Abstract
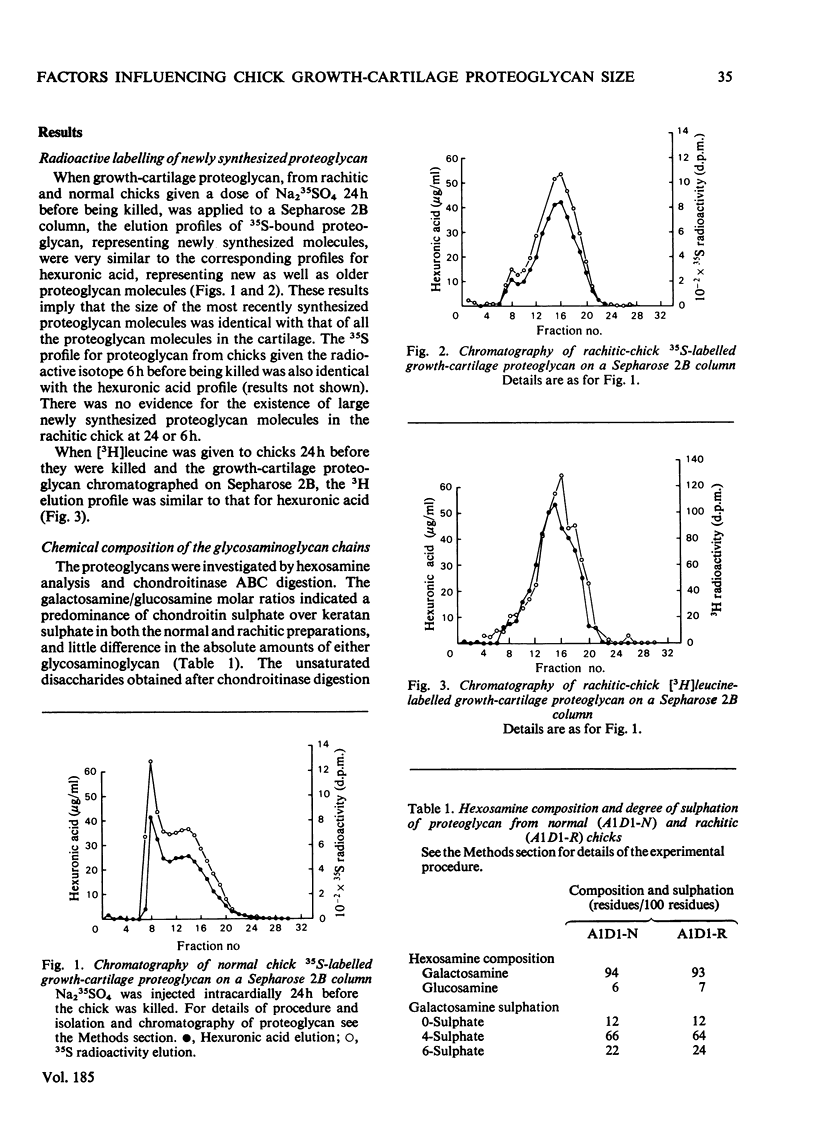
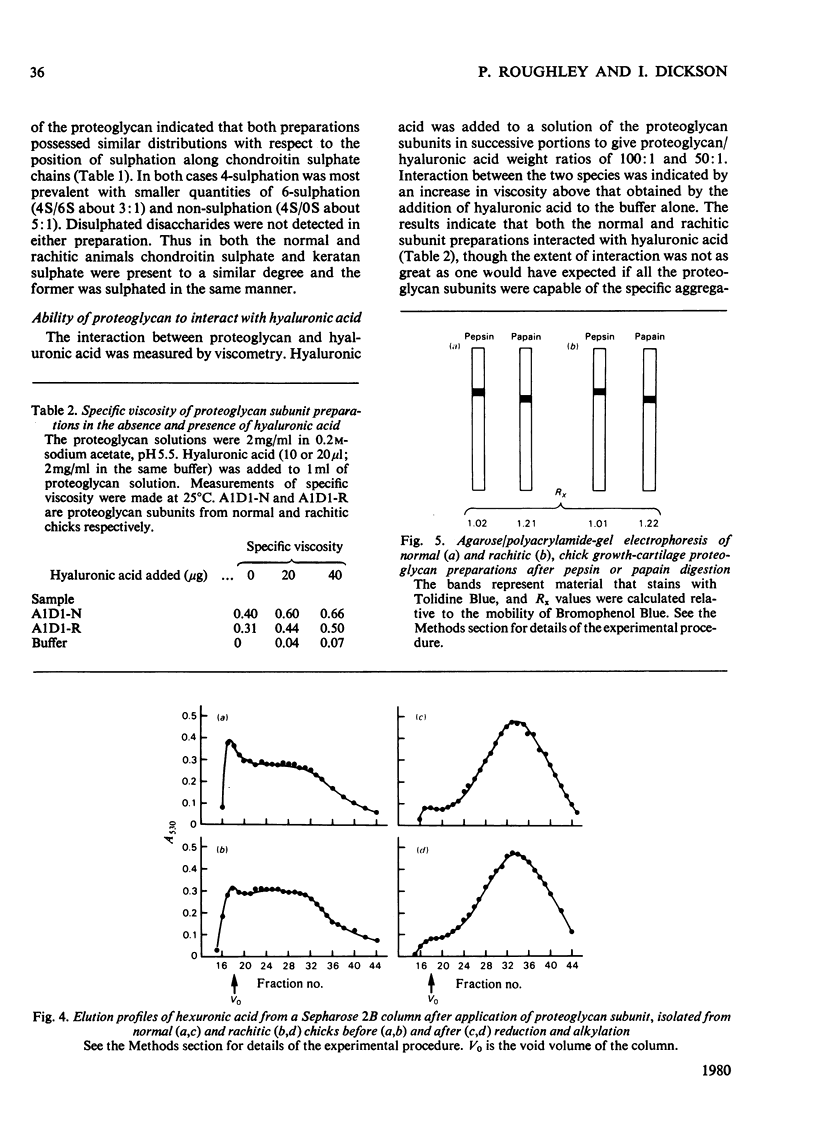
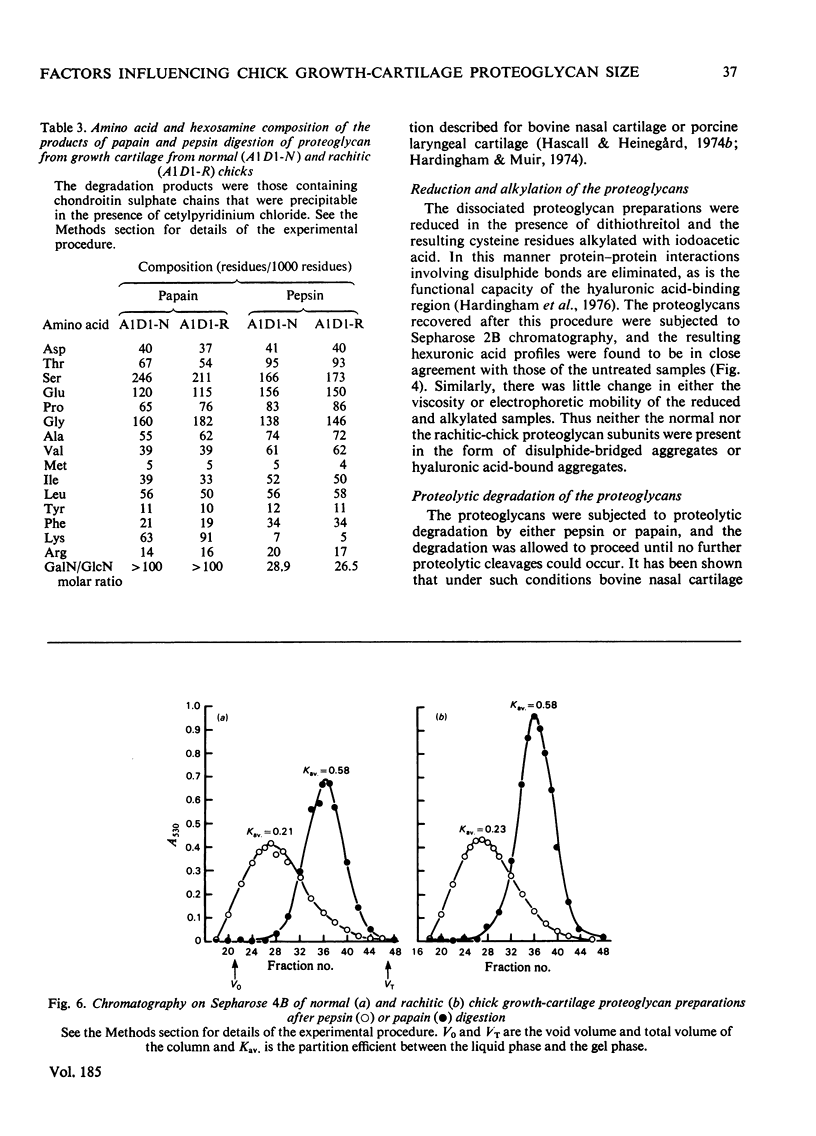
1. Proteoglycan isolated from rachitic-chick growth cartilage was of smaller size than that isolated from tissue of normal chicks. 2. The two proteoglycan populations were of similar average chemical composition and similar in the size of their chondroitin sulphate chains. 3. The size of the proteoglycans was not affected by reduction and alkylation. 4. Labelling studies in vivo with Na235SO4 and [3H]leucine suggest that the difference in size in the rachitic state results from an alteration in synthesis rather than extracellular proteolytic degradation of the normal proteoglycan, but the direct cause of this alteration remains unestablished.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Sherblom A. P. Isolation and physical characterization of hyaluronic acid prepared from bovine nasal septum by cetylpyridinium chloride precipitation. J Biol Chem. 1977 Jan 25;252(2):420–426. [PubMed] [Google Scholar]
