Abstract
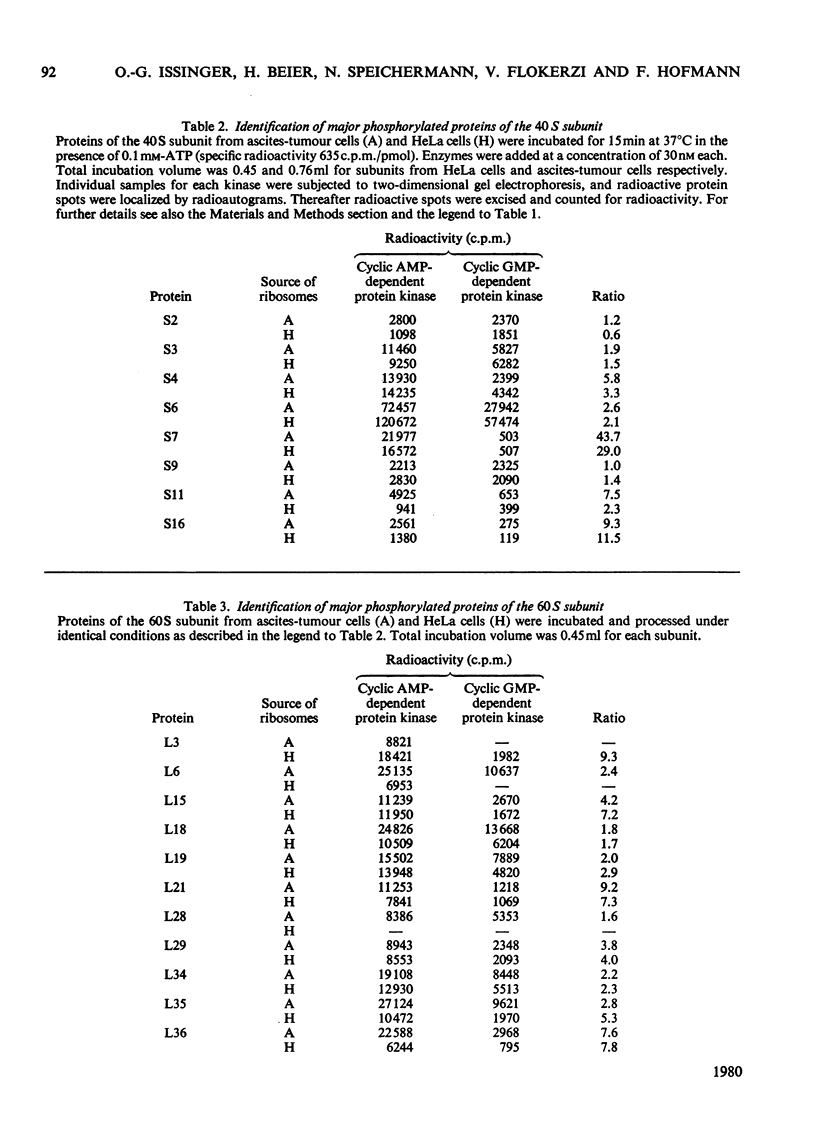
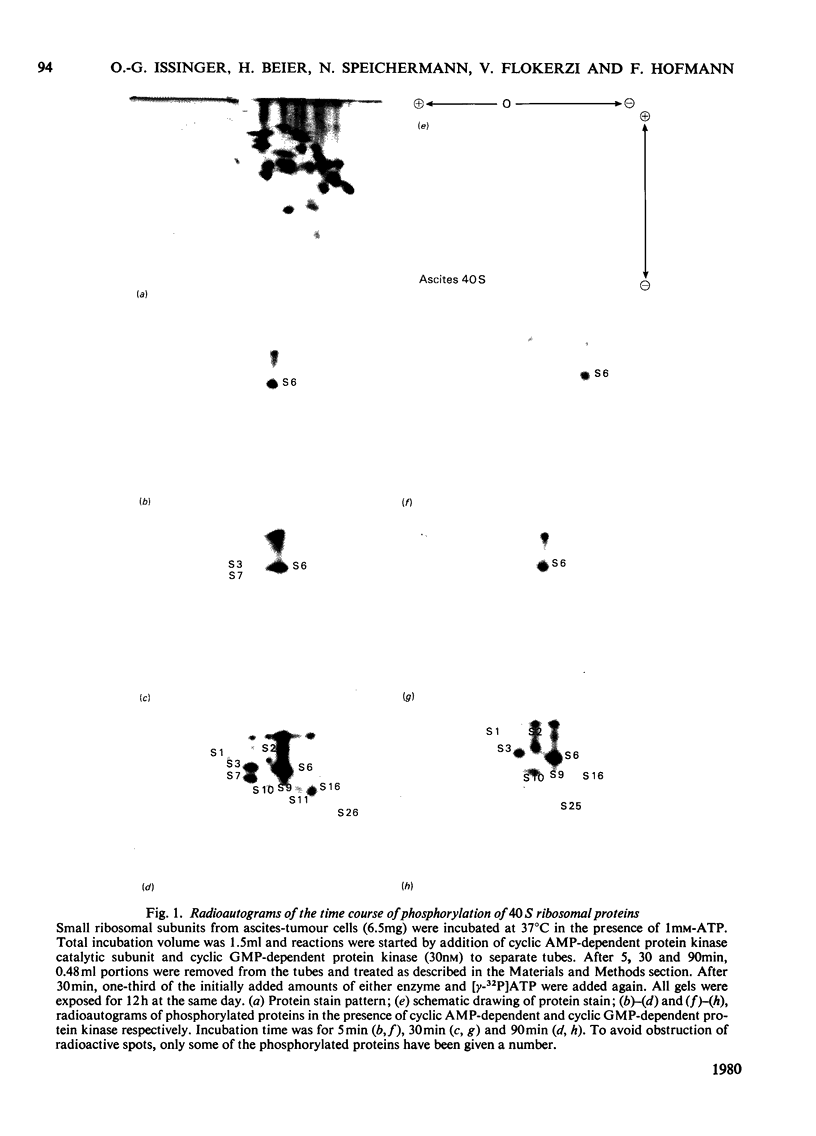
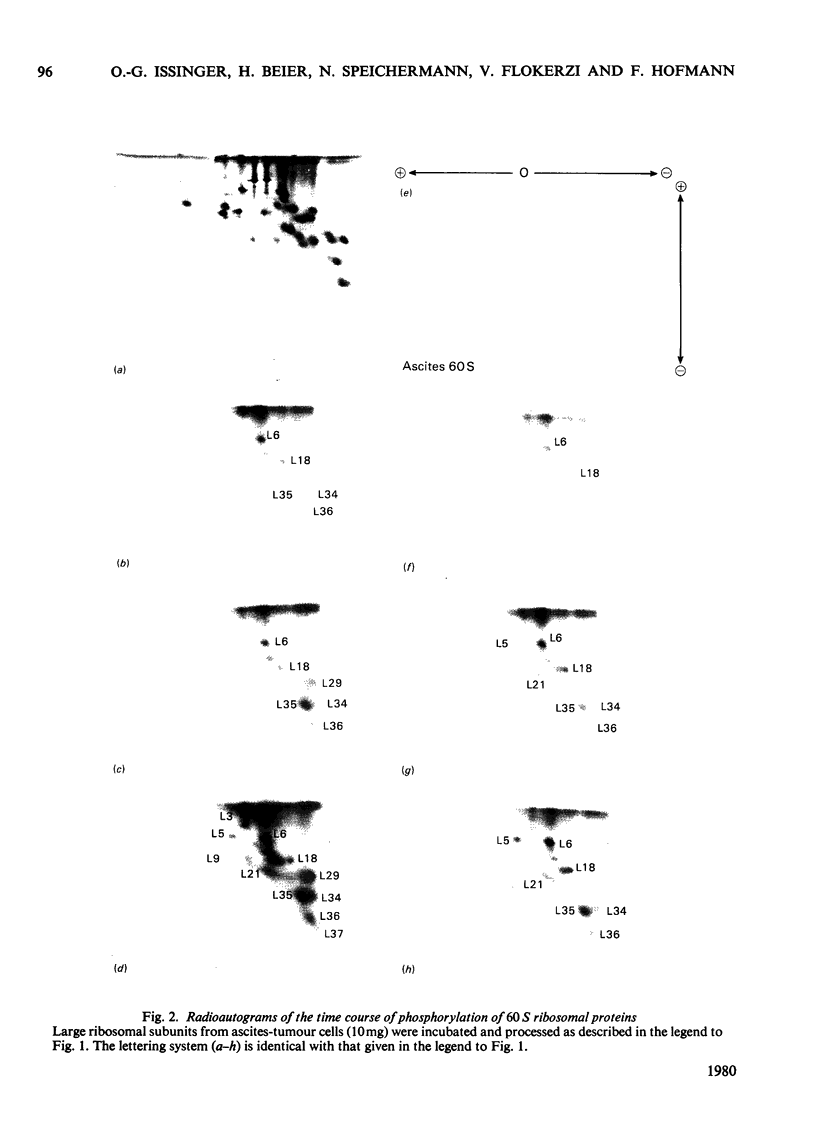
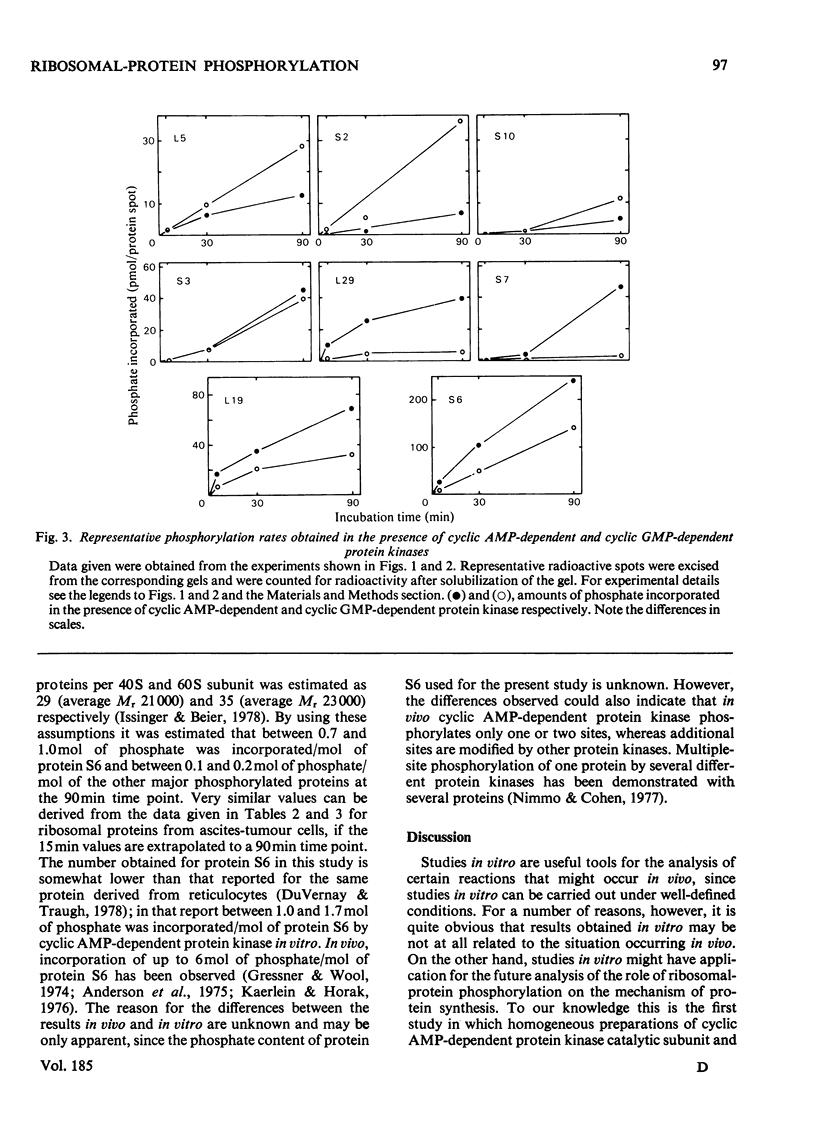
Phosphorylation of eukaryotic ribosomal proteins in vitro by essentially homogeneous preparations of cyclic AMP-dependent protein kinase catalytic subunit and cyclic GMP-dependent protein kinase was compared. Each protein kinase was added at a concentration of 30nM. Ribosomal proteins were identified by two-dimensional gel electrophoresis. Almost identical results were obtained when ribosomal subunits from HeLa or ascites-tumour cells were used. About 50-60% of the total radioactive phosphate incorporated into small-subunit ribosomal proteins by either kinase was associated with protein S6. In 90 min between 0.7 and 1.0 mol of phosphate/mol of protein S6 was incorporated by the catalytic subunit of cyclic AMP-dependent protein kinase. Of the other proteins, S3 and S7 from the small subunit and proteins L6, L18, L19 and L35 from the large subunit were predominantly phosphorylated by the cyclic AMP-dependent enzyme. Between 0.1 and 0.2 mol of phosphate was incorporated/mol of these phosphorylated proteins. With the exception of protein S7, the same proteins were also major substrates for the cyclic GMP-dependent protein kinase. Time courses of the phosphorylation of individual proteins from the small and large ribosomal subunits in the presence of either protein kinase suggested four types of phosphorylation reactions: (1) proteins S2, S10 and L5 were preferably phosphorylated by the cyclic GMP-dependent protein kinase; (2) proteins S3 and L6 were phosphorylated at very similar rates by either kinase; (3) proteins S7 and L29 were almost exclusively phosphorylated by the cyclic