the daily CBCT. If necessary, MDs reviewed and adjusted the target structures. Finally, the PTV was automatically derived from the CTV.
-
(4)
Plan selection and online quality assurance (QA): For dose calculation, a synthetic CT was generated in the background, combining the Hounsfield Units from the PL-CT and anatomy from the daily CBCT. Based on OARs and target volume contours on the CBCT, 2 RT plans were generated: a “scheduled” and an “adapted” treatment plan. The scheduled plan is a recalculation of the reference RT plan on the synthetic CT, while the adapted plan is a new optimization of the reference plan on daily anatomy with initial constraints. These 2 plans were then compared and validated by MDs and MPs. For the adapted plan, a daily independent dose calculation was conducted for QA during each session using Mobius3D version 4.0.2 (Varian).
Data collection
A logbook was maintained for all patients to document every oART session. This included influencer and target volume adaptations, treatment plan selection (scheduled vs adapted), and dosimetric parameters. For each treatment session, both the scheduled and the adapted treatment plans were evaluated by comparing the following parameters: mean dose to the heart, V20Gy for the lungs, minimal dose (D99%), maximal dose (D1%), and dose coverage (D95%) for the target volumes CTV and PTV. Additionally, the PTV size for each session was captured to assess the changes throughout the treatment. Furthermore, the timestamp of all workflow steps, including the first CBCT acquisition, target volume adaptation, plan review, acquisition of the second CBCT, and session closure were recorded to assess the time needed for each specific workflow step. The timestamp of the first CBCT was marked as the beginning and session closure as the end of every oART session. Treatment delivery time was defined as the time needed from the second CBCT to session closure.
Statistical analysis
Dosimetric parameter differences between the scheduled and adapted treatment plans were analyzed using a paired t test in SPSS version 28.0.1.1 (IBM). A P value <.05 was considered statistically significant. Box plots and linear regression analyses were employed to examine daily PTV size changes.
Results
Table 1 summarizes patient and tumor characteristics. A total of 3 female and 7 male patients with a median age of 72 years were analyzed in this study. The majority (n = 8, 80%) of tumors were adenocarcinomas (ACC) of the lower esophagus or gastroesophageal junction (GEJ), and all tumors were locally advanced (either T3 or N+). Two patients had a T3 squamous cell carcinoma of the middle esophagus. RCHT was administered in a neoadjuvant setting in 70% and with definitive intent in 30% of the cases. Radiation was delivered with a 12-beam IMRT plan in 9 (90%) patients and with a VMAT plan in 1 (10%) patient.
Table 1.
Patient characteristics
| Characteristics | All patients (n = 10) |
|---|---|
| Age, y | 72 (35-85) |
| Sex | |
| Female | 3 (30) |
| Male | 7 (70) |
| Histology | |
| Adenocarcinoma | 8 (80) |
| Squamous cell carcinoma | 2 (20) |
| Tumor localization | |
| Middle esophagus | 2 (20) |
| Lower esophagus or GEJ | 8 (80) |
| T-Stage | |
| T2 | 2 (20) |
| T3 | 8 (80) |
| N-Stage | |
| N0 | 3 (30) |
| N1 | 6 (60) |
| N2 | 1 (10) |
| M-Stage | |
| M0 | 10 (100) |
| Treatment concept | |
| Definitive RCHT | 3 (30) |
| Neoadjuvant RCHT | 7 (70) |
| Radiation dose | |
| 28 × 1.8 Gy = 50.4 Gy | 10 (100) |
| Treatment technique | |
| VMAT | 1 (10) |
| IMRT | 9 (90) |
Values represent numbers (percent) or median (range).
Abbreviations: GEJ = gastroesophageal junction; IMRT = intensity modulated radiation therapy; RCHT = radiochemotherapy; VMAT = volumetric intensity modulated arc therapy.
