Highlights
-
•
MSO are an extremely uncommon ovarian tumour with less than 200 reported cases.
-
•
We describe one of the rare reported cases during pregnancy.
-
•
Recurrence was treated with cytoreduction, thyroidectomy and radioactive iodine therapy (RAI).
-
•
The role of total thyroidectomy and RAI remains controversial. Individualized treatment is recommended.
Keywords: Malignant struma ovarii, Pregnancy, Ovarian cancer, Germ cell tumors
Abstract
Introduction
Struma ovarii are a rare type of cystic teratomas that are composed predominantly or entirely of thyroid tissue and account for less than 1 % of all ovarian tumours. Malignant presentations are even less common, accounting for approximately 5 % of struma ovarii. Due to their rarity, evidence to inform management is very limited. We report a distinctive case of a 27 year-old patient with malignant struma ovarii (MSO) during pregnancy.
Case Presentation
The patient presented with acute lower abdominal pain and was treated with a laparoscopic cystectomy for ovarian torsion. Pathology revealed a 3 cm MSO with a component of papillary thyroid carcinoma arising in an 11.2 cm mature cystic teratoma. The patient became pregnant while pathology results were pending. Given the absence of most high-risk features such as presence of surface adhesions, tumor size greater than 5–10 cm, ascites greater than 1 L, or extra-capsular extension, she was deemed suitable for close observation during pregnancy with serial ultrasounds. At term, she underwent an elective repeat cesarean section, with a concomitant completion unilateral salpingo-oophorectomy and omentectomy. Four months later, a recurrence was detected in the abdominal and pelvic lymph nodes, which was managed with cytoreductive surgery and total thyroidectomy, followed by radioactive iodine (RAI) therapy. Three years after her initial diagnosis, the patient remains well without biochemical or radiologic evidence of recurrence.
Discussion
MSO are rare and treatment should be individualized. In select cases, fertility-sparing management can be considered. The role of thyroidectomy and RAI therapy remains a topic of debate.
1. Introduction
Adnexal masses can be found in 0.05 % to 3 % of pregnancies (Eskander et al., 1953). Malignant adnexal masses in pregnancy are very rare, with an estimated risk of 0.05 % (Eskander et al., 1953, Cathcart et al., 2023). When managing ovarian masses in pregnant patients, several factors must be carefully considered, including gestational age, mass size, the risk of torsion, rupture, or obstruction during labor and delivery, and the suspicion of malignancy. The most common types of malignant adnexal masses in pregnancy are epithelial and germ cell tumors (Cathcart et al., 2023).
Malignant struma ovarii during pregnancy is exceedingly rare, with limited literature to guide clinical decisions (Lager et al., 2018, Tokuda et al., 1993, Lee et al., 2012, Im et al., 2023). In this report, we present the case of a 27-year-old patient diagnosed with malignant struma ovarii (MSO) during pregnancy. Mature cystic teratomas account for 95 % of ovarian tumours of germ cell origin. Thyroid tissue may occasionally be present in these teratomas; when the tumor is predominantly or entirely composed of thyroid tissue, it is termed struma ovarii. Struma ovarii represent approximately 3–5 % of all ovarian teratomas, and 0.3–1 % of all ovarian tumours (Addley et al., 2021). Symptoms and biochemical evidence of hyperthyroidism occur in less than 10 % of cases. Fewer than 5 % of struma ovarii are malignant. Given the rarity of MSO, this case is one of the very few reported instances occurring during pregnancy.
