Abstract
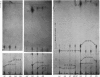
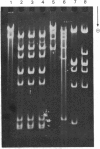
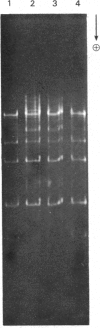
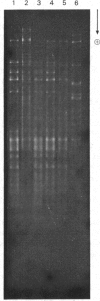
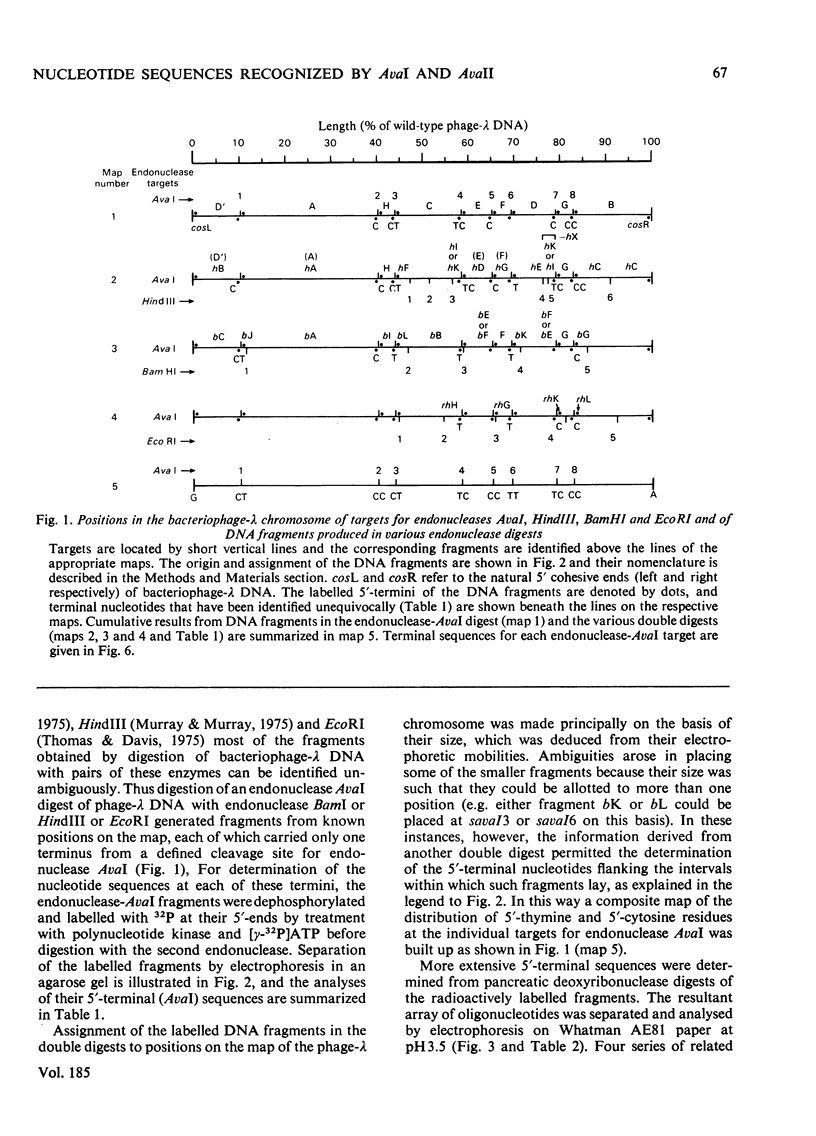
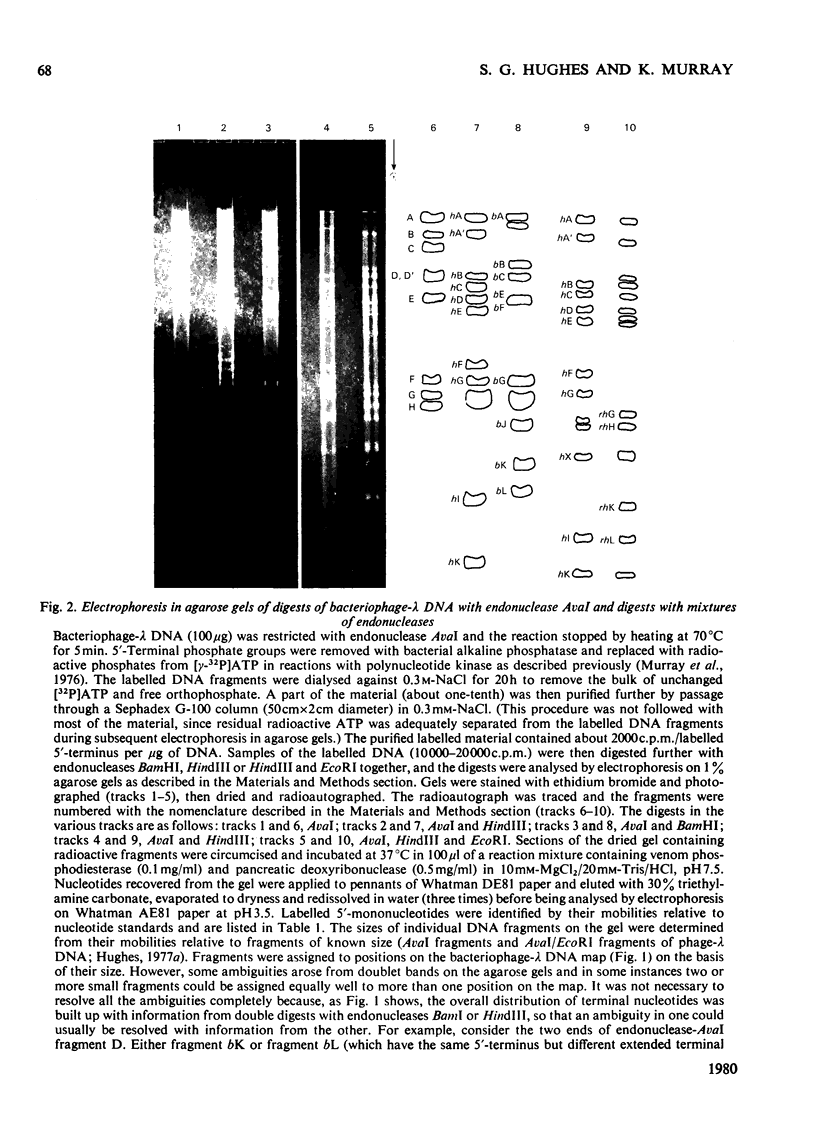
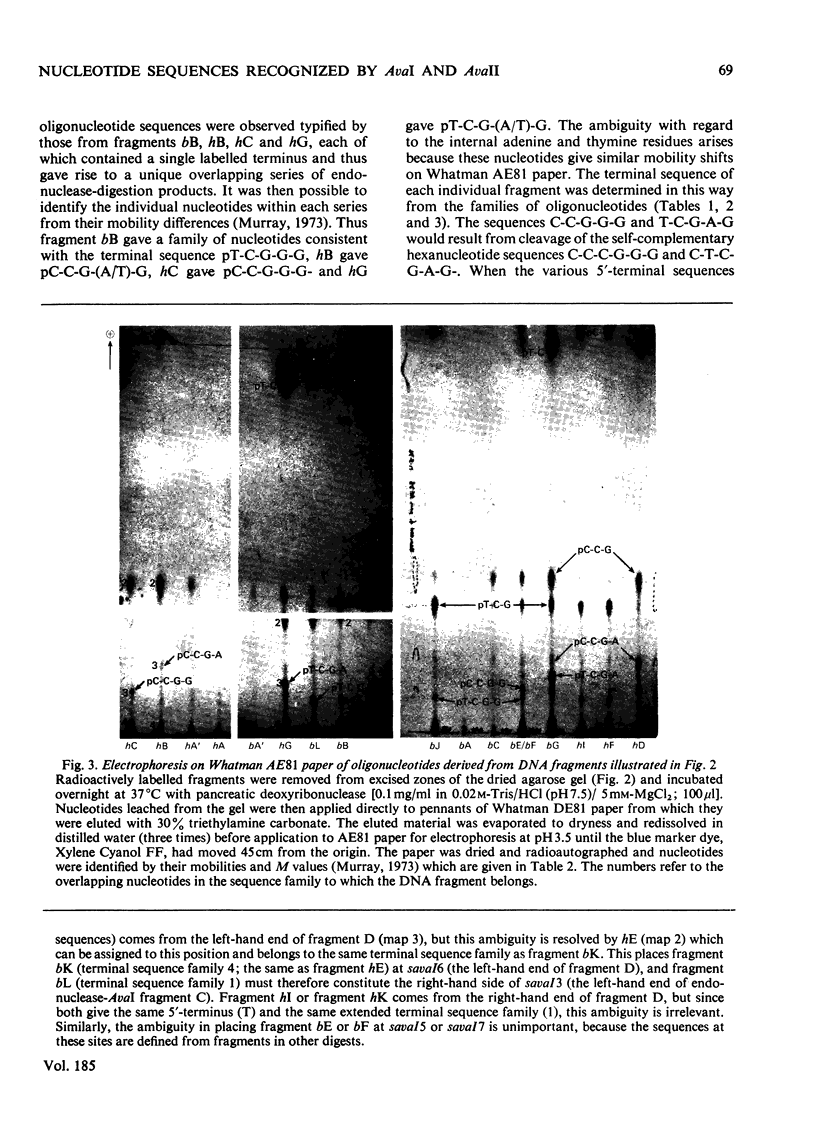
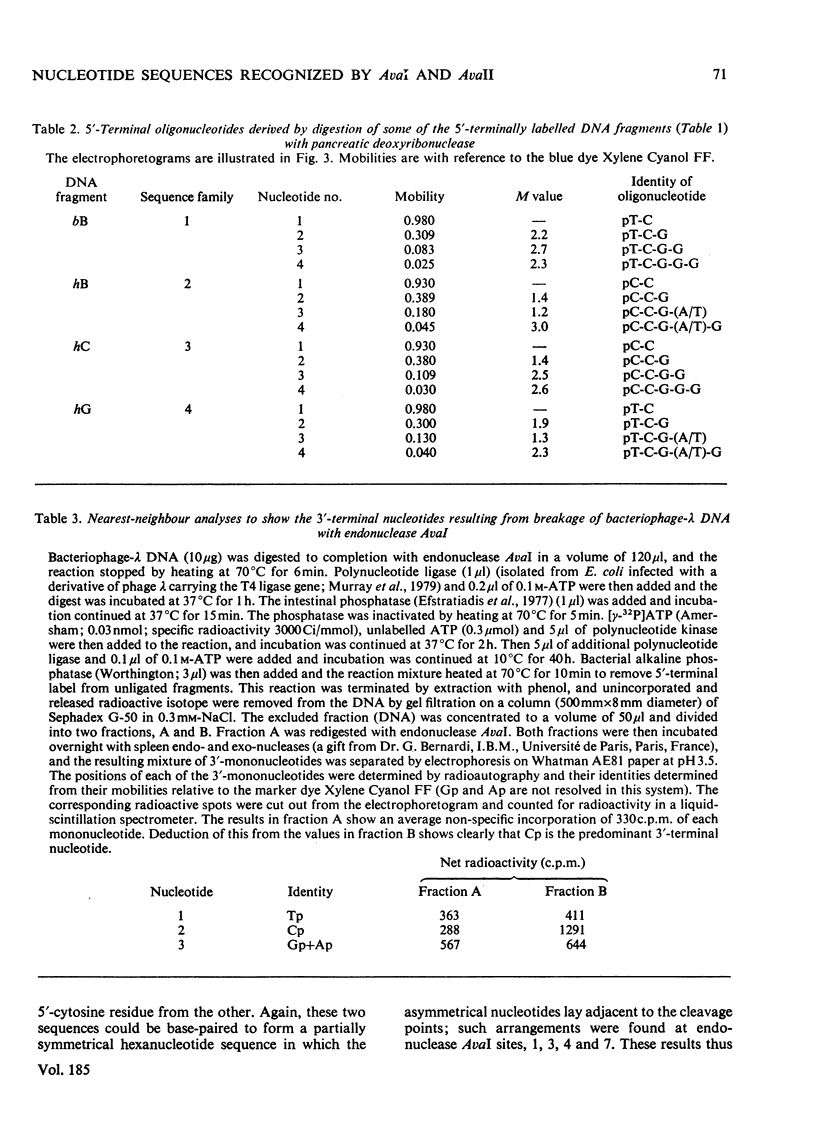
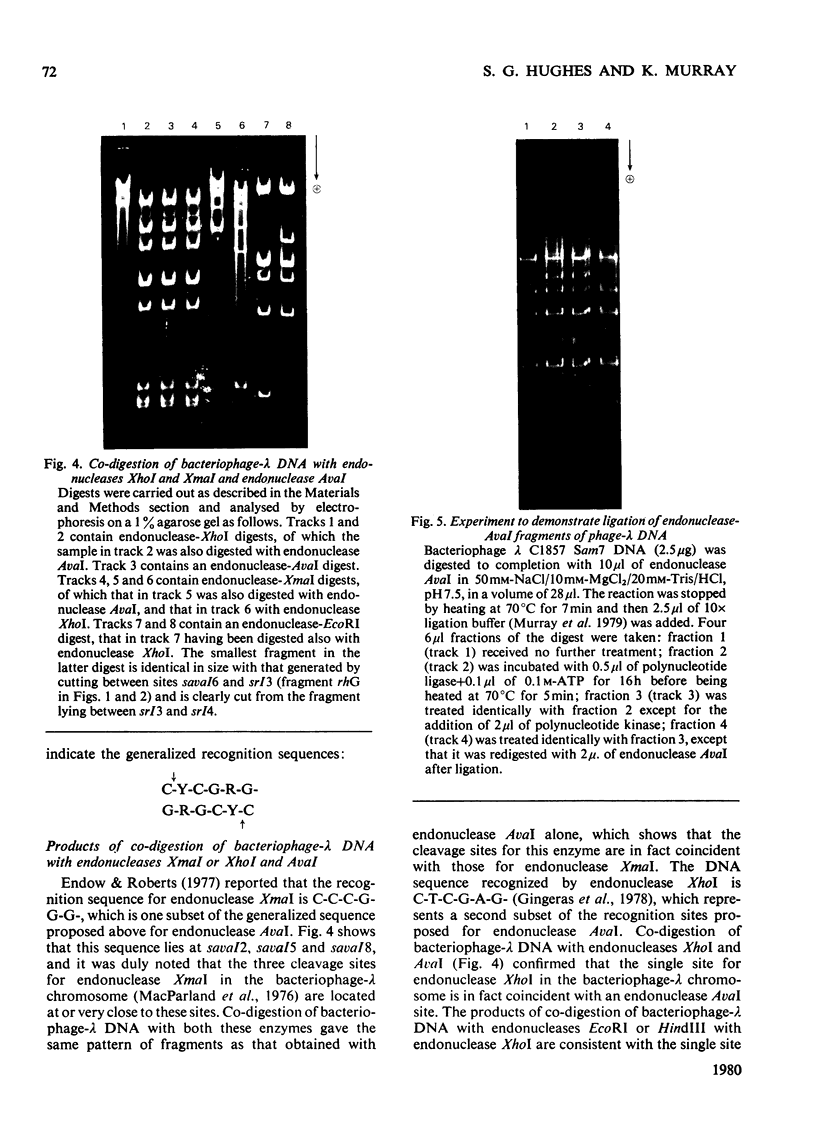
Determination of the 5'-terminal sequences flanking all the individual cleavage sites for endonuclease AvaI in bacteriophage-lambda DNA has shown that this enzyme recognizes the hexanucleotide sequences: (Formula: see text), This sequence is cut as shown by the arrows to give single-stranded 5'-tetranucleotide protrusions (cohesive ends). Endonucleases SmaI, XhoI and XmaI recognize different symmetrical subsets of this sequence and provide independent evidence for the occurrence of these subsets at particular endonuclease-AvaI cleavage sites in the bacteriophage-lambda genome. Further evidence for this