Abstract
The problem of veterinary drug residues in black-feathered silky fowl (BFS) has garnered significant attention. This study investigated the residue elimination patterns and causes of enrofloxacin (ENR), sulfachloropyrazine sodium (SPZ), and doxycycline (DOX) in BFS using ultra-high performance liquid chromatography mass spectrometry (UPLC-MS), along with the drug adsorption and melanin binding tests. The findings indicated that ENR and SPZ were metabolized slowly, DOX was metabolized faster in BFS, with optimum withdrawal time was 87.8, 37.26, and 24.7 days, respectively. The adsorption capacity of skin + fat to drugs and the binding capacity of melanin to the three drugs in BFS were in the order of ENR > SPZ > DOX, confirming that melanin's binding ability influences the drug residues in BFS. This study is highly significant for addressing the issue of veterinary drug residues in BFS.
Keywords: Black-feathered silky fowl, Enrofloxacin, Sulfachlorpyrazine sodium, Doxycycline, Withdrawal time, Melanin, Mass spectrometry
Highlights
-
•
Investigating the specific elimination pattern of drugs in the black-feathered silky fowl.
-
•
Clarified the withdrawal time of three drugs in black-feathered silky fowl.
-
•
The skin + fat of the black-feathered silky fowl has high affinity for drugs.
-
•
The high affinity of skin + fat for drugs is associated with its richness in melanin.
-
•
Melanin-drug interactions affect the rate of drug metabolism.
1. Introduction
Gallus gallus domesticus Brisson is a unique bird with medicinal, culinary, and decorative properties. It originated in Jiangxi Province, China, and has a long history of domestication in the country. Feather coloration may be categorized into two phenotypes: white-feathered silky fowl and black-feathered silky fowl (BFS). The quintessential breed of white-feathered silky fowl is the Taihe black-boned chicken, whereas the emblematic breeds of BFS include the black Phoenix chicken, the Yugan black-boned chicken, and the Xuefeng black-boned chicken. The black phoenix chicken, often referred to as BFS and dubbed “China's black treasure,” is a valuable medicinal chicken noted as “superior in medicine” in the “Compendium of Materia Medica” from the Ming Dynasty (Wu, 1999). Flesh is rich in melanin, which effectively tonifies the liver and kidneys, enhances qi and blood, increases immunity, mitigates aging, and eliminates free radicals (Chu et al., 2022). Furthermore, melanocytes can chelate metal ions and tiny molecular drugs. (Karkoszka et al., 2024). Recent research on BFS has focused primarily on its edible and medicinal properties (Pan et al., 2016), nutritional and bioactive constituents (Xiong et al., 2024), and meat quality (Yang et al., 2024). Concerns regarding quality and safety have arisen due to the recurrent incidence of excessive veterinary drug residues in BFS in recent years. In China, the primary consumers of BFS are mainly pregnant women, children, and elderly individuals. Consequently, its quality and safety are crucial to public health.
Data from China's State Administration for Market Regulation indicate that 305 batches of BFS had high veterinary drug residues between 2016 and 2021. Fluoroquinolone residues constitute 61.0 % of the total veterinary durg residuals, sulfonamides represent 36.1 %, tetracyclines constitute 2.7 %, and mephedrone accounts for 13.8 % (Shi Antong Website, 2021). Fluoroquinolones and sulfonamides are the principal pharmaceuticals susceptible to surpassing veterinary durg residue limits in China's commercially marketed BFS. The excessive accumulation of antimicrobials in animal food enters the human body via the food chain, resulting in various adverse reactions, including skin sensitization, gastrointestinal and respiratory damage, neurotoxicity, and the transfer of antibiotic resistance between multidrug-resistant and commensal bacteria, potentially disrupting the intestinal microbiota and jeopardizing human health (de Mesquita Souza Saraiva et al., 2022). Consequently, elucidating the elimination pattern of drug residues in animals and the optimal withdrawal time is essential for safeguarding food safety for the public.
Enrofloxacin(ENR), sulfachloropyrazine sodium(SPZ), and doxycycline(DOX) are fluoroquinolones, sulfonamides, and tetracyclines, respectively, that are often utilized in poultry farming and demonstrate significant therapeutic efficacy against respiratory and digestive disorders in chickens. In China “GB, 31650–2019” (Chinese Ministry of Agriculture and Rural Affairs, 2019) determined the maximum residual limits for antibiotics in broilers. The maximum residue limits(MRLs) for ENR and its metabolite ciprofloxacin(CIP) are set at 100 μg/kg for poultry muscle, 100 μg/kg for skin + fat, 200 μg/kg for liver, and 300 μg/kg for kidney. The MRLs for sulfonamides are 100 μg/kg across muscle, skin + fat, liver, and kidney. The MRLs for DOX are 100 μg/kg for muscle, 300 μg/kg for skin + fat, 300 μg/kg for liver and 600 μg/kg for the kidney. This standard aligns with EU MRLs. Establishing the antibiotic withdrawal time based on the MRLs in various tissues of animal products is essential for guaranteeing the safe application of veterinary antibiotics in agriculture. The 2020 edition of the Chinese Veterinary Pharmacopoeia (Commission of Chinese Veterinary Pharmacopoeia, 2020) prohibits the use of soluble ENR powder in Gallus gallus domesticus Brisson, because of its sluggish metabolism in white-feathered silky fowl. The withdrawal time of SPZ in chickens is 1 day, and the withdrawal time of DOX is 28 days. Except for ENR, the withdrawal time standards for the other two drugs are exclusively established for broilers, and their applicability to BFS requires further research.
Certain studies have determined that fluoroquinolones undergo slow metabolism in BFS (Chen et al., 2023), which may be attributable to their melanin-rich physiology. Prior studies on antimicrobial residues elimination in Gallus gallus domesticus Brisson have focused predominantly on white-feathered silky fowl, with fewer research on the elimination patterns of ENR, SPZ, and DOX in BFS. This study sought to investigate the withdrawal time of ENR, SPZ, and DOX in BFS and to evaluate the affinities of these three drugs in the tissues of BFS and white-feathered broilers via in vitro tissue adsorption assays. This study also sought to investigate the fundamental reason for sluggish durgs metabolism in BFS through in vitro binding experiments. The aforementioned research aims to elucidate the primary factors contributing to the elevated residue risk of BFS products, provide data to substantiate the safe application of ENR, SPZ, and DOX in BFS, and guarantee the quality and safety of their goods on the basis of scientific evidence.
2. Materials and methods
2.1. Reagents and materials
ENR (purity = 99.5 %), CIP (84.2 %), SPZ (99.8 %), and DOX (84.7 %) were acquired from the China Institute of Veterinary Drug Control. First, 10 % ENR soluble powder and 30 % sulfachloropyrazine sodium soluble powder were acquired from Hefei Zhonglong Shenli Animal Pharmaceutical Co., Ltd. Doxycycline hydrochloride (50 %) soluble powder was procured from Guangzhou Zhongguan Animal Pharmaceutical Co. Deionized water was produced using a Milli-Q system (Millipore et al.). High-performance liquid chromatography (HPLC)-grade methanol, acetonitrile, formic acid, and ethyl acetate were procured from Fisher (Fisher, USA). Analytical-grade of sodium dihydrogen phosphate monohydrate (H4NaO5P), disodium phosphate dodecahydrate (Na2HPO4), and citric acid monohydrate (C6H10O8) were procured from Tianjin Komeo Chemical Reagent Co., Ltd. (Tianjin, China). The Solid-phase extraction mixture (Oasis HLB, 200 mg, 6 mL) and hydrophilic polypropylene filter membrane (0.22 μm) were acquired from Waters (Waters, USA).