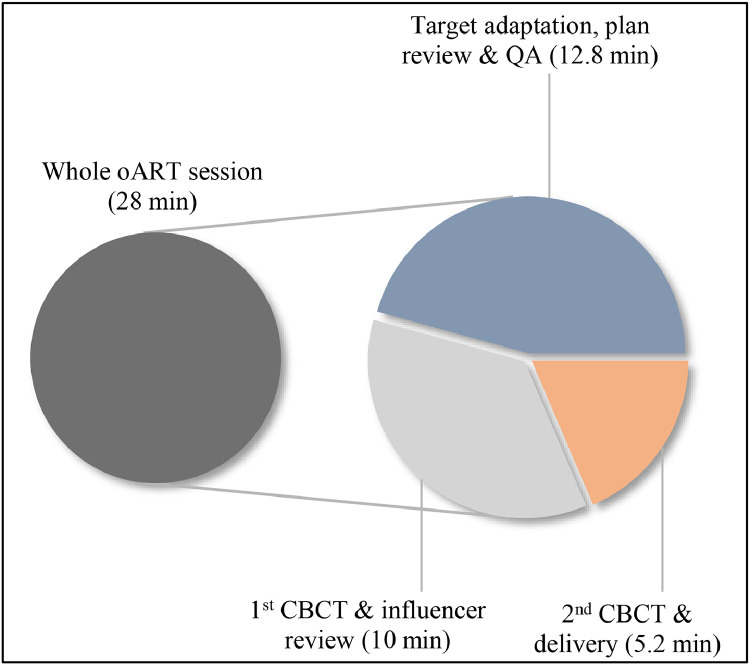
In all 280 sessions, the influencer structures were manually adapted by RTTs and/or MDs. After propagation, adaptation of the CTV was necessary in 164 sessions (59%). The adapted treatment plan was selected for delivery in 276 (99%) fractions. In the remaining 4 (1%) sessions, the scheduled plan was chosen because of the excessive dose to the lungs in the adapted plan. The median time required for the whole oART session was 28 minutes (range, 14.8-43.3; see Fig. 2) while adapting the influencer structures and target volumes took a median of 10 minutes (range, 4.9-24.1). The median time from target adaptation to reviewing the adapted plan was 12.8 minutes (range, 7.1-23.1). Overall, the plan review process was typically completed after 22.2 minutes (median, range, 9.4-36.7). Median treatment application time was 5.2 minutes (range, 3.5-10.7).
Figure 2.
Median times needed for the whole online adapted radiation therapy (oART) process and each workflow step.
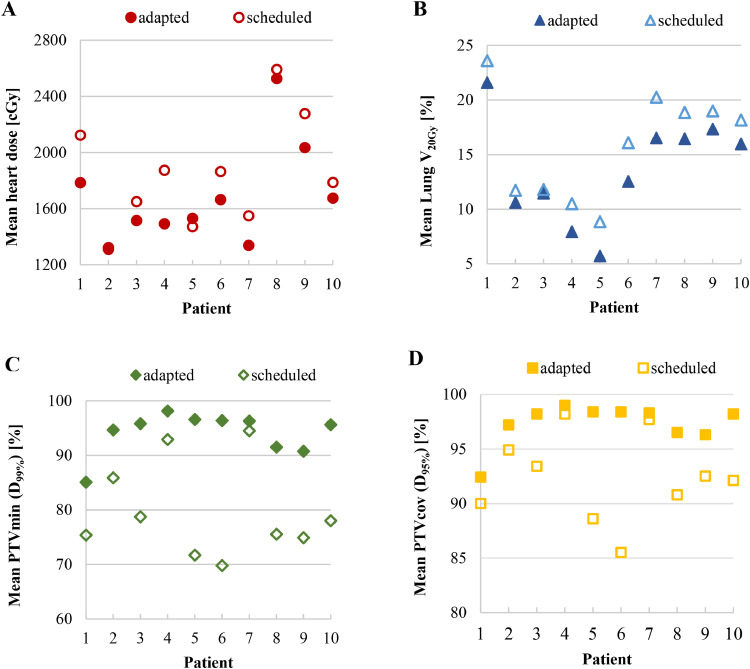
The mean dosimetric values per fraction concerning target coverage and OAR dose are summarized in Table 2, and Fig. 1 illustrates patient examples. Overall, compared to the scheduled treatment plans, the mean heart dose was significantly reduced by 9.5% (absolute, 1.6 Gy; P = .006) with the adapted treatment plans, while the mean lung V20Gy was significantly reduced by 16.9% (absolute, 2.3%; P < .001). Concomitantly, there was a significant improvement in D99%PTV and D99%CTV by 15.3% (absolute, 14.4%; P < .001) and 5.0% (absolute, 4.9%; P = .008), respectively. Additionally, a significant increase in D95%PTV by 5.1% (absolute, 5.0%; P = .003) was achieved with the adapted plan, while D95%CTV was not significantly different (P = .07). A per-patient analysis concerning the mean heart dose, mean lung V20Gy, mean D99%PTV, and mean D95%PTV is depicted in Fig. 3. For all 10 patients, a reduction in mean lung V20Gy was achieved with the adapted treatment plans while improving target coverage. Only 1 patient (patient 5) experienced a slight increase in mean heart dose with the adapted treatment plans, which was offset by a substantial improvement in target coverage.