2. Case report
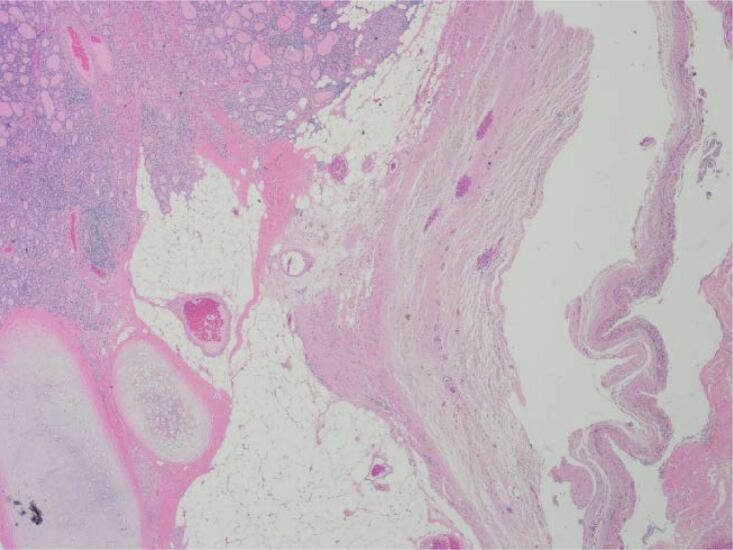
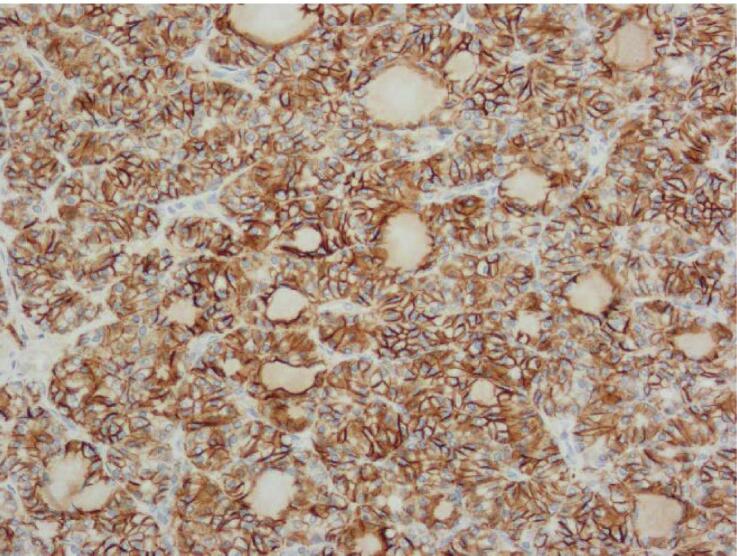
In 2021, a previously healthy 27-year-old patient presented to the emergency department of a community hospital with acute lower abdominal pain. After initial imaging investigations, she was underwent surgery with the General Gynecology team: a diagnostic laparoscopy confirmed an ovarian torsion. As there was an ovarian cyst on the torsed ovary, she underwent a unilateral cystectomy. Pathology results revealed a 3 cm struma ovarii with a component of papillary thyroid carcinoma of follicular variant, arising on the background of an 11.2 cm × 7.5 cm × 4.7 cm mature teratoma (Fig. 1). This was categorized as FIGO stage IC due to intraoperative capsule rupture. Pathologic findings included tumor necrosis and a high proliferation index, with no evidence of lymphovascular or angioinvasion. Before the pathology results were reported, the patient became pregnant.
