Abstract
This study aimed to compare the effects of dietary methionine (Met) and 2-hydroxy-4-(methylthio)-butanoate (HMTBA) on the eggshell quality of broiler breeder hens and elucidate the mechanism of Met in improving eggshell quality from the perspectives of eggshell microstructure and shell gland physiological function. A total of 720 WOD188 broiler breeder hens at 40 weeks old were assigned to 3 groups, with 8 replicates per group and 30 birds per replicate. Over 7 weeks, birds were fed a basal diet or the same diet supplemented with 0.15% Met or 0.17% HMTBA. Our findings revealed significant improvements in the Met group for egg shape index, shell thickness, breaking strength, and fracture toughness (P < 0.05), whereas the HMTBA group showed no significant improvements (P > 0.05). Met supplementation increased calcium and phosphorus levels in both serum and shell gland tissue (P < 0.05), and enhanced Ca2+ ATPase activity in shell gland tissue (P < 0.05). Histomorphological changes cluded enhanced mucosal fold dimensions and increased epithelial height in the shell gland (P < 0.05). Met also improved eggshell ultrastructure, resulting in a thicker effective layer and broader mammillae with fewer type B structures (P < 0.05). The mRNA levels for genes regulating eggshell ultrastructure, such as ovocleidin-116 (OC-116), calbindin 1 (CALB1), and integral membrane protein 2C (ITM2C), were significantly upregulated in the Met group (P < 0.05). Transcriptome analysis identified 248 differentially upregulated genes in the Met group, primarily linked to the non-canonical Wnt/Ca2+ signaling pathway, crucial for calcium ion transport and cellular proliferation. This research highlights that Met supplementation improves eggshell quality by enhancing calcium transport and cellular proliferation in uterine function, particularly through the modulation of Wnt family member 11 (WNT11) and CALB1, influencing calcium deposition and ultrastructural development.
Keywords: Eggshell quality, Eggshell ultrastructure, Methionine, Broiler breeder, Transcriptome
1. Introduction
Eggshells, vital bioceramics, not only protect embryos from physical and microbial harm but also support their growth through gas exchange and calcium provision, with quality notably diminishing towards the end of a hen's laying cycle (Feng et al., 2021). Furthermore, broiler breeders commonly face an early decrease in egg production rates, typically commencing prior to 41 weeks of age, which results in considerable economic losses. The primary cause of eggshell breakage is the aging of laying hens, likely linked to a decrease in the proliferative capacity and ion transport capability of the shell gland epithelial cells (Feng et al., 2023). The biomineralization of eggshells is a sophisticated process that entails the incorporation of calcium and bicarbonate ions into the uterine fluid, supplemented by an organic matrix. The precise coordination of these elements is vital for establishing the eggshell's ultrastructure and mechanical strength (Guru and Dash, 2014). The mechanical properties and overall quality of an eggshell are profoundly impacted by its ultrastructure, including the inner and outer membranes, mammillary layers, palisade layers, vertical crystal layer, and the cuticle. This structure is particularly affected by the aging of the shell gland in broiler breeders, especially in the late stages of laying, where alterations in the shell gland predominantly impact eggshell quality by modifying its structural components (Gloux et al., 2020).
Calcium ions are transported across the shell gland mucosal epithelium in a three-stage process involving entry from blood, intracellular transport and storage, and secretion into the uterine cavity (Kaur et al., 2013). This transport is facilitated by transient receptor potential channels and plasma membrane Ca2+-ATPase, which is ATP-dependent (Wongdee et al., 2021). The process is energy-intensive, and its efficiency declines with age due to reduced activity in energy pathways like mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling (Hao et al., 2021). Comparative transcriptome analysis in laying hens shows a significant difference in the MAPK pathway, correlating with variations in eggshell ultrastructure (Cheng et al., 2023). Calbindin 1 (CALB1) plays a critical role in intracellular calcium transport (Lu et al., 2023), and calcium pumps (Ca2+-ATPase2A2/Ca2+-ATPase2A3) and inositol 1,4,5-trisphosphate receptor (ITPR) channels are notably upregulated in high egg-producing chickens, indicating their importance in calcium handling (Jonchere et al., 2012).
Aging in shell gland mucosal epithelium is characterized by reduced Wnt/β-catenin signaling pathway activity, crucial for cell proliferation and differentiation (Ma et al., 2021). This decline is linked to bone health issues in adult mice and humans, and the non-canonical Wnt/Ca2+ pathway plays a role in balancing cell proliferation with Ca2+ regulation (Fu et al., 2019). High-egg-producing chickens exhibit enhanced Wnt signaling in their uterine section, suggesting its importance in eggshell formation (Yang et al., 2023). Aging impacts the eggshell's palisade layer, where calcium deposition is most active, potentially causing structural defects due to Ca2+ supply deficiency, though more research is needed to confirm this.
Dietary intervention, especially methionine (Met) supplementation, is an emerging strategy to mitigate uterine aging and improve eggshell quality in late-phase laying broiler breeders (Xiao et al., 2017). Methionine, which is crucial for laying hens, enhances egg production and shell quality by increasing serum Ca2+ and lengthening shell gland villi (Liu et al., 2017). This improvement may be due to Met's nourishment of shell gland epithelial cells and activation of the Wnt/β-catenin pathway, providing necessary components to the Wnt Frizzled receptor (FZD) (Nabhan et al., 2023). Methionine's metabolic by-products contribute to cysteine-rich eggshell membrane protein (CREMP), which is vital for eggshell membrane elasticity (Du et al., 2015). While Met appears to thicken the eggshell and improve its ultrastructure, its exact role in restoring biomineralization in aging shell glands requires further study. This research aims to investigate Met's impact on shell gland function, eggshell quality, and ultrastructure regulation to understand the decline in eggshell quality due to aging and lay a foundation for future strategies to enhance eggshell quality in late-phase broiler breeders.
2. Materials and methods
2.1. Animal ethics statement
In this study, the experimental protocols involving animals were authorized by the Animal Care and Use Committee of China Agricultural University (Approval No.: AW01703202-1-6). All animal experiments were conducted in compliance with the “Animal Research: Reporting of In Vivo Experiments” (ARRIVE) guidelines (https://arriveguidelines.org).
2.2. Experiment design
A total of 720 WOD 188 white feather broiler breeders at 40 wk old were randomly divided into 3 groups with 8 replicates of 30 birds each. Birds received the basal diet (CON), a basal diet supplemented with 0.15% Met, or a basal diet with an equimolar replacement of Met by 0.17% 2-hydroxy-4-(methylthio)-butanoate (HMTBA). The study spanned a duration of 7 wk. The HMTBA supplement was graciously supplied by Adisseo (#BQ4891, purity ≥88%, Rhône-Alpes, Commentry, France). We initially conducted a comparative analysis of production indices such as eggshell thickness and eggshell strength between the Met and HMTBA groups to determine the effective additive treatment group. Subsequently, we proceeded to explore the underlying mechanisms. Birds were housed in pairs in cages and all maintained good health throughout the trial, with no medical intervention required. Two weeks prior to the commencement of the experiment, a pre-feeding trial was conducted to ensure that the broiler breeders were adequately acclimatized to their respective diets. From the first day of the experiment, birds were fed according to their designated groups, a practice that was maintained until the conclusion of the trial. The basal diet (Table S1) was formulated following the nutritional guidelines for Arbor Acres Plus Parent Fast Feathering Stock 2021 in Meat Chicken Science and Technology Backyard Program in Dongchengfang Town, Zhuozhou City, Hebei Province, China.
Crude protein, gross energy and calcium in diets were determined according to GB/T6432–2018 (China National Standard 2018a), the method of 9831 (ISO, 1998), and GB/T 6436–2018 (China National Standard, 2018b), respectively. Met and Cys in diets were determined according to GB/T 15399–2018 (China National Standard 2018c). Lys, Trp, and Thr in diets were determined according to GB/T 18246–2019 (China National Standard 2019). The birds were on a restricted feeding regime of 167 g per day and accommodated in 3-tier cages. The environmental temperature within the room was kept at approximate 24 °C, alongside a daily lighting regimen of 16 h.
2.3. Sample collection
For the study, 64 egg samples (8 per replicate) were collected at the end of the 3rd and 7th weeks. Following the observation of dietary Met's superiority over HMTBA in enhancing eggshell quality, 8 birds (one from each replicate) from both the CON and Met groups were selected and humanely euthanized according to animal welfare guidelines. From these birds, blood samples were taken for serum separation via centrifugation at 3000 × g for 10 min at 4 °C, and the serum was then stored at −80 °C for future analysis. Following this, approximately 1 cm of shell gland (uterine) tissue was collected, immediately frozen in liquid nitrogen, and stored at −80 °C for RNA analysis. Furthermore, a 1-cm tissue sample from the shell gland was preserved in 4% formaldehyde for the purpose of histomorphological analysis.
2.4. Physical and mechanical properties of eggshells
Egg shape index, calculated as the length-to-width ratio of an egg, was measured using an egg shape index meter (FHK Company, Japan). The thickness of the eggshell at the blunt end, middle, and sharp end was determined using an ultrasonic thickness gauge (KDE-1061, Robotmation Co., Japan). The average value of these three measurements was used as the phenotypic value for this trait. Eggshell strength was measured with an eggshell strength tester (EFG-0502, Robotmation Co., Japan). The fracture toughness was calculated following the methodology described in previous research (Feng et al., 2023). Following the removal of contents and washing, the dried eggshell weights were recorded on an electronic balance. Subsequently, the proportion of the eggshell, defined as the ratio of the eggshell weight to the total egg weight, was calculated. Data analysis was performed using samples from 64 chickens per group (n = 64).