2.2. Animals
This work adhered to applicable animal welfare and ethical norms and received approval from the Science and Technology Ethics Committee of Ningxia University. Three hundred sixty 1-day-old BFS, consisting of equal numbers of males and females, were obtained from the Jinling Agricultural and Animal Husbandry Group in Guangxi. Before the commencement of the study, the BFS were nourished and acclimatized for 35 days. The ambient temperature was maintained at 25 ± 5 °C, with relative humidity ranging from 50 % ∼ 60 %. The diet was specifically designed to fulfill the nutritional needs of BFS, and no antimicrobial agents were utilized during the feeding duration.
2.3. Instrumental conditions
The samples were examined with an ACQUITY UPLC-Xevo TQ-S micro (Waters, USA) apparatus. The separation was conducted using an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm × 1.7 μm) at a column temperature of 40 °C. mobile phase A consisted of acetonitrile, while mobile phase B consisted of a 0.1 % aqueous solution of formic acid by volume fraction. A gradient elution protocol was implemented: 0 min, 5 % A; 0.5 min, 75 % A; 3.0 min, 10 % A;and 5.0 min,10 % A. The chromatographic analysis had a total run duration of 5.0 min, with a mobile phase flow rate of 0.3 mL/min and an injected sample volume of 1 μL. An electron spray ionization positive ion source (ESI+) and multiple response monitoring (MRM) were employed for detection. The ion source temperature was set at 100 °C, the atomization temperature at 450 °C, the ionization voltage at 3.0 kV, the gas flow rate via the cone hole at 30 L/h, and the atomizer flow rate at 1000 L/h.
2.4. Pharmaceutical residue experiment
2.4.1. Animal experiment
Following acclimatization, three hundred 35-day-old BFS, with an average weight of 0.95 ± 0.15 kg, were randomly selected for the research. Their health was evaluated, and they were then categorized into four groups. The thirty BFS in the control group received no medicine but had free access to water and frequent nourishment. Three experimental groups were established, each including ninety BFS, which were evenly distributed between male and female participants. The dosage given was the highest clinically advised amount specified in the “Veterinary Pharmacopeia of the People's Republic of China (2020 Edition)”, and the durgs were administered free access to drinking water. In experiment group I, ENR soluble powder was incorporated into the drinking water at a concentration of 75 mg/L daily, resulting in an ENR intake of 15 mg/kg body weight per day, which was administered continuously for five days. In experimental group II, Sulfachloropyrazine sodium soluble powder was added to the drinking water at 300 mg/L daily, equating to an SPZ intake of 60 mg/kg body weight per day, which was administered continuously for three days. In experimental group III, doxycycline soluble powder was introduced into the drinking water at 300 mg/L daily, corresponding to a DOX intake of 60 mg/kg body weight per day, which was administered continuously for five days. Muscle, liver, kidney, and skin + fat tissues were harvested from BFS at 4 h and at 1, 3, 5, 7, 10, 14, 20, 26, 35, 47, 58, 67, 74, and 92 days after drug treatment. Each experimental time group had six BFS (three males and three females), which underwent cervical dislocation for execution, Each sample was homogenized separately and stored at −20 °C until analysis.
2.4.2. Preparation of tissue samples
Drug residue analysis and quality control were performed using high-performance liquid chromatography-tandem mass spectrometry MOA (Ministry of Agriculture of the People's Republic of China) (2021), with modifications. Exactly 1 g (±0.01 g) of homogenized muscle, liver, kidney, skin + fat samples of BFS was weighed and transferred into 50 mL polypropylene centrifuge tubes, followed by the addition of 8 mL of Mcllvaine-Na2EDTA buffer. The mixture was vortexed, shaken vigorously, and sonicated for 20 min at 4 °C. Centrifugation was then performed for 5 min at 4 °C and 8000 rpm, and the supernatant was collected. The residue was further treated with 8 mL of phosphate buffer, vortexed, sonicated for 20 min, and centrifuged again at 4 °C and 12,000 rpm for 5 min. Both supernatants were combined, and the mixture was centrifuged at 4 °C and 15,000 rpm for 5 min, followed by purification. The solid phase extraction column (200 mg/6 mL) was conditioned with 5 mL of methanol and 5 mL of water. The supernatant was washed with 5 mL of water and 5 mL of 20 % methanol in water (1:5, V/V). Then, 10 mL of eluent (methanol: ethyl acetate:ammonia, 5:5:0.02, V/V/V) was passed through the column, and the eluate was collected in a 15 mL centrifuge tube, which was evaporated under nitrogen in a water bath at 45 °C. The residue was dissolved in 1.0 mL of a solution (methanol: acetonitrile: formic acid, 5:5:0.01, V/V/V), vortexed thoroughly, and centrifuged at 15000 rpm for 3 min. The supernatant was filtered through a 0.22 μm microporous hydrophilic and lipophilic polypropylene filter membrane and analyzed by UPLC-MS/MS. The instrumental conditions, analysis, and validation of the methodologies can be found in the supporting information section.
2.4.3. Data analysis
The withdrawal time was determined on the basis of the MRLs for chickens in the GB 31650–2019 National Standard for Food Safety Maximum Residue Limits of Veterinary Drugs in Foods. WT 1.4 software developed by the European Medicines Agency (EMA) was used to calculate the withdrawal time of the drugs in each tissue of the BFS. The 95 % tolerance limit of the population percentage at the 95 % confidence level was considered in the estimation of the withdrawal time. Statistical analysis for the calculation of means, standard deviations and ANOVA was performed via SPSS software (version 26; IBM, Armonk, NY, USA). Comparisons were considered statistically significant at the P < 0.05 level.
2.4.4. Analytical methodology and validation
Method validation was conducted on blank muscle, liver, kidney, and skin + fat of BFS. The matrix-matched standard curves spanned from 2 to 100 μg/L, with correlation coefficients (R2) computed; the limit of detection was defined as a signal-to-noise ratio S/N ≥ 3, whereas the limit of quantification was defined as S/N ≥ 10. The four tissues of BFS were spiked with standard working solutions of ENR, CIP, SPZ, and DOX at concentrations of 10.0, 50.0, and 100.0 μg/kg, respectively, and subsequently subjected to the sample preparation and analytical procedures for measurement using UPLC-MS/MS. Five samples were tested in parallel, and the recoveries and the relative standard deviations were investigated by repeating the tests for three days. All four drugs were quantified using external standard methods.
2.5. In vitro tissue adsorption assay
2.5.1. Tissue preparation
This work used and refined the in vitro tissue adsorption test as delineated by Högger (2001) and Chen et al. (2023). Six 35-day-old BFS (0.95 ± 0.15 g) and white-feathered broilers (1.8 ± 0.15 g) were chosen; none of the experimental chickens were administered ENR, SPZ, or DOX before slaughter. The experimental chickens were euthanized via spinal dislocation, and fresh skin + fat, muscle, and liver samples were promptly harvested on ice and sectioned into two mm pieces via sterilized scissors. These samples were initially rinsed in anhydrous ethanol and subsequently immersed in a PBS solution (pH = 7.4). Standard ENR, SPZ, and DOX solutions were combined with serum-containing media (10 % fetal bovine serum, 90 % DMEM) to achieve drug cultures at a concentration of 1 mg/L. A 4 g sample of each tissue was collected for the experiment. The tissue pieces were positioned in a plastic filter and subsequently transferred to a 50 mL centrifuge tube, to which 8 mL of the previously prepared drug culture solution was added. The samples were next subjected to oscillatory incubation at 41 °C, with 0.2 mL of the culture medium being extracted at 5, 15, and 30 min, as well as at 1, 2, 3, 4, 6 h, and 12 h. Following a 12 h period, the tissue pieces were removed from the growth media and preserved at −20 °C.