AMP-dependent protein kinase; (4) protein S6 and most of the other proteins were phosphorylated about two or three times faster by the cyclic AMP-dependent than by the cyclic GMP-dependent enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. M., Grundholm A., Sells B. H. Modification of ribosomal proteins during liver regeneration. Biochem Biophys Res Commun. 1975 Feb 3;62(3):669–676. doi: 10.1016/0006-291x(75)90451-9. [DOI] [PubMed] [Google Scholar]
- Barden N., Labrie F. Cyclic adenosine 3',5'-monophosphate dependent phosphorylation of ribosomal proteins from bovine anterior pituitary gland. Biochemistry. 1973 Jul 31;12(16):3096–3102. doi: 10.1021/bi00740a024. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Blat C., Loeb J. E. Effect of glucagon on phosphorylation of some rat liver ribosomal proteins in vivo. FEBS Lett. 1971 Oct 15;18(1):124–126. doi: 10.1016/0014-5793(71)80425-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon M. L., Bitte L. F., Krystosek A., Kabat D. Effect of cyclic adenosine 3':5'-monophosphate on ribosomal protein phosphorylation in reticulocytes. J Biol Chem. 1974 Jan 10;249(1):275–278. [PubMed] [Google Scholar]
- Chihara-Nakashima M., Hashimoto E., Nishizuka Y. Intrinsic activity of guanosine 3',5'-monophosphate-dependent protein kinase similar to adenosine 3',5'-monophosphate-dependent protein kinase. II. Phosphorylation of ribosomal proteins. J Biochem. 1977 Jun;81(6):1863–1867. doi: 10.1093/oxfordjournals.jbchem.a131648. [DOI] [PubMed] [Google Scholar]
- Du Vernay V. H., Jr, Traugh J. A. Two-step purification of the major phosphorylated protein in reticulocyte 40S ribosomal subunits. Biochemistry. 1978 May 30;17(11):2045–2049. doi: 10.1021/bi00604a003. [DOI] [PubMed] [Google Scholar]