2.5. Biochemical indicators of serum and uterine tissue
Approximately 300 mg of uterine tissue was collected and homogenized in physiological saline at a 1:10 ratio. The supernatant obtained from this extraction was used for the determination of protein concentration using the BCA Protein Quantification Kit (Vazyme Biotech, Nanjing, China). The levels of calcium, phosphorus, and Ca2+ ATPase in the serum and uterus were measured according to the instructions provided with the assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Readings were taken using a spectrophotometer (model 550 Microplate Reader, Bio-Rad Pacific Ltd., Hong Kong, China). Data analysis was carried out on samples from 6 chickens per group (n = 8).
2.6. Uterine histomorphology
Uterine tissue samples were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Sections of 5 mm thickness were prepared using a Leica RM2235 microtome (Leica Biosystems Inc., Buffalo Grove, IL, USA) and stained with hematoxylin and eosin (H&E) for morphological analysis. Images were captured using an microscope (Olympus Optical Co., Ltd., Beijing, China), with measurements conducted via Image J 1.54 software (Gao et al., 2024). Parameters measured included villus length, mucosal fold height and width, and epithelial cell height under 400× magnification, from 6 chickens per group (n = 6).
2.7. Immunohistochemistry
Immunohistochemical analysis was performed on deparaffinized and hydrated uterine sections, which underwent heat-induced antigen retrieval and peroxidase activity blocking. Sections were incubated with 5% bovine serum albumin, followed by overnight incubation at 4 °C with WNT family member 11 (WNT11) antibody (ImmunoWay Biotechnology, USA), with normal goat serum serving as the negative control. WNT11 immunoreactivity was detected using the Goat Anti-Rabbit immunoglobulin G (Alexa Fluor 488) kit (Wuhan Servicebio Biological Technology, China), visualized with 0.05% diaminobenzidine (DAB) in phosphate-buffered saline (PBS), and counterstained with hematoxylin. Imaging was done with a microscope (Olympus Optical Co., Ltd., Japan), with data analyzed from 6 chickens per group (n = 6).
2.8. Eggshell ultrastructure
Shell fragments from the equatorial region of each egg were carefully extracted with tweezers, maintaining a smooth fracture surface for ultrastructural analysis. The external surface and cross-sectional views of these fragments were analyzed using a scanning electron microscope (FEI Quanta 600, Thermo Fisher Scientific Ltd., USA). Following the preparation methods outlined in the referenced literature, various parameters (Jiang et al., 2021), including the effective layer thickness, mammillary layer thickness, mammillary width, and mammillary density, were measured using Image J 1.54 software. For scanning electron microscopy, eight eggshell samples from each replicate were randomly selected for analysis (n = 8).
2.9. RNA isolation and quantitative real-time PCR (qPCR)
Uterine samples were promptly placed in RNase-free centrifuge tubes and frozen in liquid nitrogen. Total RNA was isolated from 100 mg of tissue using 1 mL TRIzol Reagent (Invitrogen Life Technologies, USA), following the protocol of a referenced study. RNA transcription was performed using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China), in strict accordance with the instructions provided by the manufacturer (Gao et al., 2023b). qPCR for gene expression analysis utilized primers as shown in Table S2 and the SYBR Premix Ex Taq (TaKaRa, Dalian, China) was utilized on an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA, USA), employing a reaction volume of 20 μL.
2.10. RNA-sequencing transcriptome analysis
Total RNA from uterine samples of 6 broiler breeders per group was isolated using TRIzol Reagent, followed by DNase I treatment to remove genomic DNA. For transcriptome library preparation, 1 μg RNA per sample was processed using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) and sequenced on the Illumina Novaseq 6000 platform (LC Bio Technology Co., Ltd, Hangzhou, China). This process included mRNA purification, fragmentation, cDNA synthesis, end processing, adaptor ligation, and PCR amplification. PCR products were purified utilizing the AMPure XP system (Beckman Coulter, Beverly, USA), and the quality of the libraries was evaluated using the Agilent Bioanalyzer 2100 (Agilent Technologies, CA, USA). Clean reads were obtained by removing low-quality reads, adapters, and poly-N sequences. The quality of the data was assessed based on Q20, Q30, GC content, and sequence duplication rates. The analysis methods for the transcriptome specifically referenced the previous protocol (Lv et al., 2024). These reads were then mapped to the Gallus gallus 5.0 reference genome employing Hisat2, with functional annotations conducted using databases such as KEGG. Gene expression was quantified by fragments per klilobase of transcript per million mapped reads (FPKM), and differential expression was analyzed using DESeq2, adjusting P-values by Benjamini and Hochberg's method. Differentially expressed genes (DEGs) with fold change > 1.5 and false discovery rate < 0.05 were analyzed via KEGG using the Wallenius non-central hyper-geometric distribution in R. The visualization of results, including the volcano plot, gene set enrichment analysis (GSEA), and heatmap, was executed using R and the OmicStudio platform.
2.11. Data analysis
Data analysis was conducted using one-way ANOVA, and Duncan's multiple comparison tests were utilized. Significant differences between groups were determined using unpaired t-tests (two-tailed) with SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean values accompanied by their pooled standard error of the mean (SEM), and significance was established at P < 0.05.
3. Results
3.1. Dietary Met addition regulated eggshell mechanical properties in broiler breeders
To assess the changes in eggshell quality of late-phase broiler breeder hens, we initially compared the eggshell quality indicators of the CON group at 43 and 47 weeks (Table S3). Our findings indicated no significant differences (P > 0.05) in shell weight, egg shape index, and shell thickness between these two time points. However, a significant decrease in breaking strength and fracture toughness was observed in the eggshells at 47 weeks compared to those at 43 weeks (P = 0.005, P = 0.001).
Under conditions of strictly controlled feeding (167 g/day), the Met group exhibited a significant reduction in egg production during weeks 1 to 3 compared to the CON group (P = 0.034, Table 1). However, during weeks 4 to 7, the Met group showed a significant increase in egg production (P = 0.029). The average egg weight did not undergo significant changes throughout the feeding period (P > 0.05).
Table 1.
Effects of dietary Met and HMTBA supplementation on production performance in laying hens.1
| Item | Diets2 |
||||
|---|---|---|---|---|---|
| CON | Met | HMTBA | SEM | P-value | |
| Egg production rate, % | |||||
| wk 1–3 | 72.74a,b | 71.08b | 73.41a | 0.388 | 0.034 |
| wk 4–7 | 66.58b | 68.96a | 68.52a | 0.402 | 0.029 |
| wk 1–7 | 69.22 | 69.82 | 70.61 | 0.314 | 0.198 |
| Average egg weight, g | |||||
| wk 1–3 | 63.83 | 63.65 | 63.65 | 0.059 | 0.312 |
| wk 4–7 | 64.38 | 64.30 | 63.96 | 0.089 | 0.121 |
| wk 1–7 | 64.14 | 63.98 | 63.83 | 0.057 | 0.079 |
| Feed conversion ratio, g/g | |||||
| wk 1–3 | 3.60b | 3.69a | 3.58b | 0.018 | 0.021 |
| wk 4–7 | 3.90a | 3.77b | 3.81a,b | 0.022 | 0.041 |
| wk 1–7 | 3.76 | 3.74 | 3.71 | 0.022 | 0.361 |
HMTBA = 2-hydroxy-4-(methylthio)-butanoate.
Values are presented as mean and SEM, n = 64.
a,b Different letters represent statistically significant differences among the groups, P < 0.05.
A strict restricted feeding method was employed, with a daily feed intake of 167 g/day.
CON, the breeder hens fed with a normal diet; Met, the breeder hens fed a basal diet supplemented with Met 0.15%; HMTBA, the breeder hens fed a basal diet supplemented with 0.17% 2-hydroxy-4-(methylthio)-butanoate.
At 47 weeks, eggshell weight remained unaffected by Met treatment (P = 0.514, Table 2). Relative to the CON group, dietary inclusion of Met resulted in a notable increase in eggshell thickness and breaking strength (P = 0.001 and P = 0.007). Additionally, the egg shape index was significantly higher in the Met group than in the CON group at 47 weeks (P = 0.001). In contrast, supplementing the diet with HMTBA did not yield significant changes (P > 0.05) in eggshell weight, egg shape index, shell thickness, breaking strength, and fracture toughness at 47 weeks when compared to the CON group. In summary, HMTBA did not significantly improve eggshell quality. Therefore, we chose the Met group as the subject for our subsequent exploration of the underlying mechanisms.
Table 2.
Effects of dietary Met and HMTBA supplementation on mechanical properties of eggshell.
| Item | Diets1 |
||||
|---|---|---|---|---|---|
| CON | Met | HMTBA | SEM | P-value | |
| Egg shape index | 1.28b | 1.31a | 1.29b | 0.002 | 0.001 |
| Shell weight, g | 6.65 | 6.74 | 6.73 | 0.034 | 0.514 |
| Shell thickness, mm | 0.29b | 0.30a | 0.29b | 0.001 | 0.001 |
| Breaking strength, kg/cm2 | 3.29b | 3.58a | 3.35b | 0.039 | 0.007 |
| Fracture toughness, N/mm3/2 | 341.7b | 379.1a | 359.1b | 4.19 | 0.001 |
HMTBA = 2-hydroxy-4-(methylthio)-butanoate.