2.5.2. Pre-treatment of culture fluid
This pre-treatment test for the culture broth refers to the pre-treatment method outlined by Bahrpeyma et al. (2022). To conduct the test, dilute 0.2 mL of the culture medium sample with 0.8 mL of 0.1 % formic acid in a 25 % acetonitrile aqueous solution. The mixture was vortexed for 30 s and then incubated at 55 °C in a water bath for 15 min. The mixture was centrifuged at 15000 rpm for 5 min, and 0.5 mL of the supernatant was removed for UPLC-MS/MS analysis.
2.5.3. Pre-treatment of tissue samples
For pre-treatment of the tissue samples, skin + fat, muscle, and liver samples were homogenized. Subsequently, 1 g of homogenized tissue was transferred to a 50 mL centrifuge tube and subjected to the sample pre-treatment protocol outlined in the drug residue assay for analysis by UPLC-MS/MS.
2.5.4. Validation of the analytical methods
Standard samples at low, medium, and high concentrations (20, 60, and 100 μg/mL) were created by incorporating standard solutions of ENR, SPZ, and DOX, including blank serum, into the cell culture medium. The treatment was conducted following the experimental pre-treatment protocol for UPLC-MS/MS analysis, with six parallel samples prepared for each concentration.
2.6. Experiment on melanin binding
2.6.1. Extraction of melanin
Melanin was extracted using the methods of Pescina et al. (2012). Six BFS (males, 60 days old, 2.2 ± 0.15 kg) were taken and killed by dislocation of the cervical vertebrae. One gram of each of the skin+fat, testis, and muscle tissues (to make three parallel samples) of BFS were taken on ice and cut into pieces with scissors, immersed in 3 mL of PBS (pH = 8.0) and 0.215 mL of trypsin type I solution was added to PBS (pH = 8.0) to achieve a final enzyme activity of 1000 USP/mL. The mixture was incubated in a constant-temperature incubator at 37 °C with low-speed oscillation for 2 h and then heated to 100 °C to inactivate the enzyme for 30 min. The enzymatic process was repeated a second time. Finally, the suspension was vacuum-filtered through filter paper on a Büchner funnel. PBS (pH = 8.0) was used as the washing solution, and the filtered suspension was centrifuged at 12,000 rpm for 15 min to isolate the melanin pellet. The residue was vacuum freeze-dried and then frozen at −20 °C until use.
2.6.2. Melanin-drug binding experiments in vitro
The in vitro melanin-drug binding assay was optimized via the procedure described by Ono and Tanaka (2003). The extracted melanin was prepared a 50 μg/mL melanin suspension with phosphate buffer (pH 7.4), and the same phosphate buffer (pH 7.4) was used to prepare ENR, SPZ, and DOX as 5, 10, 30, and 50 μg/mL drug suspensions; 2 mL of the prepared melanin series of suspensions was mixed with 2 mL of different concentrations of drug suspensions and incubated at 37 °C with shaking for 16 h. At the end of the incubation, the samples were centrifuged at 15000 rpm for 15 min at 0 °C. A control group with different concentrations of drugs and a blank melanin control group were set up. The determination of drug concentration was carried out via a UV spectrophotometer (UV2600). The amount of drug adsorbed by melanin was calculated as the difference between the amount initially added (the concentration of the drug in the control samples) and the amount remaining in the supernatant, and the data were analyzed on the basis of the average of three determinations.
2.6.3. Data analyses
Manzanares et al. (2016) reported that the interaction of a drug with melanin may be examined under the premise that this interaction resembles the adsorption of a drug onto a solid and adheres to the type I Langmuir isotherm. Overall, there is a correlation between Qe, the amount of drug bound per milligram of melanin, and Ce, the concentration of free drug.
| (1) |
qmax is the maximum number of moles of melanin bound per milligram, which is necessary for the formation of a monomolecular layer, whereas KL is a constant associated with the affinity or strength of the interaction. The equation in (1) was transformed into (2):
| (2) |
This indicates that a linear correlation should be established when 1/qmax is shown as a function of 1/Ce. The extrapolated y-intercept is 1/qmax, and the slope is 1/qmax KL. Consequently, the analysis of the binding data facilitates the determination of qmax and KL for each pharmaceutical compound, establishing a foundation for comparing drug affinity to melanin (Ono & Tanaka, 2003).
3. Results and discussion
3.1. Method validation
Method validation was performed on blank muscle, liver, kidney, and skin + fat of BFS. The limit of detection (LOD) for the four drugs was 2 μg/mL, and the limit of quantification (LOQ) was 10 μg/kg in each tissue, which was based on chromatographic signals with a signal-to-noise ratio of S/N of ≥3 and (S/N) of ≥10. The drugs and their metabolites exhibited strong linear correlations within the range of 2 μg/L–100 μg/L, with correlation coefficients above 0.990, as presented in Table S1. The mean recoveries of the four tissue blanks at three spiked concentrations of 10.0 μg/kg, 50 μg/kg, and 100 μg/kg varied from 63.58 % to 119.90 %, with intra-batch RSDs between 0.52 % and 0.96 % (n = 5) and inter-batch RSDs from 0.1 % to 10.0 % (n = 15), as presented in Table S2.
3.2. Drug residue experiment
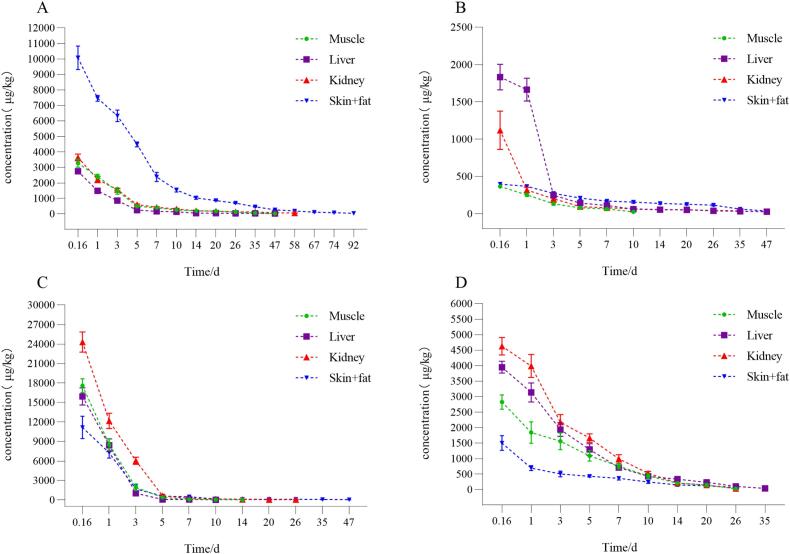
To ascertain the precise residue elimination pattern of ENR, SPZ, and DOX in BFS, the drug concentrations in this experiment were measured until the residue concentrations of the drugs and their metabolites in each tissue of BFS fell below the MRLs or were eliminated before the experiment was concluded. Fig. 1 shows the residue elimination patterns of ENR, CIP, SPZ, and DOX throughout different tissues of BFS. The concentrations of ENR and its metabolites CIP and SPZ, as well as DOX (mean ± standard deviation), in tissues at various periods, are presented in Table 1, Table 2, Table 3, Table 4.
Fig. 1.
Residual elimination of ENR (A), CIP (B), SPZ (C), and DOX (D) across different tissues in BFS.
Table 1.