Table 2.
Mean dosimetric values per fraction concerning target coverage and OAR dose for both the scheduled and adapted treatment plans including all 10 patients
| Dose/volume parameter | Scheduled mean ± SD | Adapted mean ± SD | Absolute difference mean ± SD | Relative difference (%) | P value | |
|---|---|---|---|---|---|---|
| PTV | D99% (%) | 79.7 ± 8.6 | 94.1 ± 3.9 | 14.4 ± 2.6 | +15.3 | <.001 |
| D95% (%) | 92.3 ± 3.9 | 97.3 ± 1.9 | 5.0 ± 1.2 | +5.1 | .003 | |
| D1% (%) | 103.8 ± 1.6 | 102.1 ± 0.3 | –1.7 ± 0.5 | –1.7 | .005 | |
| CTV | D99% (%) | 93.3 ± 4.5 | 98.2 ± 0.3 | 4.9 ± 1.4 | +5.0 | .008 |
| D95% (%) | 97.8 ± 1.7 | 98.9 ± 0.2 | 1.1 ± 0.5 | +1.1 | .070 | |
| D1% (%) | 103.9 ± 1.4 | 101.7 ± 0.4 | –2.2 ± 0.4 | –2.2 | <.001 | |
| Heart | Dmean (Gy) | 18.5 ± 3.9 | 16.9 ± 3.6 | –1.6 ± 0.4 | –9.5 | .006 |
| Lungs | V20Gy (%) | 15.9 ± 4.9 | 13.6 ± 4.8 | –2.3 ± 0.3 | –16.9 | <.001 |
Target dose parameters are reported in percent values, whereas 1.8 Gy (prescribed fraction dose) equals 100%.
Abbreviations: CTV = clinical target volume; Dmean = mean dose; OAR = organ at risk; PTV = planning target volume.
Figure 3.
Per-patient dosimetric differences in (A) mean heart dose, (B) mean lung V20Gy, (C) mean minimal planning target volume (PTV) dose (= PTVmin), and (D) mean PTV coverage dose (= PTVcov) comparing the adapted and the scheduled plans.
