Abstract
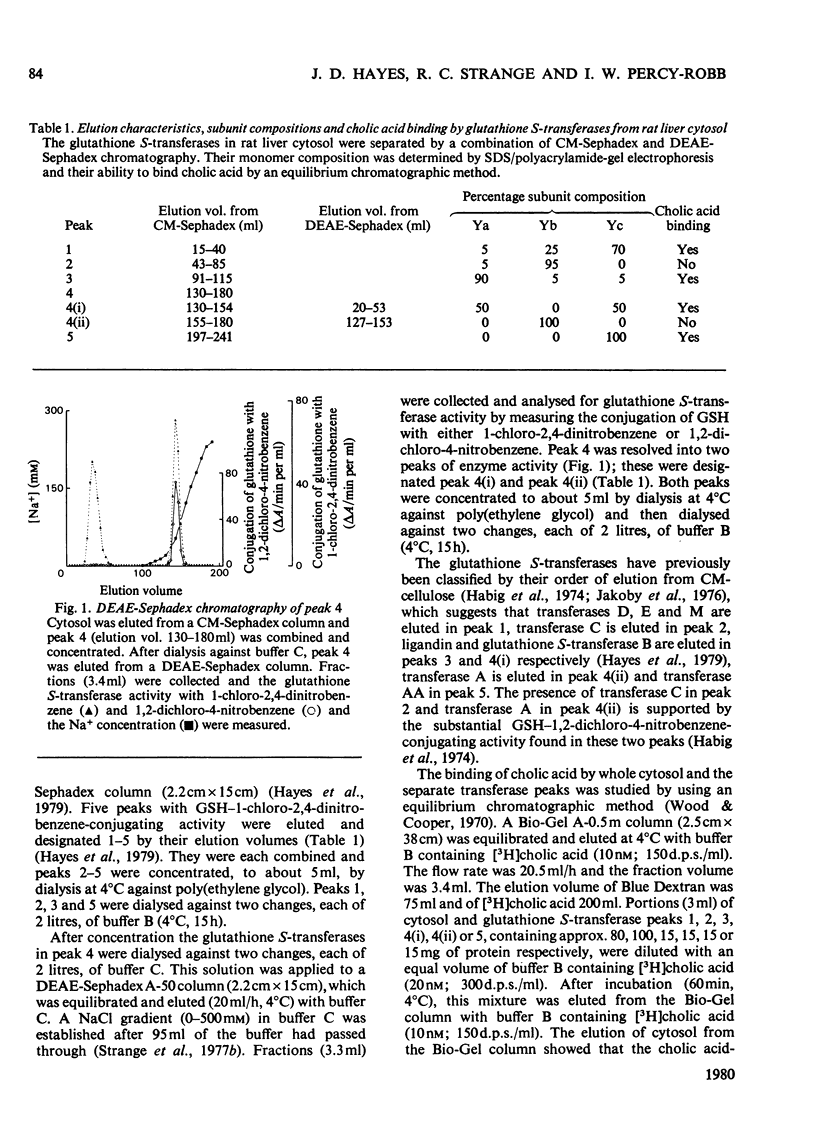
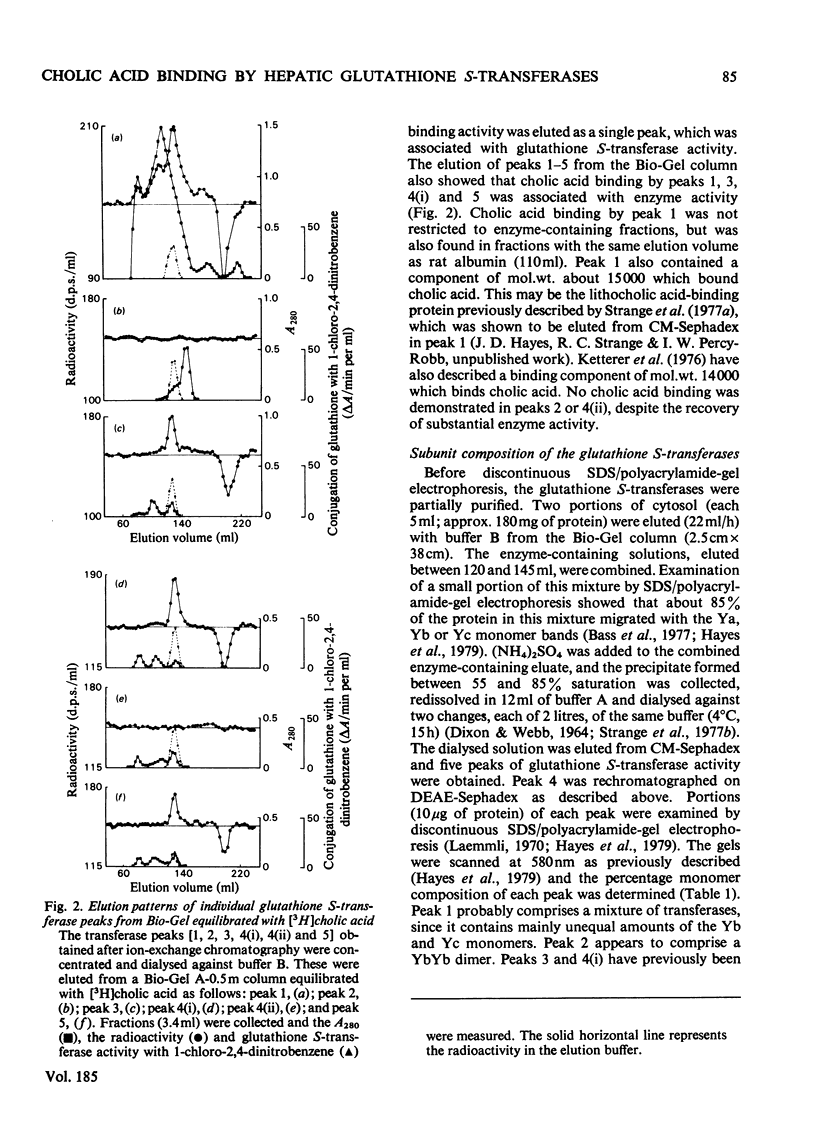
Cholic acid-binding activity in cytosol from rat livers appears to be mainly associated with enzymes having glutathione S-transferase activity; at least four of the enzymes in this group can bind the bile acid. Examination of the subunit compositions of different glutathione S-transferases indicated that cholic acid binding and the ability to conjugate reduced glutathione with 1,2-dichloro-4-nitrobenzene may be ascribed to different subunits.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Carne T., Tipping E., Ketterer B. The binding and catalytic activities of forms of ligandin after modification of its thiol groups. Biochem J. 1979 Feb 1;177(2):433–439. doi: 10.1042/bj1770433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferase AA from rat liver. Arch Biochem Biophys. 1976 Aug;175(2):710–716. doi: 10.1016/0003-9861(76)90563-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Hackney J. F., Beale D. A low-molecular-weight protein from rat liver that resembles ligandin in its binding properties. Biochem J. 1976 Jun 1;155(3):511–521. doi: 10.1042/bj1550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Chapman B. T., Johnston J. D., Nimmo I. A., Percy-Robb I. W. Partitioning of bile acids into subcellular organelles and the in vivo distribution of bile acids in rat liver. Biochim Biophys Acta. 1979 Jun 21;573(3):535–545. doi: 10.1016/0005-2760(79)90227-3. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Cramb R., Hayes J. D., Percy-Robb I. W. Partial purification of two lithocholic acid-binding proteins from rat liver 100 000g supernatants. Biochem J. 1977 Sep 1;165(3):425–429. doi: 10.1042/bj1650425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Nimmo I. A., Percy-Robb I. W. Binding of bile acids by 100 000g supernatants from rat liver. Biochem J. 1977 Mar 15;162(3):659–664. doi: 10.1042/bj1620659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. C., Cooper P. F. The application of gel filtration to the study of protein-binding of small molecules. Chromatogr Rev. 1970 Jan;12(1):88–107. doi: 10.1016/0009-5907(70)80014-x. [DOI] [PubMed] [Google Scholar]