Residual amounts of ENR in the tissues of BFS following ENR treatment.
| Time | Tissues (μg/kg), mean ± SD, (n = 6) |
|||
|---|---|---|---|---|
| skin + fat | muscle | kidney | liver | |
| 4 h | 10,072.8 ± 757.2b | 3270.9 ± 253.1a | 3641 ± 215.2a | 2758 ± 61.9d |
| 1 day | 7464.2 ± 191.0b | 2407.8 ± 145.8a | 2224.1 ± 202.9a | 1481.9 ± 90.4d |
| 3 days | 6328.9 ± 372.7b | 1480 ± 211.9a | 1562.7 ± 69.9a | 852.8 ± 51.2d |
| 5 days | 4483.4 ± 158.1b | 492.3 ± 14.1c | 601.3 ± 43.1a | 243.2 ± 23.3d |
| 7 days | 2382.2 ± 293.0b | 395.2 ± 11.8a | 424.5 ± 41.1a | 161.8 ± 11.0d |
| 10 days | 1538.1 ± 124.1b | 248.7 ± 17.0a | 321.3 ± 42.3a | 133.5 ± 16.6d |
| 14 days | 1027.1 ± 81.4b | 197.2 ± 9.4a | 197.9 ± 42.3a | 46.1 ± 5.5d |
| 20 days | 861.7 ± 24.2b | 172.4 ± 12.6a | 179.1 ± 34.9a | 43.6 ± 7.2d |
| 26 days | 698.0 ± 40.5b | 117.2 ± 22.4a | 131.8 ± 15.5a | 39.1 ± 5.3d |
| 35 days | 461.2 ± 22.7b | 54.9 ± 11.9c | 116.7 ± 23.1a | 34 ± 2.8d |
| 47 days | 264.4 ± 26.1b | 22.4 ± 4.8c | 67.85 ± 14.1a | 26.3 ± 4.6d |
| 58 days | 185.1 ± 8.9b | – | 54.2 ± 7.8a | – |
| 67 days | 107.1 ± 16.7 | – | – | – |
| 74 days | 78.6 ± 14.9 | – | – | – |
| 92 days | 35.7 ± 2.7 | – | – | – |
Different lowercase letters in the same row of the table indicate significant differences (P < 0.05), whereas identical letters denote non-significant differences (P > 0.05).
Table 2.
Residual concentrations of CIP in the tissues of BFS following ENR treatment.
| Time | Tissues (μg/kg), mean ± SD, (n = 6) |
|||
|---|---|---|---|---|
| skin + fat | muscle | kidney | liver | |
| 4 h | 396.8 ± 23.1b | 364.9 ± 15.5c | 1119.7 ± 256.6a | 1832.4 ± 170.7d |
| 1 day | 367.9 ± 13.6b | 254.4 ± 20.3c | 318.7 ± 36.7a | 1664.6 ± 153.3d |
| 3 days | 273.1 ± 19.9b | 134.9 ± 21.1c | 201.2 ± 52.3a | 246.6 ± 21.9d |
| 5 days | 209.8 ± 17.7b | 79.9 ± 8.0c | 96.9 ± 16.0a | 144.1 ± 31.8a |
| 7 days | 170.87 ± 10.6b | 63.5 ± 14.0c | 79.3 ± 11.0a | 111.9 ± 18.2d |
| 10 days | 156.2 ± 14.6b | 26.8 ± 4.6c | 67.1 ± 14.2a | 62.6 ± 6.9a |
| 14 days | 139.3 ± 11.7b | – | 56.1 ± 7.4a | 54.6 ± 6.5a |
| 20 days | 127.6 ± 11.4b | – | 53.2 ± 3.8a | 52.7 ± 6.5a |
| 26 days | 116.8 ± 15.3b | – | 43 ± 4.3a | 38.6 ± 4.6a |
| 35 days | 62.2 ± 8.1b | – | 38.8 ± 4.7a | 32.5 ± 3.9a |
| 47 days | 38.2 ± 6.6 | – | – | – |
| 58 days | – | – | – | – |
Different lowercase letters in the same row of the table indicate significant differences (P < 0.05), whereas identical letters signify non-significant differences (P > 0.05).
Table 3.
Residual concentrations of SPZ in the tissues of BFS following SPZ treatment.
| Time | Tissues(μg/kg), mean ± SD,(n = 6) |
|||
|---|---|---|---|---|
| skin+fat | muscle | kidney | liver | |
| 4 h | 11,167.16 ± 1720.25b | 17,585.0 ± 1059.2c | 24,305.1 ± 1539.6a | 15,926.6 ± 1316.8d |
| 1 day | 7259.1 ± 806.3b | 8537.4 ± 878.5c | 12,169.3 ± 1170.3a | 8423.2 ± 962.6d |
| 3 days | 1702.4 ± 615.0b | 1942.7 ± 495.4c | 6034.4 ± 550.4a | 1033.3 ± 253.9d |
| 5 days | 591.72 ± 283.15a | 376.2 ± 67.8c | 635.7 ± 123.7a | 81.8 ± 31.7d |
| 7 days | 493.07 ± 90b | 105.4 ± 39.9c | 226.7 ± 58.1a | 75.9 ± 22.2d |
| 10 days | 147.6 ± 31.3a | 43.6 ± 22.9c | 137.2 ± 25.7a | 11.8 ± 1.8d |
| 14 days | 106.4 ± 47.3b | 13.8 ± 8.23c | 69.6 ± 20.6a | – |
| 20 days | 100.3 ± 66.4b | – | 42.3 ± 16.5a | – |
| 26 days | 85.3 ± 30.55b | – | 35.3 ± 7.8a | – |
| 35 days | 69.6 ± 20.6 | – | – | – |
| 47 days | 41.8 ± 11.6 | – | – | – |
| 58 days | – | – | – | – |
Different lowercase letters in the same row of the table indicate significant differences (P < 0.05), whereas identical letters denote non-significant differences (P > 0.05).
Table 4.
Residual concentrations of DOX in the tissues of BFS following DOX treatment.
| Time | Tissues (μg/kg), mean ± SD, (n = 6) |
|||
|---|---|---|---|---|
| skin + fat | muscle | kidney | liver | |
| 4 h | 1503.5 ± 235.7b | 2826.3 ± 228.8c | 4628.4 ± 282.3a | 3950.9 ± 193.4d |
| 1 day | 692.9 ± 78.3b | 1840.7 ± 344.7c | 3990.0 ± 371.8a | 3136.6 ± 306.2d |
| 3 days | 505.6 ± 92.2b | 1559.1 ± 267.8c | 2172.5 ± 251.7a | 1937.4 ± 219.6a |
| 5 days | 431.1 ± 31.1b | 1089.3 ± 166.5c | 1662.6 ± 137.4a | 1294.6 ± 186.7d |
| 7 days | 366.5 ± 54.3b | 753.9 ± 87.0c | 991.6 ± 134.5a | 713.3 ± 76.9d |
| 10 days | 244.3 ± 45.1b | 437.7 ± 71.4c | 529.1 ± 56.1a | 430.7 ± 43.9d |
| 14 days | 154.7 ± 43.3b | 205.3 ± 31.4a | 211.3 ± 44.7a | 339.2 ± 49.4d |
| 20 days | 121.8 ± 39.7a | 145.4 ± 50.1a | 149.9 ± 21.3a | 234.3 ± 52.2d |
| 26 days | 69.0 ± 24.5b | 30.5 ± 2.0a | 32.6 ± 7.6a | 104.6 ± 14.6d |
| 35 days | – | – | – | 38.0 ± 5.8 |
| 47 days | – | – | – | – |
Different lowercase letters in the same row of the table indicate significant differences (P < 0.05), whereas identical letters denote non-significant differences (P > 0.05).