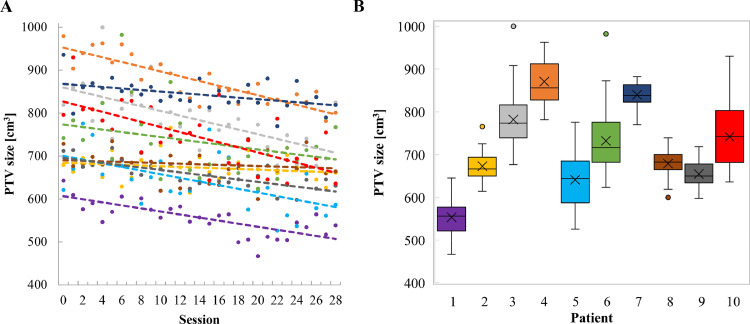
The development of PTV sizes over the course of all 28 oART sessions and daily PTV sizes of all 10 patients are displayed in Fig. 4A, B, respectively. The mean initial PTV size was 766 cm3 (range, 621-980 cm3), and the mean PTV size over 28 fractions was 717 cm3 (range, 554-871 cm3). In all but 1 patient, the mean PTV size decreased after completing the 28 oART sessions compared to the reference PTV size; the mean absolute and relative PTV size reduction was −49 cm3 (range, −109 to +20 cm3) and −6% (range, −14% to +3 %), respectively. Three patients with the greatest relative reduction in mean PTV size (patient 1, −14%; patient 4, −11%; and patient 7, −10%) also showed the largest relative reduction in mean heart dose with the adapted treatment plans (−16%, −20%, and −14%). Two of them were also among the top 3 patients who benefited most in terms of lung V20Gy. However, improvement in D99%PTV was more limited for these patients (+12.8%, +5.6%, and +1.9%). All 3 patients had ACCs of the lower esophagus/GEJ and 2 patients had T3 tumors. Similarly, the 3 patients who saw the greatest benefit in D99%PTV (patient 5, +34.7%; patient 6, +38.1%; and patient 10, +22.6%) also had ACCs of the lower esophagus or GEJ, but saw some of the smallest reduction in mean PTV size (+3%, −1%, and −7%).
Figure 4.
(A) Development of the planning target volume (PTV) size over the period of all 28 online adapted radiation therapy (oART) sessions. Each color represents a patient and the dashed lines represent linear regressions of the corresponding data points. Session 0 describes the PTV size on the reference planning computed tomography (PL-CT). (B) Box plot of the daily PTV sizes of all 10 patients. The box represents the interquartile range (IQR), the line shows the median, and the “x” indicates the mean value. Circles represent outliers beyond 1.5 × IQR.
Discussion
We report our first experiences with CBCT-based oART for patients with EC. While oART presents challenges because of its demands on resources, time, and expertise, we established a workflow with regular daily 30-minute slots in our department. We hypothesized that daily online adaptation of the RT plan for EC patients could improve the sparing of OARs and target coverage. To the best of our knowledge, no study has analyzed the potential of CBCT-based oART in real patients with EC. Boekhoff et al12 conducted an in-silico assessment of the dosimetric benefits of MRgRT for EC. They reported a significant reduction in OAR dose with MRgRT compared to the conventional image guided RT (IGRT) method. However, they employed smaller margins in the MRgRT simulation workflow, which could explain the reported reductions in PTV volumes and OAR dose. We demonstrated a significant relative and absolute reduction in the mean heart dose by 9.5% and 1.6 Gy (P = .006), respectively, for adapted plans compared to scheduled plans. Furthermore, we achieved a 16.9% reduction (absolute, 2.3%; P < .001) in mean lung V20Gy with the adapted treatment plan. Zheng et al3 demonstrated in their SEER database analysis on causes of death in EC patients that 10 years after diagnosis almost 60% of EC patients died from noncancer causes, while cardiovascular disease was the major noncancer cause of death in 43% of patients. Additionally, research on radiation-induced toxicity following RCHT for EC has revealed several associations between increased dose to the lungs and heart and an elevated risk of pulmonary and cardiac complications, along with increased mortality.14, 15, 16, 17, 18, 19 In a retrospective analysis of 560 EC patients treated with RCHT, Xu et al18 found that heart V30Gy >45% and mean lung dose >10 Gy were independently associated with worse survival, as well as cardiac and pulmonary toxicity. The authors concluded that efforts should be made to minimize radiation exposure to the heart and lungs as much as possible. We intend to conduct a prospective analysis to assess whether the reduction in mean heart dose and lung V20Gy observed in our cohort results in decreased toxicity.
In addition to reducing radiation dose to the heart and lungs, we achieved substantially improved target coverage for our first 10 EC patients treated with oART. With the adapted plans, we observed significant increases in D99%PTV and D99%CTV by 15.3% (absolute, 14.4%; P < .001) and 5% (absolute, 4.9%; P = .008), respectively. Moreover, the mean PTV coverage (= D95%PTV) was significantly improved by 5.1% (absolute, 5.0%; P = .003). These results are consistent with findings reported in the literature that analyzed oART for other tumor entities.20,21 While it is generally accepted that improved and consistent target coverage throughout the treatment course is desirable, further studies are needed to demonstrate that oART can lead to a meaningful benefit in locoregional control. We aim to report on the oncological outcomes of EC treated with oART in a larger cohort.