Values are presented as mean and SEM, n = 64.
a,b Different letters represent statistically significant differences among the groups, P < 0.05.
CON, the breeder hens fed with a normal diet; Met, the breeder hens fed a basal diet supplemented with Met 0.15%; HMTBA, the breeder hens fed a basal diet supplemented with 0.17% 2-hydroxy-4-(methylthio)-butanoate.
3.2. Dietary Met addition altered serum and uterine chemical composition, improving uterine morphology
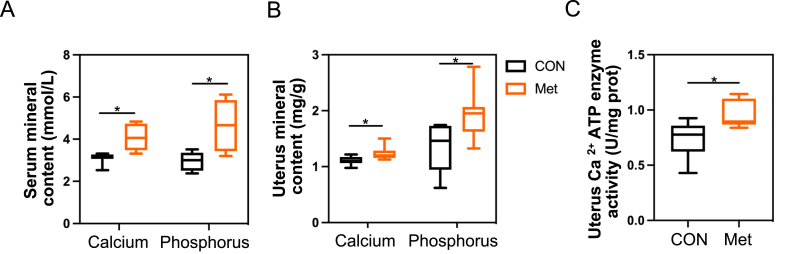
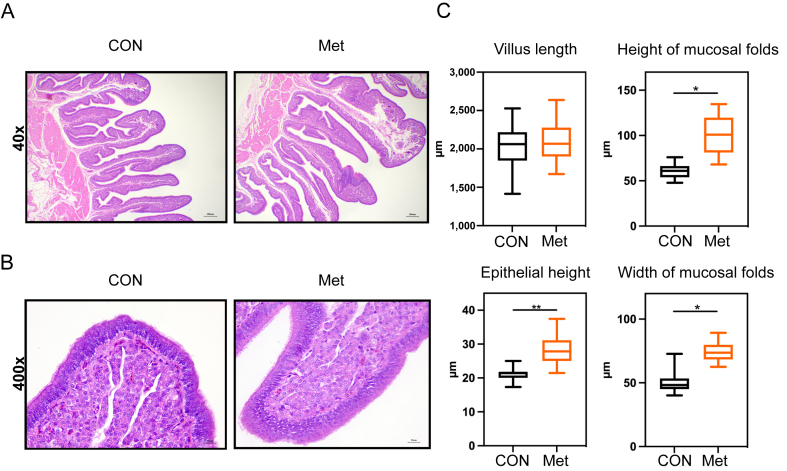
Investigating the foundation for eggshell synthesis, we observed that the levels of calcium and phosphorus in serum were significantly higher in the Met group compared to the CON group (P < 0.05, Fig. 1A and B). Similarly, in the Met group, the calcium and phosphorus contents, as well as the activity of the Ca2+ ATP enzyme in uterine tissues, were significantly elevated compared to the control group (P < 0.05, Fig. 1C). While the inclusion of Met in the diet did not result in a significant increase in the length of uterine villi (P > 0.05, Fig. 2C), it did lead to a significant increase in both the width and height of mucosal folds in the shell gland (P < 0.05, Fig. 2C). Additionally, a notable increase in the epithelial height of the shell gland mucosal epithelial cells was observed with dietary Met supplementation (P < 0.01, Fig. 2C).
Fig. 1.
Impact of additional dietary Met on calcium and phosphorus content in the serum and uterine tissue of broiler breeders. (A) Calcium and phosphorus levels in serum. (B) Calcium and phosphorus levels in uterus. (C) Ca2+ ATPase enzyme activity in uterus. Single asterisks indicate significant differences between groups (P < 0.05).
Fig. 2.
Regulatory effects of dietary Met on the morphology of shell gland tissue in broiler breeders. (A) Morphological examination of the shell gland in both CON and Met groups was conducted under 40× magnification during the late laying period, revealing notable differences. (B) A detailed morphological observation was performed under 400× magnification, providing a closer look at the cellular structure. (C) Quantitative measurements included villus length, mucosal fold height and width, and epithelial cell height. Villus length was determined from the apex of the villus to the top of the lamina propria. The height of the mucosal folds was measured as the vertical distance from the base of the epithelium to the fold's peak. The width of the mucosal folds was assessed at their broadest point using a vertical measurement. The height of the epithelial cells was evaluated at the outermost layer under 400× magnification (n = 6, with 5 measured values for each sample). Single asterisks indicate significant differences between groups (P < 0.05), and double asterisks indicate highly significant differences (P < 0.01).
3.3. Dietary Met addition modulated ultrastructure characteristics of eggshell
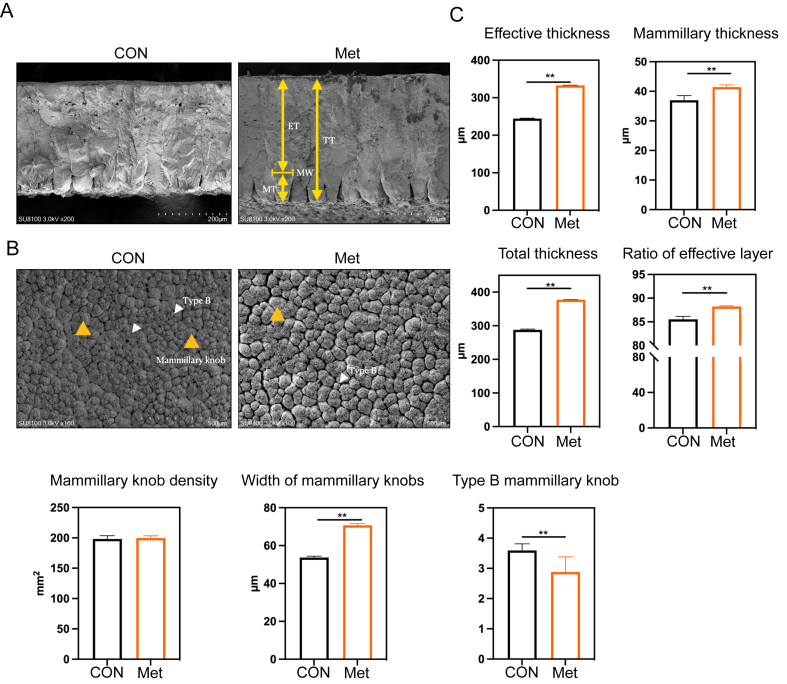
To further explore the specific factors influencing eggshell mechanical properties, scanning electron microscopy was used to analyze the eggshell ultrastructure. The density of mammillary knobs was unaffected by dietary Met supplementation (P > 0.05, Fig. 3B and C). However, Met supplementation resulted in a significant increase in effective thickness, mammillary thickness, total thickness, and the ratio of the effective layer of the eggshell (P < 0.01, Fig. 3C). Additionally, a significant expansion in the width of mammillary knobs was observed with the increased addition of Met by the end of the 7-week period (P < 0.01, Fig. 3C). Regarding the ultrastructural variations in the mammillary layer, the addition of Met led to a significant reduction in the occurrence of type B mammillae (P < 0.01, Fig. 3C).
Fig. 3.
Influence of additional dietary Met on eggshell ultrastructure. (A) Ultrastructural comparison between the control group and the Met group. (B) Observations of ultrastructural mammillary projections in both groups. (C) Measurements include effective thickness (ET), mammillary thickness (MT), total thickness (TT), ratio of effective layer, mammillary knob density, width of mammillary knobs (MW), and type B mammillary knob (Type B). These data are expressed as mean ± SEM for a sample size of n = 8. Double asterisks indicate highly significant differences (P < 0.01).
3.4. Dietary Met addition upregulated biomarker genes in the shell gland related to eggshell biomineralization
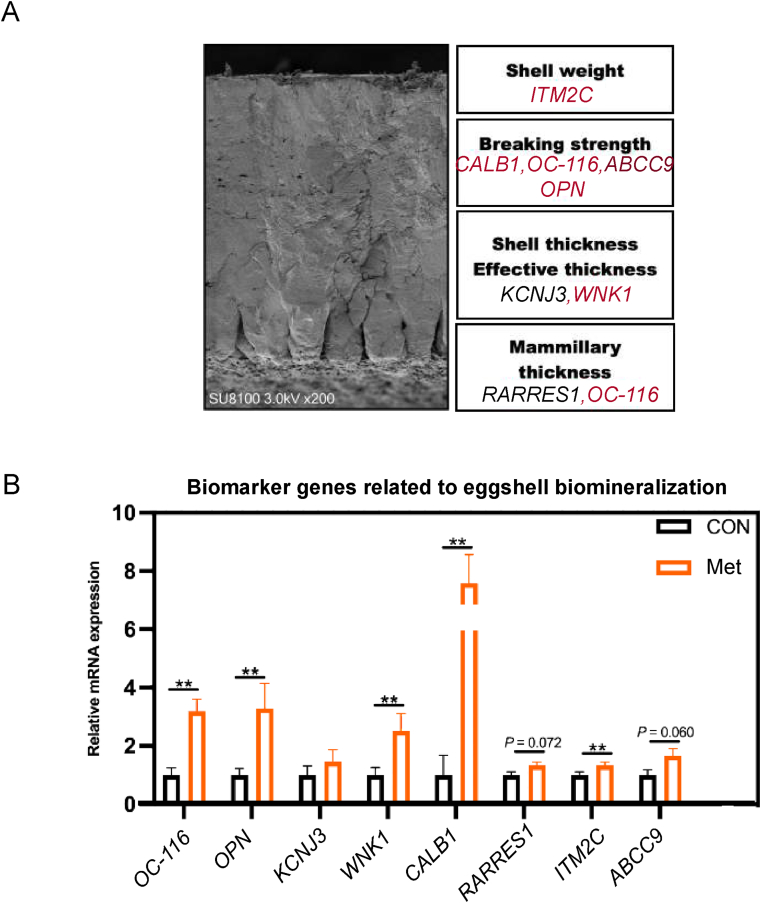
The calcification of the eggshell is a complex process influenced by the regulation of multiple genes. In the group supplemented with dietary Met, we observed elevated mRNA levels of several key genes involved in this process. These included ovocleidin-116 (OC-116), osteopontin 3 (OPN3), WNK lysine deficient protein kinase 1 (WNK1), calbindin 1 (CALB1), and integral membrane protein 2C (ITM2C), which all showed significant upregulation (P < 0.01, Fig. 4B).