The residual clearance of ENR and its metabolite CIP in the tissues of BFS was protracted, with ENR residues persisting in the skin + fat of BFS on day 92, indicating significantly prolonged elimination compared with that in other tissues. Residues of CIP, a metabolite of ENR, were eliminated from the skin + fat by day 58. ENR and CIP exhibited identical sequences of residue removal in BFS, with skin + fat > kidney > liver > muscle. At 74 days, the ENR concentrations in the skin + fat were below the MRLs set by China and the EU. The withdrawal timeof ENR in the muscle of BFS was determined to be 39.82 days, that in the liver was 14.86 days, that in the kidney was 16.68 days, and that in the skin + fat was 87.8 days, utilizing the WT 1.4 program(Fig. S1). The aggregation of all the parameters indicates that the withdrawal time for ENR in BFS is 87.8 days. Research on the residual patterns of fluoroquinolones in Gallus gallus domesticus Brisson has focused mostly on white-feathered silky fowl. Simultaneously, fewer studies have been undertaken on BFS, which constitute a similar share of the Chinese market as white-feathered silky fowl. Li (2019) reported that the durations of ENR withdrawal from the muscle, liver, kidney, and skin + fat of white-feathered silky fowl following 5 days of oral treatment with 75 mg/L soluble ENR powder were 114.5, 50.6, 101.4 and 133.9 days, respectively. Yuan et al. (2023) reported that the withdrawal times of ENR in Taihe black-boned chickens was 284 days, demonstrating its unsuitability for this breed. The elimination period of ENR in the skin + fat of BFS was significantly shorter than that of white-feathered silky fowl at the same dosage and method of administration in the current investigation. Researchers have posited that melanin is the primary factor contributing to the protracted clearance of drug residues, as evidenced by the observation that white-feathered silky fowl have greater melanin concentrations than their BFS (Tu et al., 2023). ENR has been prohibited in China because of its sluggish metabolism in Taihe black-boned chickens. This investigation revealed that the residual elimination period of ENR in BFS was shorter than that in white-feathered silky fowl and was concluded before penning. ENR is a prevalent chick opener in the poultry sector; therefore, in the absence of superior alternatives, it is anticipated that the current study will assist the government in conducting a more comprehensive investigation into the application of ENR in white-feathered silky fowl and BFS, as well as facilitating a more thorough safety evaluation of ENR.
The removal of SPZ residue from different tissues of BFS was investigated. SPZ was determined to have the longest elimination period from the skin + fat, followed by the kidney, muscle, and liver. The withdrawal time for SPZ in BFS was 37.26 days for skin + fat, 16.43 days for kidney, 13.55 days for muscle, and 9.39 days for liver (Fig. S2). The results indicate that the withdrawal time for SPZ in BFS exceeds the advised duration of 1 day for broiler chickens. Consequently, a safety evaluation is essential before the utilization of sulfonamides in BFS. There are few studies on SPZ in BFS, but studies on the residue patterns of SPZ and similar drugs in other avian species have been reported. Łebkowska-Wieruszewska and Kowalski (2012) reported that the best withdrawal time for SPZ (50 mg/kg b.w. /day) in broilers and turkeys were 14 days and 21 days, respectively. Research involving identical sulfonamide drugs in Taihe and Yugan black-boned chickens revealed that the administration of 160 mg/L SPZ in the drinking water of Taihe black-boned chickens for 5 days necessitated a calculated withdrawal time of 14 days, with the skin + fat exhibiting the longest elimination duration (Yuan et al., 2023). Similarly, the withdrawal time for SPZ at the same dosage and administration method in Yugan black-boned chickens was 3 days across all tissues, with skin + fat still demonstrating the longest elimination time (Zhao et al., 2023). These findings indicate that SPZ, akin to ENR, are slowly metabolized in the skin + fat of BFS.
The concentration of DOX was elevated in kidney and liver compared with muscle and skin + fat of BFS, with the liver exhibiting the longest metabolism duration and completely metabolizing the substance over 47 days. The residual concentrations in the skin + fat, muscle, and kidney decreased below the MRLs at 10, 26, and 10 days, respectively. The residue elimination pattern of DOX in BFS remains unexamined. Based on the MRLs established by China and the EU, the withdrawal time of DOX in BFS skin + fat was determined to be 14.57 days in the withdrawal time 1.4 software, 22.51 days in the liver, 11.09 days in the kidney, and 24.7 days in the muscle (Fig. S3); consequently, the withdrawal time of DOX in BFS should be no less than 24.7 days. Several studies have examined the use of DOX in various avian species. Monir et al. (2021) reported that DOX persists in the liver of broiler chickens for a longer duration than in their muscle and skin. Croubels et al. (1998) reported that DOX requires 12 days to be eliminated from the liver of turkeys and 17 days from their muscles. Hsiao et al. (2016) reported that DOX requires 5 days to be eliminated from the consumable tissues of broiler chickens. The withdrawal time for all avian tissues in these investigations were shorter than those for BFS. The suggested withdrawal time for all BFS tissues in our investigation were less than the 28-day withdrawal time mandated for DOX (Chinese Veterinary Pharmacopoeia 2020 edition), suggesting that DOX may be utilized safely in BFS production. In contrast to SPZ and ENR, DOX is expelled more rapidly from the skin + fat of BFS. Nonetheless, the clearance of DOX from the skin + fat tissues of the BFS remains relatively slower than that in other avian species.
The residue elimination experiments indicated that the withdrawal time for ENR and SPZ exceeded the stipulated durations, whereas the best withdrawal time for DOX was within the designated timeframe. ENR and SPZ have the most prolonged withdrawal time in the skin + fat of BFS. This outcome is probably associated with the melanin-rich characteristic of BFS, which can chelate small-molecule drugs (Otręba et al., 2012). Conversely, DOX is digested rather swiftly in BFS, perhaps because of the differential binding affinity of melanin for the drug.
3.3. In vitro adsorption assay for pharmaceuticals
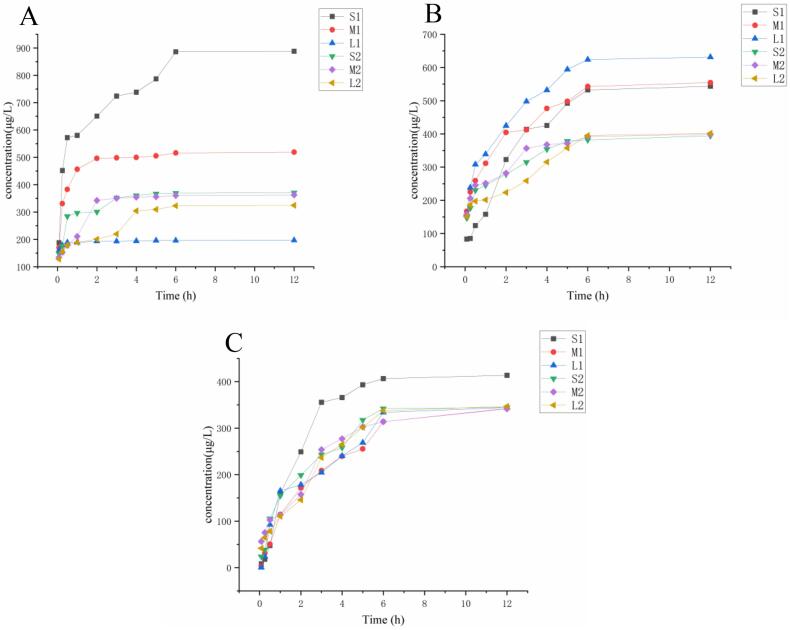
ENR, SPZ, and DOX exhibited strong linearity within the range of 2–100 μg/L with correlation values above 0.99. The recoveries of ENR, SPZ, and DOX in serum-containing cell cultures varied from 70.66 % to 107.63 %, with a relative standard deviation (RSD) of less than 8.3 % (n = 6), as presented in Table S3. The concentrations of free ENR, SPZ, and DOX in skin + fat, muscle, and liver cultures of BFS and white-feathered broilers are shown in Table S4 to S6. The cumulative quantity of drug in each tissue and the free drug in the serum-containing culture media constituted 73.8 % to 91.25 % of the starting drug amount, as shown in Tables S7-S8. This finding indicates that a segment of the drug was allocated to the tissues, which aligns with the findings of Chen et al. (2023). Simultaneously, disparities in adsorption capabilities across different tissues and drugs in BFS and white-feathered broilers were observed, and an in vitro tissue adsorption experiment further evaluated the affinities of the three drugs, ENR, SPZ, and DOX, in skin + fat, muscle, and liver tissues. The drug concentration in each tissue culture mixture remained stable after 30 min of incubation and then gradually decreased. The free drug concentration in the culture solution was continually incubated and measured for up to 12 h, revealing that the drug concentration in each tissue culture solution reached a steady state after 6 h. Fig. 2 illustrates the adsorption of 1 μg/L ENR, SPZ and DOX in the tissues of BSF and white-feathered broilers.