structure came from the demonstration that DNA fragments generated by endonuclease AvaI can be ligated to form a discrete set of larger molecules and from nearest-neighbor analysis which showed that cytosine residues occurred at the 3'-side of cleavage points. The observation that endonuclease AvaII recognized a subset of the sites recognized by AsuI [Hughes, Bruce & Murray (1979) Biochem. J. 185, 59-63[ led to the deduction that AvaII recognize the pentanucleotide sequence: (Formula: see text), and breaks internucleotide bonds at the positions indicated by the arrows.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K. Restriction and modification in B. subtilis. Nucleotide sequence recognised by restriction endonuclease R. Bsu R from strain R. Mol Gen Genet. 1975 Dec 30;143(1):25–33. doi: 10.1007/BF00269417. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Schreier P. H., Buchel D. E. Nucleotide sequence of the attachment site of coliphage lambda. Nature. 1977 Dec 22;270(5639):757–760. doi: 10.1038/270757a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow S. A., Roberts R. J. Two restriction-like enzymes from Xanthomonas malvacearum. J Mol Biol. 1977 May 25;112(3):521–529. doi: 10.1016/s0022-2836(77)80198-8. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Myers P. A., Olson J. A., Hanberg F. A., Roberts R. J. A new specific endonuclease present in Xanthomonas holcicola, Xanthomonas papavericola and Brevibacterium luteum. J Mol Biol. 1978 Jan 5;118(1):113–122. doi: 10.1016/0022-2836(78)90247-4. [DOI] [PubMed] [Google Scholar]
- Haggerty D. M., Scheif R. F. Location in bacteriophage lamdba DNA of cleavage sites of the site-specific endonuclease from Bacillus amyloliquefaciens H. J Virol. 1976 May;18(2):659–663. doi: 10.1128/jvi.18.2.659-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heininger K., Hörz W., Zachau H. G. Specificity of cleavage by restriction nuclease from Bacillus subtilis. Gene. 1977 Jul;1(5-6):291–303. doi: 