Fig. 4.
Regulatory effects of additional dietary Met on mRNA expression related to biomineralization in the shell gland of broiler breeders in the late laying period. (A) Genes associated with various aspects of biomineralization, with light red indicating significant upregulation and dark red indicating a trend towards upregulation (P < 0.1). (B) Biomarker genes related to eggshell biomineralization. OC-116 = ovocleidin-116; KCNJ3 = potassium inwardly rectifying channel subfamily J member 3; CALB1 = calbindin 1; RARRES1 = retinoic acid receptor responder 1; ITM2C = integral membrane protein 2C; WNK1 = WNK lysine deficient protein kinase 1; ABCC9 = ATP binding cassette subfamily C member 9. These data are expressed as mean ± SEM for a sample size of n = 6. Double asterisks indicate highly significant differences (P < 0.01).
3.5. Dietary Met addition altered the transcriptome of the shell gland region
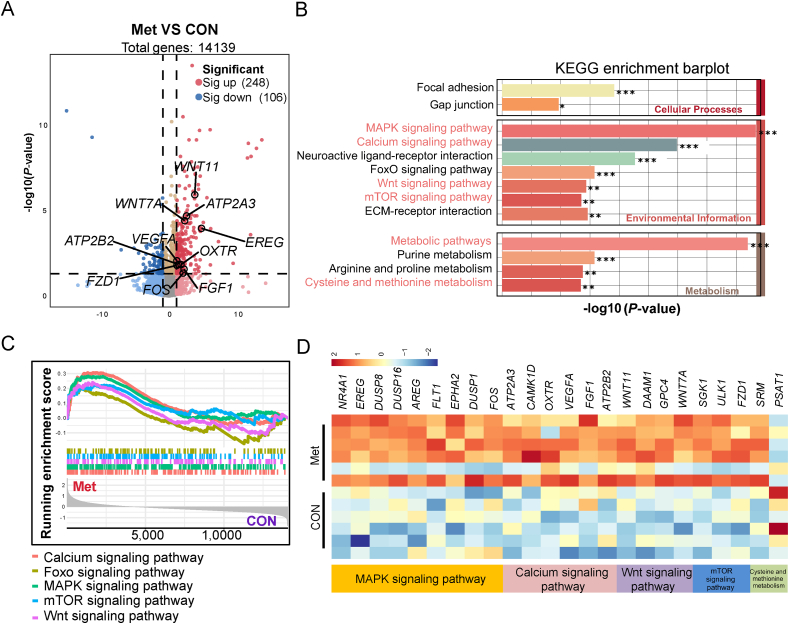
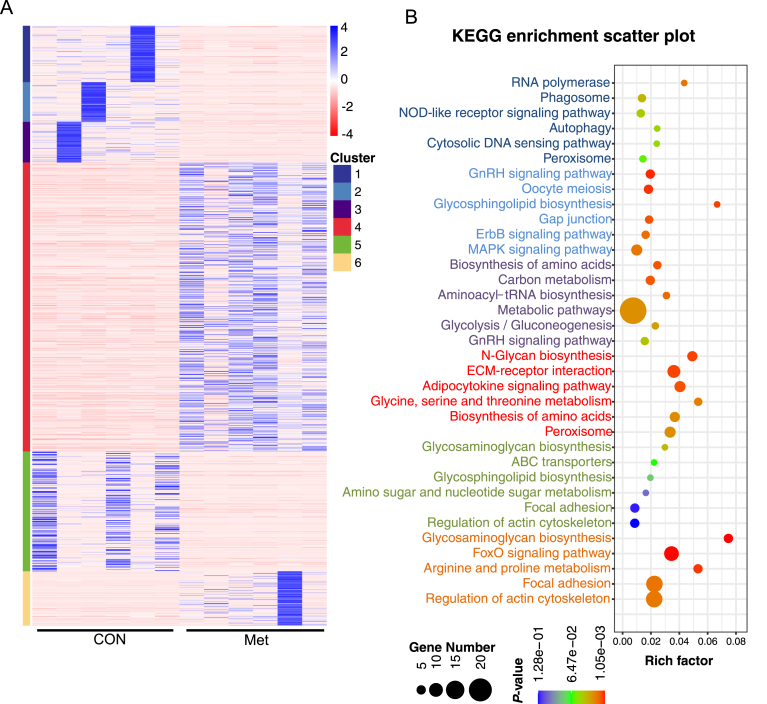
A total of 739,940,606 clean reads, amounting to 80.99 Gb of clean data, were divided into 2 groups. Each sample produced over 46 million clean reads (Table S4). Around 5699 × 108 reads, accounting for more than 94% of the total raw reads, were uniquely aligned to the Gallus gallus genome. The RNA sequencing results were validated by achieving that over 95.5% of bases had a quality score of ≥ Q30, and the GC content of the libraries was close to the anticipated 50%. These findings underscore the reliability of our RNA sequencing data. We detected a total of 354 DEGs in the uterus when comparing the CON and Met groups (Fig. 6A). Of these, 248 were significantly upregulated, and 106 were downregulated in the Met group compared to the CON group. Further analysis using the k-means algorithm categorized the DEGs into 6 distinct clustering patterns. Notably, 170 DEGs in clusters 1 and 4 showed typical up-regulation in the CON group (Fig. S1). These genes were predominantly associated with pathways such as Phagosome, NOD-like receptor signaling, Autophagy, and Adipocytokine signaling, as revealed by KEGG enrichment analysis (Fig. 5C). The upregulation of genes like macrophage receptor with collagenous structure (MARCO) and Toll-like receptor 2 (TLR2) suggests inflammation associated with uterine aging. Additionally, the increased expression of autophagy related 16 like 2 (ATG16L2), autophagy related 3 (ATG3), and mitochondrial calcium uniporter (MCU) highlights the link between uterine aging, autophagy onset, and reduced energy utilization (Table S5).
Fig. 6.
RNA-Seq analysis and validation in broiler breeders of the CON and Met groups (n = 6). (A) Differentially expressed genes (DEGs) with a log2|fold change| > 1.5 at a false discovery rate (FDR) < 0.05. (B) KEGG pathway analysis of DEGs in the uterus of the Met group relative to the CON group. Genes differentially expressed in the Met group compared to the CON group were significantly enriched (P < 0.05). (C) Gene set enrichment analysis (GSEA) based on the Reactome enrichment of DEGs. (D) DEGs in the KEGG pathways of the top 5. Single asterisks indicate significant differences between groups (P < 0.05), and double asterisks indicate highly significant differences (P < 0.01). Triple asterisks indicate extremely significant differences (P < 0.001). NR4A1 = nuclear receptor subfamily 4 group A member 1; EREG = epiregulin; DUSP = dual specificity phosphatase; AREG = amphiregulin; FLT1 = fms related tyrosine kinase 1; EPHA2 = eph receptor A2; FOS = fos proto-oncogene, ap-1 transcription factor subunit; ATP2A3 = ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 3; OXTR = oxytocin receptor; CAMK1D = calcium/calmodulin dependent protein kinase ID; VEGFA = vascular endothelial growth factor A; FGF1 = fibroblast growth factor 1; ATP2B2 = ATPase plasma membrane Ca2+ transporting 2; WNT = Wnt family member; DAAM1 = dishevelled associated activator of morphogenesis 1; GPC4 = glypican 4 = GPC4; SGK1 = serum/glucocorticoid regulated kinase 1; ULK1 = unc-51 like autophagy activating kinase 1; FZD1 = frizzled class receptor 1; SRM = spermidine synthase; PSAT1 = phosphoserine aminotransferase 1.
Fig. 5.
RNA-seq analysis and validation in CON and Met group broiler breeders (n = 6). (A) Heatmap of the different gene clustering. (B) Top 10 significantly enriched KEGG.