Fig. 2.
Adsorption of 1 μg/L ENR (A), SPZ (B), and DOX (C) by each tissue of BFS and white-feathered broilers. The drug concentration is the difference between the initial concentration of the drug incubation solution and the concentration of the free drug after incubation; S1, M1, and L1 represent the skin + fat, muscle, and liver of the BFS, respectively; S2, M2, and L2 represent the skin + fat, muscle, and liver of the white-feathered broilers, respectively.
Tests on the adsorption of ENR by different tissues demonstrated that the free ENR content in the culture solution of the skin + fat and muscle tissues of BFS was lower than that in the culture solution of white-feathered broilers. These findings suggest that the affinity of ENR for the skin + fat and muscle tissues of BFS is more pronounced than that for white-feathered broilers. The affinity of each tissue for ENR was rated from strongest to weakest as follows: skin + fat (BFS) > muscle (BFS) > skin + fat (white-feathered broilers) > muscle (white-feathered broilers) > liver (white-feathered broilers) > liver (BFS). The adsorption capacity of BFS for ENR in skin + fat and muscle tissue cultures significantly exceeded that of white-feathered broilers. The distribution pattern of melanin across various tissues of BFS was observed as skin + fat > muscle > liver, with liver melanin being exceedingly minimal (Ye, 2023).This finding partially supports the hypothesis that melanin serves as the principal substance for drug adsorption. In the BFS tissue culture mixture, the concentration of free SPZ was lower than that in white-feathered broilers. BFS had a greater attraction effect on SPZ than white-feathered broilers did. In BFS, the hierarchy of tissue affinity for SPZ was liver > muscle > skin + fat. In white-feathered broilers, the adsorption capacity of each tissue was the same. The adsorption capacity of each tissue for SPZ in BFS was inferior to that of ENR. This outcome aligns with the extended elimination pattern of ENR relative to SPZ in the residual elimination test. The adsorption capacity of DOX in the skin + fat tissues of BFS was marginally superior to that in other tissues, whereas the concentration of free DOX in muscle and liver tissues paralleled that in white-feathered broilers, both of which exhibited a consistent declining trend. The DOX adsorption capacity of BFS tissues was relatively inferior to that of ENR and SPZ, and overall, the DOX adsorption capacity aligned with that of white-feathered broilers, corroborating the finding that DOX metabolism in BFS tissues was not sluggish during the DOX residue elimination test.
The experimental results indicated that the affinity of the BFS tissues for the three drugs was ranked as ENR > SPZ > DOX in descending order, with the skin + fat tissues exhibiting the highest affinity and following the same pattern. This outcome resembles the adsorption of glucocorticoids in several human tissues examined by Högger (2001). Drug binding is influenced by a specific mechanism in all tissues, although there are variations in drug affinity across various tissues. This work examined the differential affinity of BFS and white-feathered broilers tissues for three drugs via an in vitro tissue adsorption test. The skin + fat of BFS exhibited greater affinities for SPZ and ENR. The drug's high affinity for the tissue possesses dual characteristics: it facilitates sustained exocytosis of the compound into the animal's circulation at a low rate, thereby minimizing adverse reactions, while simultaneously prolonging the drug's action in the target tissue, manifesting as a decreased metabolic rate. This finding elucidates, to a degree, the sluggish metabolism and and elevated residue risk linked to the utilization of ENR and CIP in the breeding of BFS. In vitro tissue adsorption assays are conducted under the premise that the drug can interact with proteins within the tissue and that membrane lipids or other macromolecular constituents of the tissue also participate in this interaction (Kurz & Fichtl, 1983). The skin + fat of the BFS, which exhibited the highest affinity for ENR and SPZ in this study, is notably characterized by its melanin content (Tian et al., 2015), a macromolecular biopolymer known to chelate metal ions and small molecular compounds. The high affinity of drugs for skin + fat tissues may be attributed to this factor, and elucidating the interaction between melanin and drugs in BFS could represent a significant advancement in mitigating the risk of drug residues in BFS.
3.4. Melanin-binding experiment
The most notable characteristic of BFS is its high melanin content, namely, real melanin, which is mostly located in the connective tissues of eumelanin, including the epidermis, dermis, epimysium, fasciculus, and perivascular region (Tian et al., 2015), with no melanin present in the liver. In 1959, Hobbs et al. (1959) reported that prolonged high dosages of chloroquine and phenothiazines resulted in choroidal retinopathy (Davidorf, 1973), suggesting that the harmful effects of certain drugs are associated with their strong affinity for the pigment melanin. Rimpelä et al. (2017) reported that drug binding to melanin is a significant biological occurrence that influences the pharmacokinetics and pharmacodynamics in melanin-dense tissues. Robbie et al. (2013) demonstrated through microradiography autoradiography that small-molecule drugs can bind to melanin, resulting in their accumulation and retention in pigmented tissues. They reported that melanin binding influences the local pharmacokinetics and pharmacodynamics of these drugs and that melanin binding can serve as a therapeutic strategy for targeting the drug to pigmented tissues and extending its half-life. Rok et al. (2019) established that tetracycline and melanin may chelate to form complexes, resulting in the deposition of the drug or its metabolites in pigmented tissues. These investigations indicate that melanin-rich tissues can bind antibiotics and prolong their half-life. The residual elimination patterns and in vitro tissue adsorption studies of ENR, SPZ, and DOX in BFS indicate that all three drugs necessitate extended elimination half-lives and withdrawal time in melanin-rich skin + fat tissue. The melanin binding test is optimal for determining the melanin in BFS affinity with the drug.
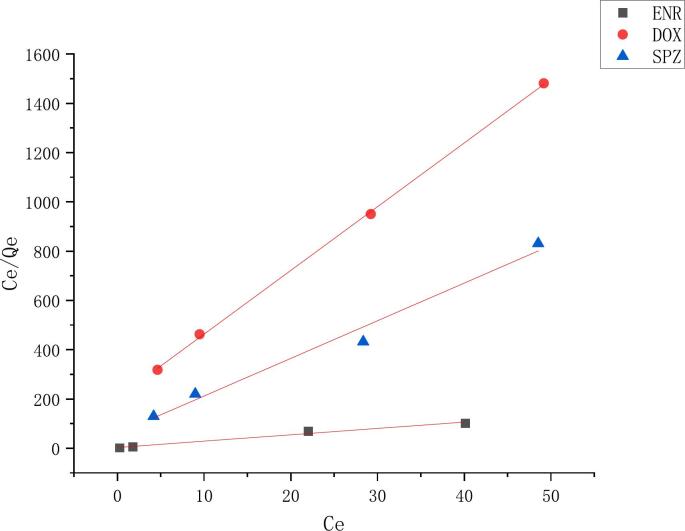
Analysis of several incubation durations for durg binding to melanin in BFS revealed that binding plateaus after 16 h. Therefore, the incubation time was 16 h for several drugs in this experiment. This work employed curve fitting on the basis of the Langmuir binding isotherm for a single binding site to ascertain the binding capacity (qmax) and dissociation constant (KL) (Koeberle et al., 2003). Fig. 3 shows the Langmuir binding curve of the melanin-durg interaction, demonstrating adherence to the Langmuir model with an R2 value over 0.98. The binding curves and associated parameters indicated varying affinities among the three drugs and melanin; ENR exhibited the highest affinity (KL = 1326.27 × 10 −4), followed by SPZ (KL = 11.07 × 10 −4), while DOX demonstrated the lowest affinity with melanin (KL = 1.89 × 10 −4). The order of maximum binding was ENR > SPZ > DOX. The drug-melanin binding capacity characteristics are presented in Table 5.