Recently, several studies have also reported on the potential for margin reduction using CBCT-based oART in various tumor entities; however, data on EC are lacking.22, 23, 24 With daily adapted treatment plans, interfraction uncertainties are minimized, and systematic errors are transformed into more random variations. Consequently, PTV margins could potentially be reduced using oART.25 Additionally, contouring guidelines for IMRT in EC suggest that the CTV is extended up to 1 cm into the lungs and 0.5 cm into the heart and liver. If there is no infiltration of these OARs and the boundary with the esophagus is clearly distinguishable on daily CBCT, the CTV could potentially be cropped from these OARs using oART. Incorporating both of these potential margin reductions would result in drastically smaller target volumes for EC; however, care must be taken to avoid target undercoverage because of the remaining intrafraction uncertainties. Boekhoff et al26 observed a generally modest intrafraction shift (<0.5 cm), but noted a high interpatient variability. We performed a second CBCT immediately before dose delivery for position verification and used a 0.5 cm CTV to PTV margin, thus limiting the risk of target undercoverage caused by intrafraction motion. As one of our aims in this study was to demonstrate the feasibility of oART for patients with EC, we used the same margins for treatment planning as in the nonadaptive IGRT workflow, and we preferred to analyze the potential of margin reduction in CBCT-based oART for EC in a prospective study. Consequentially, observing adequate CTV coverage in our cohort with scheduled plans demonstrates that the default margin concept applied in nonadaptive IGRT treatments seems to be reasonable.
We noted substantial changes in daily PTV sizes, even though patients were instructed to fast for ≥4 hours before the treatment. Target definition is influenced not only by changes in daily anatomy and tumor size but also by the treating MD, introducing a degree of interobserver variability. Moreover, the breathing phase in which the CBCT is performed likely also contributes to volume variations. We are currently assessing the use of oART during breath-hold in combination with a surface scanning system. This approach should reduce volume variations and, as a secondary benefit, enhance CBCT image quality. However, the PTV size tended to decrease during the course of oART for almost all patients, as shown in Fig. 4A. As the scheduled plan is based on the initial PTV size from the reference PL-CT, an unnecessarily large volume would often be irradiated with the scheduled plan. Conversely, target coverage of the adapted, smaller target volume appears to be excellent, naturally, if an excessively large volume were to be irradiated with the scheduled plan. Yet, we simultaneously achieved improved target coverage and OAR sparing for most of our patients with the adapted plans. Our data suggest that patients with the greatest reduction in PTV size benefit most in terms of OAR dose reduction, but only to a limited extent with regard to target coverage. On the other hand, those patients with the greatest improvement in target coverage showed the smallest PTV size reduction. It seems plausible that concerning target coverage tumor shrinkage plays a subordinate role, while organ or tumor mobility may be more significant. Our findings remain hypothesis-generating, given the small sample size and the fact that 80% of our patients had ACCs of the lower esophagus or GEJ. However, we presume patients with tumors located in the lower esophagus/GEJ that regress under treatment are more likely to profit from oART, because of the resulting smaller target volumes and the greater mobility of the lower esophagus.
Particularly for abdominal tumors, CBCT quality still presents challenges for oART. Although the majority of our patients (80%) had lower esophageal or GEJ tumors, there was no need to repeat CBCT acquisition because of poor image quality across all 280 fractions. This may primarily be attributed to the target volume definition in EC. The CTV can be contoured without difficulties given the contrast between lungs and mediastinum, as well as the anatomic area between stomach and liver (Fig. 1A, B for patient examples). We manually corrected the CTV volume in almost 60% of the fractions; however, we did not analyze the dosimetric differences between automatically generated and manually adjusted target volumes. Mao et al20 evaluated the auto-contouring and dose distributions for oART in patients with locally advanced lung cancers. They found that manual corrections of contours do not result in meaningful dosimetric changes compared to the automatic contouring process. We will consider this point for our further investigations.