- Dickson I. R., Roughley P. J. A comparative study of the proteoglycan of growth cartilage of normal and rachitic chicks. Biochem J. 1978 Jun 1;171(3):675–682. doi: 10.1042/bj1710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Isemura M., Ikenaka T. Beta-Elimination and sulfite addition reaction of chondroitin sulfate peptidoglycan and the peptide structure of the linkage region. Biochim Biophys Acta. 1975 Nov 10;411(1):11–21. doi: 10.1016/0304-4165(75)90280-9. [DOI] [PubMed] [Google Scholar]
- Koseki M., Kimura A., Tsurumi K. Micro determination of unsaturated disaccharide formed by the action of acidic glycosaminoglycan-endoeliminases. An application of the thiobarbituric acid method to the assay of D-gluco-4-enepyranosyluronic acid-containing disaccharides. J Biochem. 1978 Feb;83(2):553–558. doi: 10.1093/oxfordjournals.jbchem.a131943. [DOI] [PubMed] [Google Scholar]
- Roughley P. J. A comparative study of the glycosaminoglycan-peptides obtained after degradation of cartilage proteoglycan by different proteinases, and their use in the characterization of different proteoglycans. Connect Tissue Res. 1978;6(3):145–153. doi: 10.3109/03008207809152624. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan heterogeneity and the pathway of proteolytic degradation. Biochem J. 1977 Dec 1;167(3):639–646. doi: 10.1042/bj1670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno N., Anno K., Yaegashi Y., Okuyama T. Microheterogeneity of chondroitin sulfates from various cartilages. Connect Tissue Res. 1975;3(1):87–96. doi: 10.3109/03008207509152345. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman L., Ber A., Allalouf D. Use of thin-layer chromatography in the separation of disaccharides resulting from digestion of chondroitin sulphates with chondroitinases. J Chromatogr. 1977 Jun 11;136(2):342–347. doi: 10.1016/s0021-9673(00)86291-3. [DOI] [PubMed] [Google Scholar]