Fig. 3.
Langmuir plot: ENR, DOX, SPZ versus melanin binding curves, Ce is the concentration of drug at equilibrium reached (μg/mL), Ce/Qe is the concentration of drug at equilibrium over the amount of melanin binding drug (μg/g).
Table 5.
Parameters related to the drug-melanin binding capacity.
| Durg | Qmax (mg/g) | KL | R2 | mean square |
|---|---|---|---|---|
| ENR | 0.3872 | 1326.27× 10 −4 | 0.9833 | 60.34 |
| SPZ | 0.06543 | 11.07× 10 −4 | 0.9824 | 2570.7216 |
| DOX | 0.03864 | 1.89× 10 −4 | 0.9996 | 162.2356 |
Numerous melanin types exist in nature. Ono and Tanaka (2003) investigated the binding properties of fluoroquinolones to synthetic levodopa melanin and calculated the affinity and binding capacity using Langmuir isothermal adsorption models. The study revealed that fluoroquinolones exhibited differing affinities for synthetic melanin, with CIP and sparfloxacin demonstrating greater potency than the other fluoroquinolones examined. The present study on fluoroquinolones, sulfonamides and tetracyclines revealed that ENR has a stronger affinity for melanin than SPZ and DOX. Jakubiak et al. (2019) found that all these known physicochemical and morphological differences between squid melanin, synthetic melanin, and mammalian melanin affect the binding parameters, and thus the translation from in vitro binding data to in vivo pharmacokinetics. Consequently, varying interactions between pharmaceuticals and distinct species of melanin for the same pharmaceutical agent; hence, a comprehensive study on the link between melanin species and drugs is essential to elucidate the mechanisms behind their interactions.
This work investigated the affinity and binding parameters of BFS melanin with three drugs: ENR, SPZ, and DOX. This research involved the extraction of BFS melanin for the drug binding assay and the comparison of the affinities of various medicines and BFS melanin. The study aimed to confirm the correlation between the slow elimination of drug residues in BFS melanin and the melanin itself. The high melanin affinity for ENR and SPZ in BFS is one of the main reasons for the slow metabolism of the drugs in the body. Although the in vitro melanin binding assay may not accurately predict toxic reactions from drug-melanin interactions (Leblanc et al., 1998), it remains a cost-effective method to anticipate the residual elimination patterns of drugs in melanin-rich tissues of BFS, thereby averting unnecessary economic losses for BFS producers.
4. Conclusion
This study revealed that ENR and its metabolites CIP and SPZ were metabolized slowly in skin + fat tissues of BFS, far exceeding the withdrawal time by the drug. DOX exhibited protracted removal in skin + fat tissues compared with other tissues in different broiler breeds; nevertheless, the withdrawal time of DOX in all tissues of BFS conformed to the 28-day standard established for the drug. To further examine the causes of the sluggish metabolism of the three drugs in BFS, in vitro tissue adsorption tests and melanin binding studies were conducted on BFS. The outcomes of the in vitro tissue adsorption test and melanin binding test were congruent with the withdrawal time patterns of the drugs in the drinking water drug delivery experiment, and the capacity of melanin to bind the drugs was associated with the elimination pattern of drug residues in the bodies of the BFS.The greater the binding affinity is, the prolonged the elimination duration of the drug residues. The rate of residue removal of ENR in BFS was greater than that in white-feathered silky fowl (Taihe black-boned chicken). The sluggish metabolism of SPZ and ENR may be ascribed to the robust binding affinity of melanin in BFS for the medication. Consequently, to ensure the quality and safety of products, it is necessary to establish a corresponding standard system for the safe use of commonly used clinical drugs in BFS. Mass spectrometry and drug-melanin binding assays has the potential to become a more economical way to predict the elimination pattern of drugs in BFS.
CRediT authorship contribution statement
Yongqin Li: Writing – original draft, Validation, Software, Methodology, Funding acquisition, Data curation, Conceptualization. Jiawei Du: Methodology, Formal analysis, Data curation. Qi Yang: Supervision, Funding acquisition. Ruiqian Li: Methodology, Formal analysis. Shuangyuan Jin: Methodology, Investigation, Formal analysis. Xuelian Guo: Software. Xueyan Wang: Validation. Wen Zhang: Validation, Formal analysis. Lihua Xu: Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that this research was conducted in the absence of any.
commercial or financial relationships that could be considered as a potential conflict.
of interest.
Acknowledgments
We thank the College of Animal Science and Technology of Ningxia University and Ningxia Institute of Veterinary Durg and Feed Control for their support and assistance in this experiment. This work was financially supported by the College of Animal Science and Technology of Ningxia University and the Ningxia Livestock Product Safety and Quality Monitoring Innovation Team Project (Grant No.: 2022BSB03108).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101994.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Bahrpeyma S., Reinisalo M., Hellinen L., Auriola S., del Amo E.M., Urtti A. Mechanisms of cellular retention of melanin bound drugs: Experiments and computational modeling. Journal of Controlled Release. 2022;348:760–770. doi: 10.1016/j.jconrel.2022.05.059. [DOI] [PubMed] [Google Scholar]
- Chen Z., Liu W., Wu Q., Li Z., Tan L., Ding H., Liu W., Shen X. The withdrawal time of danofloxacin and difloxacin and in vitro binding phenomenon to melanin in black-boned silky fowl. Journal of Food Science. 2023;111 doi: 10.1111/1750-3841.16753. [DOI] [PubMed] [Google Scholar]
- Chinese Ministry of Agriculture and Rural Affairs . 2019. GB 31650–2019 national food safety standard-maximum residue limits for veterinary drugs in foods. [Google Scholar]
- Chu M., Liu Y.Q., Si Y., Yu H., Ye Y.Q., Zhao H. Tyrosine hydroxylase-immunopositive cells and melanin inthe mesencephalon ofyugan black-bonefowl. Journal of Microscopy and Ultrastructure. 2022;10(1):20. doi: 10.4103/jmau.jmau_50_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission of Chinese Veterinary Pharmacopoeia . China Agricultural Press; 2020. Veterinary pharmacopoeia of the People’s Republic of China; p. 473. 241,274. [Google Scholar]
- Croubels S., Vermeersch H., de Backer P., Santos M.D., Remon J.P., van Peteghem C. Liquid chromatographic separation of doxycycline and 4-epidoxycycline in a tissue depletion study of doxycycline in turkeys. Journal of Chromatography. B, Biomedical Sciences and Applications. 1998;708(1–2):145–152. doi: 10.1016/s0378-4347(97)00644-0. 24. [DOI] [PubMed] [Google Scholar]
- Davidorf F.H. Thioridazine pigmentary retinopathy. Archives of Ophthalmology. 1973;90(3):251–255. doi: 10.1001/archopht.1973.01000050253014. [DOI] [PubMed] [Google Scholar]
- Hobbs H.E., Sorsby A., Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2(7101):478–480. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- Högger P. Comparison of the tissue affinity of glucocorticoids to human lung, nasal, and skin tissue in vitro. Arzneimittelforschung. 2001;51(10):825–831. doi: 10.1055/s-0031-1300121. [DOI] [PubMed] [Google Scholar]