Depending on the anatomic site, the time slots needed for oART vary substantially. They can range from 12 minutes for prostate cancer to more than 35 minutes for pancreatic carcinoma.27 For EC, we reported a median time of 28 minutes. We interpret this as a reasonable time slot for initial experiences in EC patients. As noted in the literature, daily replanning time depends on the radiation delivery technique, as VMAT plans require more time for reoptimization than IMRT plans.27 As reported in this study, 9 out of 10 patients were treated with IMRT plans, not only because of faster reoptimization time but also because of superior plan quality compared to VMAT plans. The quality of adapted plans was deemed acceptable according to our internal guidelines, which are used to analyze conventional IGRT treatments. We performed independent daily online QA with Mobius3D (Varian), for all adapted plans, and we never detected a significant discrepancy between dose calculations from ETHOS TPS compared to Mobius3D. Zhao et al28 showed that daily plan QA may not be necessary for every fraction of oART and recommended establishing a workflow with periodic QA. This adjustment could further reduce the time needed for an oART treatment.
There are limitations to this work. First, it is limited by the small number of patients, and the retrospective nature of the study introduces inherent biases. Second, influencer contouring and target volume adaptation are to some extent at the discretion of the MD present at the oART session. This inevitable interobserver variability was not further analyzed in this study. Third, we neither used 4-dimensional PL-CT nor reduced margins for target volumes, which could have presumably led to even more pronounced dosimetric differences. However, as previously mentioned, we aimed to demonstrate the feasibility of oART for EC, and we preferred to focus on the latter points in a prospective trial.
Conclusions
In this study, we showed for the first time the feasibility and successful implementation of CBCT-based oART for patients with EC. We demonstrated significant dosimetric benefits related to critical dose-volume parameters of the heart and lungs, along with improved target coverage using oART in this patient cohort. As a next step, we aim to conduct further studies to assess the toxicity profile, oncological outcomes, and the potential of margin reduction in oART for patients with EC.
Disclosures
The Department of Radiation Oncology, Inselspital, Bern University Hospital, has long-term collaborations with Varian (A Siemens Healthineers Company, Erlangen, Germany). None of the authors has any affiliation with Varian.
Acknowledgments
Nicolas Bachmann was responsible for statistical analysis.
Footnotes
Sources of support: This work had no specific funding.
Data sharing statement: Research data are not available at this time.
References
- 1.Obermannová R, Alsina M, Cervantes A, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992–1004. doi: 10.1016/j.annonc.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa G, Banik P, Thawani R, et al. Comparison of neoadjuvant regimens for resectable gastroesophageal junction cancer: a systematic review of randomized clinical trials across three decades. J Gastrointest Oncol. 2022;13:1454–1466. doi: 10.21037/jgo-22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Zhang A, Xiao Y, et al. What causes death in esophageal cancer patients other than the cancer itself: a large population-based analysis. J Cancer. 2022;13:3485–3494. doi: 10.7150/jca.78004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann L, Poulsen PR, Ravkilde T, et al. Setup strategies and uncertainties in esophageal radiotherapy based on detailed intra- and interfractional tumor motion mapping. Radiother Oncol. 2019;136:161–168. doi: 10.1016/j.radonc.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Feng A, Gu H, Chen H, et al. Account for the full extent of esophagus motion in radiation therapy planning: a preliminary study of the IRV of the esophagus. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.734552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nardone V, Giugliano FM, Reginelli A, et al. 4D CT analysis of organs at risk (OARs) in stereotactic radiotherapy. Radiother Oncol. 2020;151:10–14. doi: 10.1016/j.radonc.2020.06.048. [DOI] [PubMed] [Google Scholar]