- Eikenberry E. F., Bickle T. A., Traut R. R., Price C. A. Separation of large quantities of ribosomal subunits by zonal ultracentrifugation. Eur J Biochem. 1970 Jan;12(1):113–116. doi: 10.1111/j.1432-1033.1970.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Speichermann N., Hofmann F. A guanosine 3':5'-monophosphate-dependent protein kinase from bovine heart muscle. Purification and phosphorylation of histone I and IIb. J Biol Chem. 1978 May 25;253(10):3395–3399. [PubMed] [Google Scholar]
- Gill G. N., Holdy K. E., Walton G. M., Kanstein C. B. Purification and characterization of 3':5'-cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3918–3922. doi: 10.1073/pnas.73.11.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. Effect of experimental diabetes and insulin on phosphorylation of rat liver ribosomal protein S6. Nature. 1976 Jan 15;259(5539):148–150. doi: 10.1038/259148a0. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. Influence of glucagon and cyclic adenosine 3':5'-monophosphate on the phosphorylation of rat liver ribosomal protein S6. J Biol Chem. 1976 Mar 10;251(5):1500–1504. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y., Hamana K., Iwai K. Studies on the sites in histones phosphorylated by adenosine 3':5'-monophosphate-dependent and guanosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1976 Oct 25;251(20):6287–6293. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Horak I., Schiffmann D. Acidic phosphoproteins of HeLa and rat 60 S ribosomal subunits. FEBS Lett. 1977 Oct 1;82(1):82–84. doi: 10.1016/0014-5793(77)80890-9. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traut R. R. A modified two-dimensional gel system for the separation and radioautography of microgram amounts of ribosomal proteins. Methods Enzymol. 1974;30:526–539. doi: 10.1016/0076-6879(74)30052-3. [DOI] [PubMed] [Google Scholar]