- Hsiao P.F., Chang S.K., Hsu T.H., Li K.P., Chou C.C. Pharmacokinetics and tissue depletion of doxycycline administered at high dosage to broiler chickens via the drinking water. Acta Veterinaria Hungarica. 2016;64(4):472–481. doi: 10.1556/004.2016.044. [DOI] [PubMed] [Google Scholar]
- Jakubiak P., Lack F., Thun J., Urtti A., Alvarez-Sánchez R. Influence of melanin characteristics on drug binding properties. Molecular Pharmaceutics. 2019;16(6):2549–2556. doi: 10.1021/acs.molpharmaceut.9b00157. [DOI] [PubMed] [Google Scholar]
- Karkoszka M., Rok J., Wrześniok D. Melanin biopolymers in pharmacology and medicine—skin pigmentation disorders, implications for drug action, adverse effects and therapy. Pharmaceuticals. 2024;17:521. doi: 10.3390/ph17040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle M.J., Hughes P.M., Skellern G.G., Wilson C.G. Binding of memantine to melanin: Influence of type of melanin and characteristics. Pharmaceutical Research. 2003;20:1702–1709. doi: 10.1023/a:1026116208008. [DOI] [PubMed] [Google Scholar]
- Kurz H., Fichtl B. Binding of drugs to tissues. Drug Metabolism Reviews. 1983;14(3):467–510. doi: 10.3109/03602538308991397. [DOI] [PubMed] [Google Scholar]
- Łebkowska-Wieruszewska B., Kowalski C.J. Residue depletion of sulfachlorpyrazine in edible tissues of broiler chickens. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2012;30(2):272–277. doi: 10.1080/19440049.2012.747005. [DOI] [PubMed] [Google Scholar]
- Leblanc B., Jezequel S., Davies T., Hanton G., Taradach C. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regulatory Toxicology and Pharmacology. 1998;28(2):124–132. doi: 10.1006/rtph.1998.1243. [DOI] [PubMed] [Google Scholar]
- Li Z.L. South China Agricultural University; 2019. Elimination of Enrofloxacin and ciprofloxacin residues in Uggs. [DOI] [Google Scholar]
- Manzanares J.A., Rimpelä A.K., Urtti A. Interpretation of ocular melanin drug binding assays. Alternatives to the model of multiple classes of independent sites. Molecular Pharmaceutics. 2016;13(4):1251–1257. doi: 10.1021/acs.molpharmaceut.5b00783. [DOI] [PubMed] [Google Scholar]
- de Mesquita Souza Saraiva M., Lim K., do Monte D.F.M., Givisiez P.E.N., Alves L.B.R., de Freitas Neto O.C.…Gebreyes W.A. Antimicrobial resistance in the globalized food chain: A one health perspective applied to the poultry industry. Brazilian Journal of Microbiology. 2022;53(1):465–486. doi: 10.1007/s42770-021-00635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOA (Ministry of Agriculture of the People’’s Republic of China) 2021. National food safety standard - determination of tetracyclines, sulfonamides and quinolones residues in animal foods by liquid chromatography-tandem mass spectrometry. GB 31658.17–2021. Beijing, China. (in Chinese) [Google Scholar]
- Monir H.H., Fayez Y.M., Nessim C.K., Michael A.M. When is it safe to eat different broiler chicken tissues after administration of doxycycline and tylosin mixture? Journal of Food Science. 2021;86(3):1162–1171. doi: 10.1111/1750-3841.15640. [DOI] [PubMed] [Google Scholar]
- Ono C., Tanaka M. Binding characteristics of fluoroquinolones to synthetic levodopa melanin. The Journal of Pharmacy and Pharmacology. 2003;55(8):1127–1133. doi: 10.1211/002235703322277168. [DOI] [PubMed] [Google Scholar]
- Otręba M., Rok J., Buszman E., Wrześniok D. Regulacja melanogenezy: rola cAMP i MITF [Regulation of melanogenesis: the role of cAMP and MITF] Postepy Hig Med Dosw (Online) 2012;66:33–40. [PubMed] [Google Scholar]
- Pan H., Song S., Ma Q., Wei H., Ren D., Lu J. Preparation, identification, and antioxidant properties of black-bone silky fowl (Gallus gallus domesticus Brisson) Iron (II)-oligopeptide chelate. Food Technology and Biotechnology. 2016;54(2):164–171. doi: 10.17113/ftb.54.02.16.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescina S., Santi P., Ferrari G., Padula C., Cavallini P., Govoni P., Nicoli S. Ex vivo models to evaluate the role of ocular melanin in trans-scleral drug delivery. European Journal of Pharmaceutical Sciences. 2012;46(5):475–483. doi: 10.1016/j.ejps.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Rimpelä A.K., Reinisalo M., Hellinen L., Grazhdankin E., Kidron H., Urtti A., del Amo E.M. Implications of melanin binding in ocular drug delivery. Advanced Drug Delivery Reviews. 2017;126:23–43. doi: 10.1016/j.addr.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Robbie S.J., Lundh von Leithner P., Ju M., Lange C.A., King A.G., Adamson P.…Shima D.T. Assessing a novel depot delivery strategy for noninvasive administration of VEGF/PDGF RTK inhibitors for ocular neovascular disease. Investigative Ophthalmology & Visual Science. 2013;54(2):1490–1500. doi: 10.1167/iovs.12-10169. [DOI] [PubMed] [Google Scholar]
- Rok J., Rzepka Z., Respondek M., Beberok A., Wrześniok D. Chlortetracycline and melanin biopolymer - the risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chemico-Biological Interactions. 2019;303:27–34. doi: 10.1016/j.cbi.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Shi Antong Website . 2021. Food sampling information and analysis. Retrieved from https://www.Eshian.com/sat/foodsampling/. Accessed June 12, 2024. [Google Scholar]
- Tian Y.G., Xu D.L., Liao C.Y. Morphological and distributional characteristics of melanocytes in taihe black-boned chicken. China Poultry. 2015;37(01):5–8. doi: 10.16372/j.issn.1004-6364.2015.01.002. [DOI] [Google Scholar]
- Tu Y.J., Zhang M., Ju X.J., Liu Y.F., Ji G.G., Shan Y.J.…Shu J.T. Comparative study on the early growth and development pattern, slaughtering performance, and meat quality of three kinds of black-feathered silky fowl. Chinese Journal of Animal Science. 2023;59(1):168–174. doi: 10.19556/j.0258-7033.20211228-07. [DOI] [Google Scholar]
- Wu H.H. Introduction of Yugan black-feathered silky fowl. China Poultry. 1999;01:51. doi: 10.16372/j.issn.1004-6364.1999.01.035. [DOI] [Google Scholar]
- Xiong G., Chen W., Jiang K., Liu S., Li J., Liao X. Integrated transcriptome and proteome analysis reveal the unique molecular features and nutritional components of the muscles in Chinese taihe black-boned chicken. PLoS One. 2024;19(3) doi: 10.1371/journal.pone.0299385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Tang C., Ma B., Zhao Q., Jia Y., Meng Q.…Zhang J. Identification of characteristic bioactive compounds in silkie chickens, their effects on meat quality, and their gene regulatory network. Foods. 2024;13(6):969. doi: 10.3390/foods13060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.H. Ningxia University; 2023. Melanin distribution and deposition characteristics of Guyuan black chicken and effects of tyrosine on its tissue melanin content and expression of genes related to melanin synthesis. [DOI] [Google Scholar]
- Yuan L., Wu H., Wang J., Zhou M., Zhang L., Xiang J.…Zhang D. Pharmacokinetics, withdrawal time, and dietary risk assessment of enrofloxacin and its metabolite ciprofloxacin, and sulfachloropyridazine-trimethoprim in taihe black-boned chicken. Journal of Food Science. 2023;88(4):1743–1752. doi: 10.1111/1750-3841.16501. [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang D., Lan J., Sun X. Tissue residue distribution and withdrawal time estimation of trimethoprim and sulfachloropyridazine in Yugan black-bone fowl (Gallus gallus domesticus Brisson) Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2023;40(8):981–991. doi: 10.1080/19440049.2023.2232884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.