- 7.Qiu B, Lu S, Wang B, et al. Quantifying the interfractional motion of esophagus using daily cone beam computed tomography with oral contrast during radiation therapy for locally advanced non-small cell lung cancer. Pract Radiat Oncol. 2020;10:e339–e347. doi: 10.1016/j.prro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee SL, Bassetti M, Meijer GJ, Mook S. Review of MR-guided radiotherapy for esophageal cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.628009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defize IL, Boekhoff MR, Borggreve AS, et al. Tumor volume regression during neoadjuvant chemoradiotherapy for esophageal cancer: a prospective study with weekly MRI. Acta Oncol. 2020;59:753–759. doi: 10.1080/0284186X.2020.1759819. [DOI] [PubMed] [Google Scholar]
- 10.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boekhoff M, Defize I, Borggreve A, et al. An in-silico assessment of the dosimetric benefits of MR-guided radiotherapy for esophageal cancer patients. Radiother Oncol. 2021;162:76–84. doi: 10.1016/j.radonc.2021.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Wu AJ, Bosch WR, Chang DT, et al. Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys. 2015;92:911–920. doi: 10.1016/j.ijrobp.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86:885–891. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konski A, Li T, Christensen M, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 2012;104:72–77. doi: 10.1016/j.radonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pao TH, Chang WL, Chiang NJ, et al. Cardiac radiation dose predicts survival in esophageal squamous cell carcinoma treated by definitive concurrent chemotherapy and intensity modulated radiotherapy. Radiat Oncol. 2020;15:221. doi: 10.1186/s13014-020-01664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Guo L, Liao Z, et al. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin Transl Radiat Oncol. 2019;17:17–23. doi: 10.1016/j.ctro.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas M, Defraene G, Lambrecht M, et al. NTCP model for postoperative complications and one-year mortality after trimodality treatment in oesophageal cancer. Radiother Oncol. 2019;141:33–40. doi: 10.1016/j.radonc.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Mao W, Riess J, Kim J, et al. Evaluation of auto-contouring and dose distributions for online adaptive radiation therapy of patients with locally advanced lung cancers. Pract Radiat Oncol. 2022;12:e329–e338. doi: 10.1016/j.prro.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Shelley CE, Bolt MA, Hollingdale R, et al. Implementing cone-beam computed tomography-guided online adaptive radiotherapy in cervical cancer. Clin Transl Radiat Oncol. 2023;40 doi: 10.1016/j.ctro.2023.100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen A, Zhong X, Lin MH, Nwachukwu C, Albuquerque K, Hrycushko B. Improved dosimetry with daily online adaptive radiotherapy for cervical cancer: waltzing the pear. Clin Oncol (R Coll Radiol) 2024;36:165–172. doi: 10.1016/j.clon.2024.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Junfang Y, Wang W, Zhang FQ. Reducing CTV to PTV margins with daily adaptive radiotherapy in the postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2023;117:e552. [Google Scholar]
- 24.Brennsæter JA, Dahle TJ, Moi JN, Svanberg IF, Haaland GS, Pilskog S. Reduction of PTV margins for elective pelvic lymph nodes in online adaptive radiotherapy of prostate cancer patients. Acta Oncol. 2023;62:1208–1214. doi: 10.1080/0284186X.2023.2252584. [DOI] [PubMed] [Google Scholar]
- 25.van der Bijl E, Remeijer P, Sonke JJ, van der Heide UA, Janssen T. Adaptive margins for online adaptive radiotherapy. Phys Med Biol. 2022;67:19. doi: 10.1088/1361-6560/ac9175. [DOI] [PubMed] [Google Scholar]
- 26.Boekhoff MR, Lagendijk JJW, van Lier A, Mook S, Meijer GJ. Intrafraction motion analysis in online adaptive radiotherapy for esophageal cancer. Phys Imaging Radiat Oncol. 2023;26 doi: 10.1016/j.phro.2023.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Schaal D, Curry H, et al. Review of cone beam computed tomography based online adaptive radiotherapy: current trend and future direction. Radiat Oncol. 2023;18:144. doi: 10.1186/s13014-023-02340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Stanley DN, Cardenas CE, Harms J, Popple RA. Do we need patient-specific QA for adaptively generated plans? Retrospective evaluation of delivered online adaptive treatment plans on Varian Ethos. J Appl Clin Med Phys. 2023;24:e13876. doi: 10.1002/acm2.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]