In contrast, genes in clusters 2, 3, 5, and 6 were significantly upregulated in the Met group. Top KEGG pathways enriched in these clusters included the MAPK, calcium, Wnt, and mTOR signaling pathways, with 12, 8, 5, and 5 DEGs, respectively, in the environmental information processing category (Fig. 6B). Significant enrichments in the metabolism category were observed in pathways like metabolic pathway and cysteine and Met metabolism (P < 0.01). Gene set enrichment analysis (GSEA) based on Reactome enrichment further validated these results, with GSEA plots demonstrating positive enrichment of gene signatures associated with these KEGG-identified functional pathways (Fig. 6C). The MAPK signaling pathway was enriched with genes such as epiregulin (EREG), amphiregulin (AREG), fms-related tyrosine kinase 1 (FLT1), EPH receptor A2 (EPHA2); the calcium signaling pathway with ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 3 (ATP2A3), calcium/calmodulin-dependent protein kinase 1D (CAMK1D), ATPase plasma membrane Ca2+ transporting 2 (ATP2B2); the Wnt signaling pathway with WNT11, WNT7A; and the mTOR pathway with Frizzled receptor 1 (FZD1), serum/glucocorticoid regulated kinase 1 (SGK1) (Fig. 6D).
3.6. Dietary Met addition activates the Wnt/Ca2+ signaling pathway
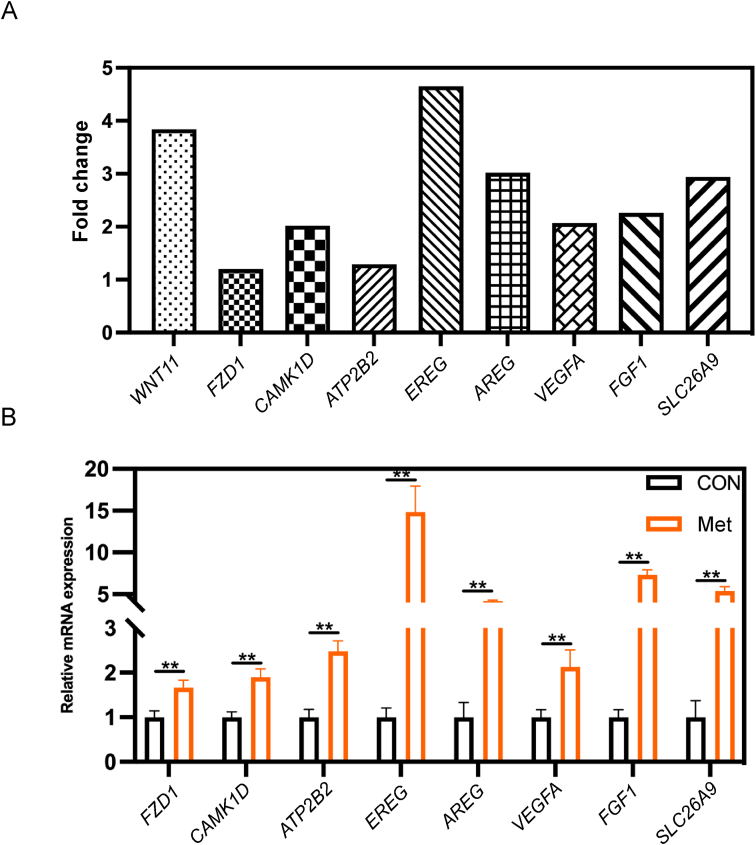
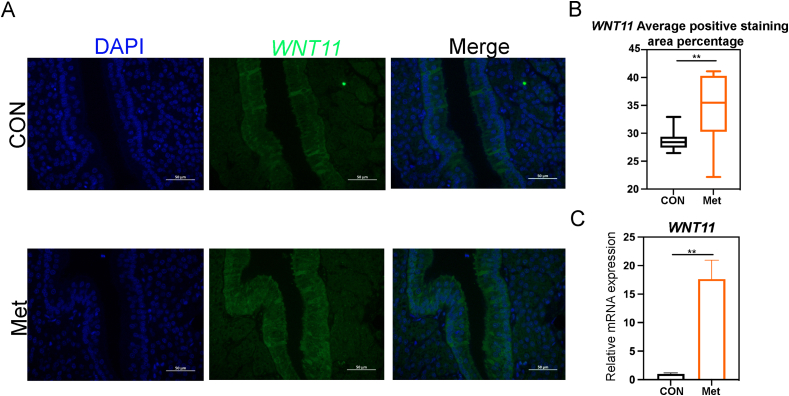
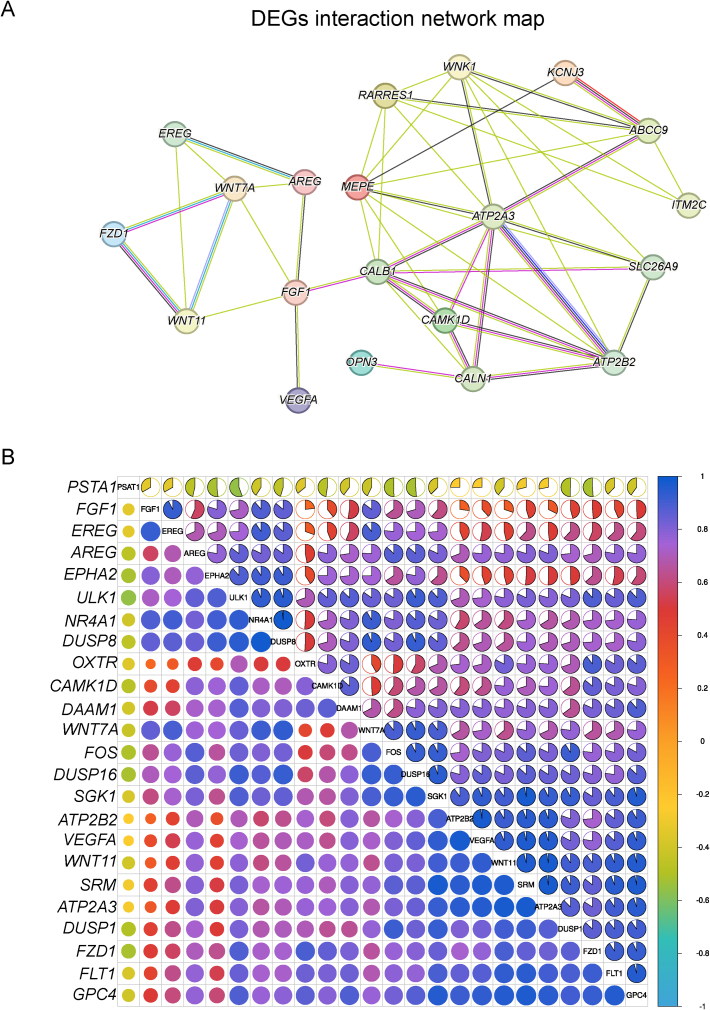
Following the KEGG enrichment results, we identified 9 DEGs with significant roles in their respective pathways, using the criterion of |log2 fold change| > 1. These genes were WNT11, FZD1, and CAMK1D in the Wnt signaling pathway; ATP2B2, vascular endothelial growth factor A (VEGFA), and FGF1 (fibroblast growth factor 1) in the calcium signaling pathway; and EREG, AREG, VEGFA, FGF1, and solute carrier family 26 member 9 (SLC26A9) in the MAPK signaling pathway (Fig. 7A). To corroborate the transcriptome data, qPCR was performed on selected genes. The relative RNA expression levels observed were in agreement with the results of the transcriptome analysis, thereby validating their significance (Fig. 7B). WNT11, a pivotal gene in the non-canonical Wnt/Ca2+ signaling pathway, showed a significant upregulation in mRNA expression in the shell gland of the Met group (P < 0.01, Fig. 8C). Immunofluorescence analysis of the shell gland tissue indicated a significantly higher average positive staining area percentage for WNT11 in the Met group compared to the CON group (P < 0.01, Fig. 8A and B). Lastly, we performed a correlation analysis and a protein-protein interaction network analysis between the marker genes associated with eggshell formation and the upregulated genes from the transcriptome (Fig. 9A). This analysis revealed a significant positive correlation between each pair of genes, with the exception of the phosphoserine aminotransferase 1 (PSAT1) gene (P < 0.01, Fig. 9B).
Fig. 7.
RT-qPCR validation of differentially expressed genes (DEGs). (A) Fold change analysis of transcriptome results for DEGs in the Met group compared to the CON group. (B) Relative mRNA expression of selected genes. FZD1 = Frizzled receptor 1; CAMK1D = calcium/calmodulin-dependent protein kinase 1D; ATP2B2 = ATPase plasma membrane Ca2+ transporting 2; EREG = epiregulin; AREG = amphiregulin; VEGFA = vascular endothelial growth factor A; FGF1 = fibroblast growth factor 1; SLC26A9 = solute carrier family 26 member 9. These data are expressed as mean ± SEM for a sample size of n = 6. Double asterisks indicate highly significant differences (P < 0.01).
Fig. 8.
Regulatory effects of dietary Met on epithelial cell proliferation in the shell gland of broiler breeders in the late laying period. (A) Immunofluorescence identification of Wnt family member 11 (WNT11) expression in the shell gland epithelial cells of the CON and Met groups. (B) Comparative analysis of the average positive staining area percentage for WNT11. (C) Relative mRNA expression of WNT11. These data are expressed as mean ± SEM for a sample size of n = 6. Double asterisks indicate highly significant differences (P < 0.01).
Fig. 9.