- Issinger O. G., Kiefer M. C., Traut R. R. Specificity of ATP-dependent and GTP-dependent protein kinases with respect to ribosomal proteins of Escherichia coli. Eur J Biochem. 1975 Nov 1;59(1):137–143. doi: 10.1111/j.1432-1033.1975.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Issinger O. G. Phosphorylation of acidic ribosomal proteins from rabbit reticulocytes by a ribosome-associated casein kinase. Biochim Biophys Acta. 1977 Jul 15;477(2):185–189. doi: 10.1016/0005-2787(77)90234-9. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kaerlein M., Horak I. Phosphorylation of ribosomal proteins in HeLa cells infected with vaccinia virus. Nature. 1976 Jan 15;259(5539):150–151. doi: 10.1038/259150a0. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo J. C., Sperry P. J., Gill G. N., Steinberg D. Activation of hormone-sensitive lipase and phosphorylase kinase by purified cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4843–4847. doi: 10.1073/pnas.74.11.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlicki W., Grankowski N., Gasior E. Isolation and properties of two protein kinases from yeast which phosphorylate casein and some ribosomal proteins. Eur J Biochem. 1978 Mar 15;84(2):493–498. doi: 10.1111/j.1432-1033.1978.tb12191.x. [DOI] [PubMed] [Google Scholar]
- Kuo J. F. Guanosine 3':5'-monophosphate-dependent protein kinases in mammalian tissues. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4037–4041. doi: 10.1073/pnas.71.10.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastick S. M., Nielsen P. J., McConkey E. H. Phosphorylation of ribosomal protein S6 in suspension cultured HeLa cells. Mol Gen Genet. 1977 Apr 29;152(3):223–230. doi: 10.1007/BF00693074. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. Characterization of the acidic phosphorprotein of eukaryotic ribosomes using a new system of two-dimensional gel-electrophoresis. Biochim Biophys Acta. 1978 Jun 22;519(1):213–223. doi: 10.1016/0005-2787(78)90074-6. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. The phosphorylation of ribosomal proteins L14 and S3 in Krebs II ascites cells. Biochim Biophys Acta. 1978 Jun 22;519(1):224–223. doi: 10.1016/0005-2787(78)90075-8. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Rankine A. D., Coia A. A. The phosphorylation of ribosomal protein S6 in baby hamster kidney fibroblasts. Biochem Biophys Res Commun. 1976 Aug 23;71(4):966–974. doi: 10.1016/0006-291x(76)90749-x. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. Adenosine 3':5'-cyclic monophosphate- and guanosine 3':5'-cyclic monophosphate-dependent protein kinases: possible homologous proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3239–3243. doi: 10.1073/pnas.74.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. Purified cyclic GMP-dependent protein kinase catalyzes the phosphorylation of cardiac troponin inhibitory subunit (TN-1). J Biol Chem. 1978 Jan 25;253(2):337–339. [PubMed] [Google Scholar]
- Lincoln T. M., Dills W. L., Jr, Corbin J. D. Purification and subunit composition of guanosine 3':5'-monophosphate-dependent protein kinase from bovine lung. J Biol Chem. 1977 Jun 25;252(12):4269–4275. [PubMed] [Google Scholar]
- Loeb J. E., Blat C. Phosphorylation of some rat liver ribosomal proteins and its activation by cyclic AMP. FEBS Lett. 1970 Sep 24;10(2):105–108. doi: 10.1016/0014-5793(70)80427-6. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Rankine A. D., Leader D. P., Coia A. A. The phosphorylation of the ribosomal proteins of Krebs II ascites cells. Biochim Biophys Acta. 1977 Jan 20;474(2):293–307. doi: 10.1016/0005-2787(77)90203-9. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Roberts S., Ashby D. Ribosomal protein phosphorylation in rat cerebral cortex in vitro. Influence of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):288–296. [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. Two-dimensional polyacrylamide gel electrophoresis of eukaryotic ribosomal proteins. Methods Enzymol. 1974;30:506–526. doi: 10.1016/0076-6879(74)30051-1. [DOI] [PubMed] [Google Scholar]
- Stahl J., Welfle H., Bielka H. Studies on proteins of animal ribosomes. XIV. Analysis of phosphorylated rat liver ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis. FEBS Lett. 1972 Oct 1;26(1):233–236. doi: 10.1016/0014-5793(72)80580-5. [DOI] [PubMed] [Google Scholar]
- Takai Y., Nishiyama K., Yamamura H., Nishizuka Y. Guanosine 3':5'-monophosphate-dependent protein kinase from bovine cerebellum. Purification and characterization. J Biol Chem. 1975 Jun 25;250(12):4690–4695. [PubMed] [Google Scholar]
- Traugh J. A., Porter G. G. A comparison of ribosomal proteins from rabbit reticulocytes phosphorylated in situ and in vitro. Biochemistry. 1976 Feb 10;15(3):610–616. doi: 10.1021/bi00648a025. [DOI] [PubMed] [Google Scholar]
- Treloar M. A., Treloar M. E., Kisilevsky R. Ethionine and the phosphorylation of ribosomal protein S6. J Biol Chem. 1977 Sep 10;252(17):6217–6221. [PubMed] [Google Scholar]
- Ventimiglia F. A., Wool I. G. A kinase that transfers the gamma-phosphoryl group of GTP to proteins of eukaryotic 40S ribosomal subunits. Proc Natl Acad Sci U S A. 1974 Feb;71(2):350–354. doi: 10.1073/pnas.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]