10.1016/0378-1119(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Hughes S. G. A map of the cleavage sites for endonuclease AvaI in the chromosome of bacteriophage lambda. Biochem J. 1977 Jun 1;163(3):503–509. doi: 10.1042/bj1630503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. G., Bruce T., Murray K. The isolation and characterization of a sequence-specific endonuclease from Anabaena subcylindrica. Biochem J. 1980 Jan 1;185(1):59–63. doi: 10.1042/bj1850059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McParland R. H., Brown L. R., Pearson G. D. Cleavage of lambda DNA by a site-specific endonuclease from Serratia marcescens. J Virol. 1976 Sep;19(3):1006–1011. doi: 10.1128/jvi.19.3.1006-1011.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Hughes S. G., Brown J. S., Bruce S. A. Isolation and characterization of two sequence-specific endonucleases from Anabaena variabilis. Biochem J. 1976 Nov;159(2):317–322. doi: 10.1042/bj1590317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Murray K. Nucleotide sequence analysis with polynucleotide kinase and nucleotide "mapping" methods. 5'-Terminal sequences of deoxyribonucleic acid from bacteriophages lambda and 424. Biochem J. 1973 Mar;131(3):569–582. doi: 10.1042/bj1310569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Tiollais P. Defective bacteriophage lambda chromosome, potential vector for DNA fragments obtained after cleavage by Bacillus amyloliquefaciens endonuclease (BamI). FEBS Lett. 1975 Aug 1;56(1):7–11. doi: 10.1016/0014-5793(75)80099-8. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Shimatake H., Brady C., Rosenberg M. Sequence of Cro gene of bacteriophage lambda. Nature. 1977 Nov 17;270(5634):274–275. doi: 10.1038/270274a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Church G. M. The cleavage site of the restriction endonuclease Ava II. Nucleic Acids Res. 1978 Jul;5(7):2313–2319. doi: 10.1093/nar/5.7.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]