Interaction relationships between biomarkers related to eggshell biomineralization and selected differentially expressed genes (DEGs). (A) Interaction network between biomarkers and selected DEGs, where the lines represent existing interaction relationships. The greater the number of lines, the stronger and more numerous the interactions between the genes. (B) Correlation analysis between biomarkers and selected DEGs. The intensity of the color indicates the strength of the correlation, n = 6. FZD1 = Frizzled receptor 1; CAMK1D = calcium/calmodulin-dependent protein kinase 1D; ATP2B2 = ATPase plasma membrane Ca2+ transporting 2; EREG = epiregulin; AREG = amphiregulin; VEGFA = vascular endothelial growth factor A; FGF1 = fibroblast growth factor 1; ABCC9 = ATP binding cassette subfamily C member 9; SLC26A9 = solute carrier family 26 member 9; OC-116 = ovocleidin-116; OPN3 = osteopontin 3; WNK1 = WNK lysine deficient protein kinase 1; CALB1 = calbindin 1; ITM2C = integral membrane protein 2C; MEPE = matrix extracellular phosphoglycoprotein; KCNJ3 = ATP-Sensitive inward rectifier potassium channel 3; RARRES1 = retinoic acid receptor responder 1; WNT11 = wnt family member 11; PSTA1 = prostate specific transcript 1; EPHA2 = EPH receptor A2; ULK1 = Unc-51 like autophagy activating kinase 1; NR4A1 = nuclear receptor subfamily 4 group a member 1; DUSP8 = dual specificity phosphatase 8; OXTR = oxytocin receptor; DAAM1 = dishevelled associated activator of morphogenesis 1; FOS = Fos proto-oncogene, AP-1 transcription factor subunit; SGK1 = serum/glucocorticoid regulated kinase 1.
4. Discussion
The inadequate quality of eggshells in broiler breeder chickens, a known factor leading to internal contamination of hatching eggs, may adversely affect hatchability (Noetzold et al., 2020). Eggshells need to possess optimal hardness to protect developing embryos from external environmental harm (Damaziak et al., 2023). Our study underscores the significance of improving eggshell quality through nutritional planning in broiler breeders. Uterine aging has been identified as a key factor in the deterioration of eggshell quality in laying hens (Feng et al., 2020). Previous research indicates that dietary Met supplementation can enhance eggshell quality, mainly characterized by increased shell thickness and heightened antioxidant levels in serum (Liu et al., 2022). However, the effectiveness of Met in broiler breeders and its influence on eggshell formation and quality still need to be fully understood. In this study, we first evaluated the effects of Met and its hydroxy analogue on improving eggshell quality, selecting Met as the superior nutritional additive for regulating eggshell quality. We found that Met supplementation in broiler breeder chickens' diets enhances ion transport, as evidenced by increased calcium and phosphorus concentrations in serum and shell gland tissue. This supplementation appears to counteract functional deficiencies in eggshell biomineralization, positively affecting both the ultrastructure and strength of eggshells.
The aging process of the uterus is linked to a reduction in the proliferation and energy supply of epithelial cells, leading to a thinning of the uterine lining (Park and Sohn, 2018), and we observed that 47-week-old chickens showed notable decreases in eggshell weight and thickness compared to 43-week-old chickens. A key aspect of uterine aging is the diminished capacity for calcium ion transport, critical for eggshell calcification. This element is pivotal in the eggshell calcification process and is intimately associated with the calcium content in the uterus and the expression of the uterine calcium transport protein CALB1 (Shet et al., 2018). Our findings reveal that Met supplementation in late-laying broilers significantly increases calcium ion content in serum and uterine tissues, with an upregulation of the calcium transport protein CALB1. This indicates enhanced calcium transport, essential for eggshell formation. The calcium ion content in serum and shell gland tissue serves as a direct indicator reflecting the transport of calcium ions (Ren et al., 2019). Contrastingly, HMTBA did not significantly improve eggshell quality. This may be due to its orientation towards the transsulfuration pathway, leading to antioxidant metabolite production rather than direct eggshell quality enhancement (Nie et al., 2007). The lack of effect on eggshell quality might also stem from breed-specific metabolic pathways, as broilers have more advanced liver and intestinal development than laying hens, potentially altering HMTBA metabolism (Han et al., 2020). Furthermore, oxidative stress may not be the primary cause of uterine aging in late-stage broiler breeders. Breed differences could also play a role, as broilers typically exhibit more advanced liver and intestinal development than laying hens. Consequently, the dosage of HMTBA used in our study might have been predominantly metabolized and absorbed in the liver and intestines, failing to affect the shell glands significantly. Future studies should investigate the use of increased HMTBA dosages to assess its potential effects on eggshell quality in broiler breeders.
In the late laying period, laying hens undergo notable alterations in uterine morphology, characterized by a substantial reduction in the length and height of villi and the area of mucosal folds. These morphological changes can markedly impact the efficiency of ion transport from the epithelial cells of the shell gland during eggshell formation, as well as affect secretion pathways to the extracellular space. A notable manifestation of these alterations is the observed reduction in calcium and phosphorus content in eggshell and serum (Attia et al., 2020). Our research has demonstrated that dietary Met supplementation beneficially affects eggshell quality, notably enhancing the phosphorus levels in the serum and uterus of broiler breeder chickens. Furthermore, it led to an improvement in the shell gland epithelial cells and augmented the dimensions (width and height) of wrinkles, likely aiding in the efficient transport of calcium throughout the eggshell calcification process. The marked elevation in Ca2+ ATPase activity within the shell gland tissue offers a plausible rationale for these enhancements. Furthermore, the eggshell membrane safeguards eggshell quality by forming a protective layer that prevents external contaminants from entering the egg (Kulshreshtha et al., 2022). The elasticity of the eggshell membrane, which is highly dependent on CREMP, suggests its critical protective function (Du et al., 2015). The role of Met in one-carbon metabolism, leading to cysteine formation, may enhance overall eggshell quality. The study highlights Met's potential in improving not just the mechanical attributes of the eggshell but also in fortifying its protective mechanisms.
The ultrastructure of eggshells, formed in the distal oviduct uterus (shell gland), is pivotal in determining their mechanical properties (Guru and Dash, 2014). Factors such as the size, shape, and orientation of the crystals in the eggshell calcification layer, the gap size between mammillary bodies and palisade layer crystals, and the relative thickness of the mammillary layer compared to the palisade layer are all crucial determinants of eggshell quality (Feng et al., 2020). Consistent with prior research, our study observed significant alterations in eggshell ultrastructure during the late laying period in broiler breeder chickens, a process influenced by uterine aging (Feng et al., 2023). These changes included reduced thickness and increased width of the mammillary layer, diminished effectiveness of the layers, and delayed fusion of mammillary caps, notably marked by an increase in type B mammillae. The delayed fusion of mammillae, indicative of a less dense and looser structure, increases the eggshell's susceptibility to rupture. Type B mammillae, with their smaller caps than typical mammillae, lead to a more relaxed structure and reduced fracture resistance (Samiullah et al., 2013). Our findings suggest that dietary Met supplementation can mitigate the early fusion of mammillae. This enhancement in the binding capacity between mammillary knobs potentially hinders crack propagation from force points, thereby improving the eggshell's mechanical properties. The effective layer, which includes the palisade layer, vertical crystal layer, and cuticle, constitutes a crucial ultrastructural element that dictates the strength of the eggshell (Jiang et al., 2021). As laying hens age, there is a notable thinning in this effective layer of the eggshell ultrastructure (Feng et al., 2020). This study demonstrates that additional Met supplementation can counter this thinning. An increase in the thickness of the effective layer contributes to enhanced eggshell strength, reducing the likelihood of fractures and thereby improving the overall mechanical properties of the eggshell.
Eggshell ultrastructure formation involves a complex interaction between minerals, organic substances, and a gene network in uterine epithelial cells (Duan et al., 2016). Dysregulated gene expression in the uterus during late laying is linked to declines in eggshell quality. Key genes like ABCC9, KCNJ3, and WNK1, which govern ion transport, correlate with eggshell thickness (Duan et al., 2016; Feng et al., 2020). ABCC9 regulates cell membrane potential via ATP concentration sensitivity, while KCNJ and WNK1 manage potassium ion utilization (Gao et al., 2023a; Hardege et al., 2018). Our research shows that dietary Met supplementation boosts eggshell thickness by influencing these genes. ITM2C, RARRES1, and OC-116, involved in protein regulation and biomineralization, are also upregulated by Met, enhancing mammillary thickness and antimicrobial activities (Duan et al., 2016; Poyatos Pertinez et al., 2020). This complex regulatory mechanism likely aids the reassembly of mineral elements, contributing to the optimization of eggshell structure and strength. Past research has indicated that dietary supplementation with Met enhances intestinal health in laying hens (Gong et al., 2023), which could be a contributing factor to enhanced eggshell thickness, strength, and toughness. The observed increase in eggshell thickness may be linked to enhanced calcium and phosphorus absorption, given the heightened activity in calcium metabolism during the laying period. Significant increases in CALB1 and OPN mRNA levels in Met-supplemented broiler breeders were observed. These proteins are vital for calcium ion transport and eggshell calcification, impacting eggshell strength (Shet et al., 2018). Our findings suggest that dietary Met can mitigate uterine aging effects, promoting efficient calcium transport and deposition, which is crucial for eggshell formation. However, the specific mechanisms of Met's influence on these processes require further exploration.
In this study, RNA sequencing was used to investigate how dietary Met affects the transcriptome of the shell gland, focusing on the regulation of epithelial cells essential for eggshell biomineralization. We identified key cell clusters and genes responsible for secreting biomineralization precursors. Notably, genes like ATP2B1 and ATP2B2 were found to be crucial in calcium ion transport for eggshell formation (Gloux et al., 2019). Our analysis identified eight DEGs associated with the Calcium signaling pathway, crucial for the transport of calcium ions into the epithelial cells of the shell gland, their subsequent secretion into the uterine fluid, and the formation of the eggshell. CAMK1D, influenced by intracellular calcium levels, modulates cell function and affects protein phosphorylation, interacting with CALB proteins to facilitate transport and promote cell proliferation (Zhou et al., 2019b). ATP2A3, a calcium pump on the endoplasmic reticulum membrane, maintains intracellular calcium concentration by transporting ions to storage sites (Li et al., 2023), while ATP2B2, a plasma membrane calcium pump, expels calcium ions to the extracellular space, vital for eggshell mineralization (Zhang et al., 2019). Additionally, bicarbonate is transported from uterine epithelial cells to the uterine fluid through passive transport, mediated by SLC26A9 (Nii et al., 2018). Our findings highlight that Met supplementation augments calcium utilization, enhancing these calcium transport pathways. The ATP-dependent operation of these pumps is further supported by the upregulated MAPK signaling pathway in the Met-supplemented group. DEGs like VEGFA and FGF1, significant in the calcium signaling pathway, bind to MAPK pathway receptors, indirectly regulating calcium release and modulation. EREG and AREG, associated with the EGFR, influence cell proliferation and growth by activating the RAS/RAF/MEK/ERK signaling pathway (Zhou et al., 2019a).
In our study, we observed a notable enrichment of the Wnt signaling pathway in the Met-supplemented group, a pathway known for its close association with cell proliferation (Zhou et al., 2019c). Notably, we identified significant differences in genes within the Wnt family, including WNT7A and WNT11, which are integral to the non-canonical Wnt/Ca2+ signaling pathway. This pathway differs from the canonical Wnt pathway primarily in regulating intracellular calcium ion concentration, which facilitates the release of these ions (Krishnamurthy and Kurzrock, 2018). Notably, the binding of WNT11 to the FZD1 receptor activates this pathway. In the Met group, we detected a significant upregulation of FZD1 expression. The extracellular N-terminal of the FZD1 receptor, containing a cysteine-rich domain, is believed to be influenced by cysteine supplementation, which Met can provide, thereby promoting its synthesis and expression (Nabhan et al., 2023). Immunofluorescence analysis revealed a marked increase in the Wnt11-positive area in the shell gland epithelial cells of the Met group, underscoring the activation of the Wnt/Ca2+ signaling pathway under Met supplementation. However, it is important to note that current research only covers the relationship between the Wnt/Ca2+ signaling pathway and the MAPK pathway. We constructed an interaction network of DEGs through STING and correlation analysis methods. These genes, associated with eggshell biomineralization and ultrastructure formation, exhibited a close and significantly positive correlation. In summary, dietary Met supplementation may activate the Wnt/Ca2+ signaling pathway, mainly through the interaction between WNT11 and FZD1 receptors. This activation triggers the release of intracellular calcium ions. The study finds that changes in calcium ion levels interact with the CAMKII kinase, influencing the MAPK pathway, which is essential in calcium regulation. This leads to enhanced shell gland cell proliferation, improved calcium transport, and upregulated genes like OPN, WNK1, and OC-116. These effects improve the aging uterine shell gland's structure and increase tissue calcium-phosphorus levels, resulting in eggshells with greater thickness and improved mechanical properties.
5. Conclusion
In conclusion, dietary Met supplementation for broiler breeders in late-phase laying is more effective than HMTBA in improving eggshell quality. Dietary Met significantly improves uterine morphology, leading to a thicker effective layer in the ultrastructure of the eggshell. Methionine is crucial in improving uterine function and biomineralization of eggshells, particularly enhancing cell proliferation and calcium transport. This effect is largely mediated through the Wnt/Ca2+ signaling pathway, which is essential for calcium deposition and the development of the eggshell ultrastructure. Our findings highlight the potential of Met to counteract the age-related decline in uterine function and eggshell quality.
Author contributions
Mingkun Gao: conceptualization, methodology, sampling, experimentation, data curation, writing the original draft, and editing; Dongli Li: sampling, methodology and funding acquisition; Xiaomin Li: sampling and methodology; Youying Chen: experimentation and data curation; Aiqiao Liu: methodology and funding acquisition; Wei Nie: methodology; Zhonghua Ning: methodology and funding acquisition; Yuming Guo: project administration, funding acquisition, writing-review and editing. Zengpeng Lv: conceptualization, methodology, project administration, funding acquisition, writing-review and editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was funded by the National Key R&D Program of China (2021YFD1300404), the China Agriculture Research System program (CARS-40 and CARS-41), the Beijing Natural Science Foundation (NO. 6222036), and the Meat Chicken Science and Technology Backyard Program in Dongchengfang Town, Zhuozhou, Hebei Province, China (CAUZ2024013). We would like to express our sincere gratitude to Huadu Yukou Poultry Industry Co., Ltd. China for providing excellent breeding conditions.
Footnotes
Peer review under the responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2024.04.026.
Contributor Information
Yuming Guo, Email: guoyum@cau.edu.cn.
Zengpeng Lv, Email: lvzengpeng310@cau.edu.cn.
Appendix. Supplementary data
The following is the Supplementary data to this article:
References
- Attia Y.A., Al-Harthi M.A., Abo El-Maaty H.M. Calcium and cholecalciferol levels in late-phase laying hens: effects on productive traits, egg quality, blood biochemistry, and immune responses. Front Vet Sci. 2020;7:389. doi: 10.3389/fvets.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Li X., Liu Y., Ma Y., Zhang R., Zhang Y., Fan C., Qu L., Ning Z. DNA methylome and transcriptome identified key genes and pathways involved in speckled eggshell formation in aged laying hens. BMC Genom. 2023;24:31. doi: 10.1186/s12864-022-09100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2018. Determination of crude protein in feeds - kjeldahl method. GB/T 6432-2018. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2018. Determination of calcium in feeds. GB/T 6436-2018. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2018. Determination of sulfur amino acids in feeds - ion exchange chromatography. GB/T 15399-2018. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2019. Determination of amino acids in feeds. GB/T 18246-2019. [Google Scholar]
- Damaziak K., Marzec A., Riedel J., Wojcik W., Pstrokonski P., Szudrowicz H., Gozdowski D. Effect of pearl Guinea fowl eggshell ultrastructure and microstructure on keets hatchability. Poultry Sci. 2023;102 doi: 10.1016/j.psj.2023.102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Hincke M.T., Rose-Martel M., Hennequet-Antier C., Brionne A., Cogburn L.A., Nys Y., Gautron J. Identifying specific proteins involved in eggshell membrane formation using gene expression analysis and bioinformatics. BMC Genom. 2015;16:792. doi: 10.1186/s12864-015-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Sun C., Shen M., Wang K., Yang N., Zheng J., Xu G. Genetic architecture dissection by genome-wide association analysis reveals avian eggshell ultrastructure traits. Sci Rep. 2016;6 doi: 10.1038/srep28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Lu M., Ma L., Zhang H., Wu S., Qiu K., Min Y., Qi G., Wang J. Uterine inflammation status modulates eggshell mineralization via calcium transport and matrix protein synthesis in laying hens. Anim Nutr. 2023;13:411–425. doi: 10.1016/j.aninu.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., Wu S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J Anim Sci Biotechnol. 2021;12:72. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang H.J., Wu S.G., Qi G.H., Wang J. Uterine transcriptome analysis reveals mrna expression changes associated with the ultrastructure differences of eggshell in young and aged laying hens. BMC Genom. 2020;21:770. doi: 10.1186/s12864-020-07177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Liu X., Tan L., Cui Z., Zheng Y., Liang Y., Li Z., Zhu S., Yeung K.W.K., Feng X., Wang X., Wu S. Photoelectric-responsive extracellular matrix for bone engineering. ACS Nano. 2019;13:13581–13594. doi: 10.1021/acsnano.9b08115. [DOI] [PubMed] [Google Scholar]
- Gao J., Mcclenaghan C., Matreyek K.A., Grange D.K., Nichols C.G. Rapid characterization of the functional and pharmacological consequences of cantu syndrome k(atp) channel mutations in intact cells. J Pharmacol Exp Therapeut. 2023;386:298–309. doi: 10.1124/jpet.123.001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Liao C., Fu J., Ning Z., Lv Z., Guo Y. Probiotic cocktails accelerate baicalin metabolism in the ileum to modulate intestinal health in broiler chickens. J Anim Sci Biotechnol. 2024;15:25. doi: 10.1186/s40104-023-00974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang J., Lv Z. Supplementing genistein for breeder hens alters the growth performance and intestinal health of offspring. Life (Basel) 2023;13 doi: 10.3390/life13071468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloux A., Le Roy N., Brionne A., Bonin E., Juanchich A., Benzoni G., Piketty M.L., Prie D., Nys Y., Gautron J., Narcy A., Duclos M.J. Candidate genes of the transcellular and paracellular calcium absorption pathways in the small intestine of laying hens. Poultry Sci. 2019;98:6005–6018. doi: 10.3382/ps/pez407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloux A., Le Roy N., Meme N., Piketty M.L., Prie D., Benzoni G., Gautron J., Nys Y., Narcy A., Duclos M.J. Increased expression of fibroblast growth factor 23 is the signature of a deteriorated ca/p balance in ageing laying hens. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Mahmood T., Mercier Y., Xu H., Zhang X., Zhao Y., Luo Y., Guo Y. Dietary methionine sources and levels modulate the intestinal health status of broiler chickens. Anim Nutr. 2023;15:242–255. doi: 10.1016/j.aninu.2023.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru P.S., Dash S. Sorption on eggshell waste--a review on ultrastructure, biomineralization and other applications. Adv Colloid Interface Sci. 2014;209:49–67. doi: 10.1016/j.cis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Han H.L., Zhang J.F., Yan E.F., Shen M.M., Wu J.M., Gan Z.D., Wei C.H., Zhang L.L., Wang T. Effects of taurine on growth performance, antioxidant capacity, and lipid metabolism in broiler chickens. Poultry Sci. 2020;99:5707–5717. doi: 10.1016/j.psj.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao E.Y., Wang D.H., Chen Y.F., Zhou R.Y., Chen H., Huang R.L. The relationship between the mtor signaling pathway and ovarian aging in peak-phase and late-phase laying hens. Poultry Sci. 2021;100:334–347. doi: 10.1016/j.psj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardege I., Long L., Al Maskari R., Figg N., O'shaughnessy K.M. Targeted disruption of the kcnj5 gene in the female mouse lowers aldosterone levels. Clin Sci (Lond) 2018;132:145–156. doi: 10.1042/CS20171285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (International Organization for Standardization) International Organization for Standardization; Geneva, Switzerland: 1998. Animal feeding stuffs, animal products, and faeces or urine-Determination of gross calorific value- Bomb calorimeter method. Standard 9831. [Google Scholar]
- Jiang Q., Sun J., He Y., Ma Y., Zhang B., Han Y., Wu Y. Hydroxychloride trace elements improved eggshell quality partly by modulating uterus histological structure and inflammatory cytokines expression in aged laying hens. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonchere V., Brionne A., Gautron J., Nys Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol. 2012;12:10. doi: 10.1186/1472-6793-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Rathgeber B.M., Thompson K.L., Macisaac J. Uterine fluid proteins and egg quality characteristics for 2 commercial and 2 heritage laying hen lines in response to manipulation of dietary calcium and vitamin d3. Poultry Sci. 2013;92:2419–2432. doi: 10.3382/ps.2012-02983. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy N., Kurzrock R. Targeting the wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha G., Diep T., Hudson H.A., Hincke M.T. High value applications and current commercial market for eggshell membranes and derived bioactives. Food Chem. 2022;382 doi: 10.1016/j.foodchem.2022.132270. [DOI] [PubMed] [Google Scholar]
- Li L.L., Liu Z.P., Liu C.A., Elnesr S.S., Guo S.S., Ding B.Y., Zou X.T. Research note: disturbance of intracellular calcium signal in salpingitis simulation of laying hens. Poultry Sci. 2023;102 doi: 10.1016/j.psj.2022.102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lin X., Zhou X., Wan D., Wang Z., Wu X., Yin Y. Effects of dynamic feeding low and high methionine diets on egg quality traits in laying hens. Poultry Sci. 2017;96:1459–1465. doi: 10.3382/ps/pew398. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang D., Zhao L., Zhang J., Huang S., Ma Q. Effect of methionine deficiency on the growth performance, serum amino acids concentrations, gut microbiota and subsequent laying performance of layer chicks. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Wu Y., Fu M., Wu Y., Wang Z., Lin J., Yang Q. Calb1: anovel antiviral factor in chicken ileal mucus. Int J Biol Macromol. 2023;253 doi: 10.1016/j.ijbiomac.2023.127007. [DOI] [PubMed] [Google Scholar]
- Lv Z., Fan H., Gao M., Zhang X., Li G., Fan Y., Ning Z., Guo Y. The accessible chromatin landscape of lipopolysaccharide-induced systemic inflammatory response identifying epigenome signatures and transcription regulatory networks in chickens. Int J Biol Macromol. 2024;266 doi: 10.1016/j.ijbiomac.2024.131136. [DOI] [PubMed] [Google Scholar]
- Ma Z., Jiang K., Wang D., Wang Z., Gu Z., Li G., Jiang R., Tian Y., Kang X., Li H., Liu X. Comparative analysis of hypothalamus transcriptome between laying hens with different egg-laying rates. Poultry Sci. 2021;100 doi: 10.1016/j.psj.2021.101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan A.N., Webster J.D., Adams J.J., Blazer L., Everrett C., Eidenschenk C., Arlantico A., Fleming I., Brightbill H.D., Wolters P.J., Modrusan Z., Seshagiri S., Angers S., Sidhu S.S., Newton K., Arron J.R., Dixit V.M. Targeted alveolar regeneration with frizzled-specific agonists. Cell. 2023;186:2995–3012 e15. doi: 10.1016/j.cell.2023.05.022. [DOI] [PubMed] [Google Scholar]
- Nie W., Guo Y.M., Yang Y., Yuan J.M., Wang Z. Comparison of the efficacy of methionine and its hydroxy analogus in diets of laying hens. J Chin Soc Anim Sci. 2007;9:22–24. [Google Scholar]
- Nii T., Isobe N., Yoshimura Y. Effects of interleukin-1 beta and -6 on the expression of ion transporters involved in eggshell mineralization in cultured hen uterine mucosal tissue. J Poultry Sci. 2018;55:142–149. doi: 10.2141/jpsa.0170138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzold T.L., Vieira S.L., Favero A., Horn R.M., Silva C.M., Martins G.B. Manganese requirements of broiler breeder hens. Poultry Sci. 2020;99:5814–5826. doi: 10.1016/j.psj.2020.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.A., Sohn S.H. The influence of hen aging on eggshell ultrastructure and shell mineral components. Korean J Food Sci Anim Resour. 2018;38:1080–1091. doi: 10.5851/kosfa.2018.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyatos Pertinez S., Wilson P.W., Icken W., Cavero D., Bain M.M., Jones A.C., Dunn I.C. Transcriptome analysis of the uterus of hens laying eggs differing in cuticle deposition. BMC Genom. 2020;21:516. doi: 10.1186/s12864-020-06882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Sun W., Liu Y., Li Z., Han D., Cheng X., Yan J., Yang X. Dynamics of serum phosphorus, calcium, and hormones during egg laying cycle in hy-line brown laying hens. Poultry Sci. 2019;98:2193–2200. doi: 10.3382/ps/pey572. [DOI] [PubMed] [Google Scholar]
- Samiullah Chousalkar KK., Roberts J.R., Sexton M., May D., Kiermeier A. Effects of egg shell quality and washing on salmonella infantis penetration. Int J Food Microbiol. 2013;165:77–83. doi: 10.1016/j.ijfoodmicro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Shet D., Ghosh J., Ajith S., Awachat V.B., Elangovan A.V. Efficacy of dietary phytase supplementation on laying performance and expression of osteopontin and calbindin genes in eggshell gland. Anim Nutr. 2018;4:52–58. doi: 10.1016/j.aninu.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongdee K., Chanpaisaeng K., Teerapornpuntakit J., Charoenphandhu N. Intestinal calcium absorption. Compr Physiol. 2021;11:2047–2073. doi: 10.1002/cphy.c200014. [DOI] [PubMed] [Google Scholar]
- Xiao X., Wang Y., Liu W., Ju T., Zhan X. Effects of different methionine sources on production and reproduction performance, egg quality and serum biochemical indices of broiler breeders. Asian-Australas J Anim Sci. 2017;30:828–833. doi: 10.5713/ajas.16.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Cai J., Rong L., Yang S., Li S. Transcriptome identification of genes associated with uterus-vagina junction epithelial folds formation in chicken hens. Poultry Sci. 2023;102 doi: 10.1016/j.psj.2023.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang Y., Zhang C., Xiong M., Rajput S.A., Liu Y., Qi D. The differences of gonadal hormones and uterine transcriptome during shell calcification of hens laying hard or weak-shelled eggs. BMC Genom. 2019;20:707. doi: 10.1186/s12864-019-6017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Yan Q., Zou Q.Y., Zhong X.Q., Tyler C.T., Magness R.R., Bird I.M., Zheng J. Sexual dimorphisms of preeclampsia-dysregulated transcriptomic profiles and cell function in fetal endothelial cells. Hypertension. 2019;74:154–163. doi: 10.1161/HYPERTENSIONAHA.118.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang Y., Liu Y., Zeng H., Xu H., Lian F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. J Cell Biochem. 2019;120:2198–2212. doi: 10.1002/jcb.27530. [DOI] [PubMed] [Google Scholar]
- Zhou J.Y., Wang Z., Zhang S.W., Lin H.L., Gao C.Q., Zhao J.C., Yang C., Wang X.Q. Methionine and its hydroxyl analogues improve stem cell activity to eliminate deoxynivalenol-induced intestinal injury by reactivating wnt/beta-catenin signaling. J Agric Food Chem. 2019;67:11464–11473. doi: 10.1021/acs.jafc.9b04442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.