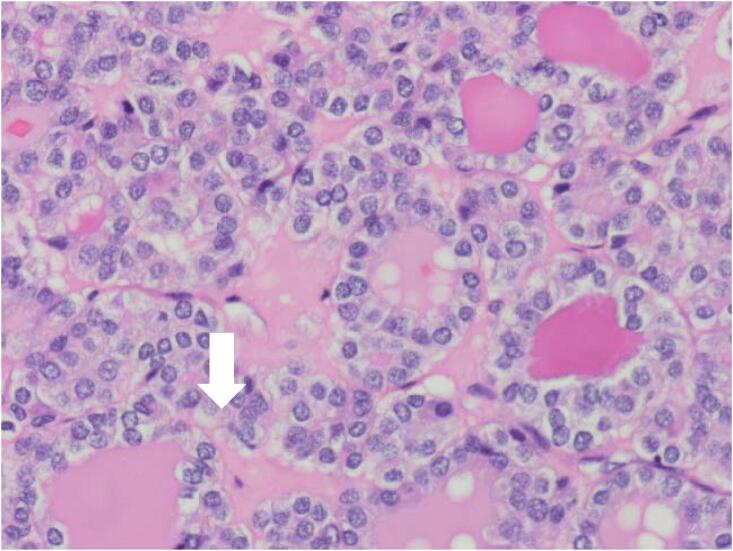
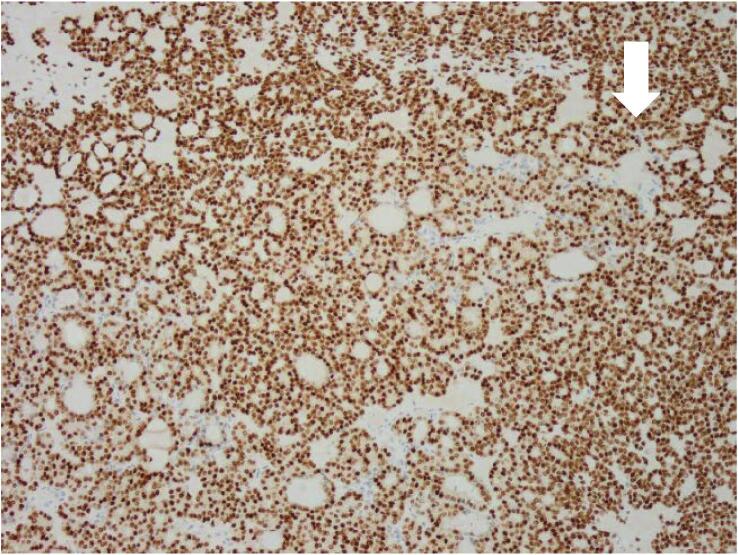
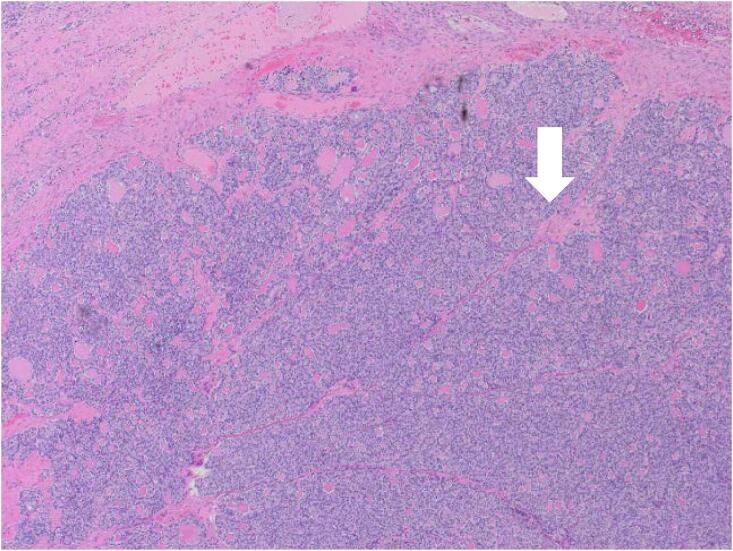
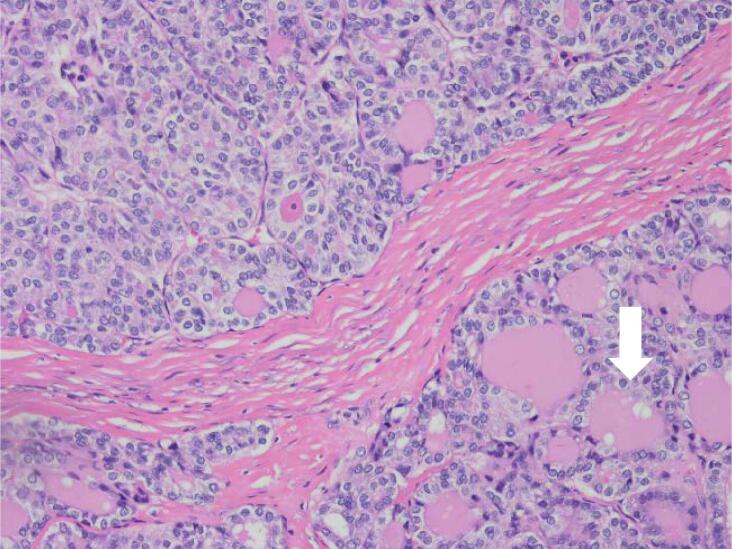
Fig. 1.
Histopathology findings. A: Hemotoxylin and Eosin (H&E) stain X20 magnification: Mature cystic teratoma with proliferative thyroid tissue, consistent with struma ovarii. B: H&E ×20 magnification: Abnormal proliferation of the struma ovarii component with small glands and infiltrative borders. C: H&E X200 magnification: Neoplastic proliferation of thyroid tissue forming follicles with ground-glass nuclei and colloid in the lumen. D: H&E X400 magnification: Neoplastic proliferation of the thyroid tissue forming follicles with ground glass type nuclei with colloid in the lumen. E: TTF-1 Immunohistochemistry X20 magnification: Neoplastic struma ovarii cells show positive nuclear staining. F: Thyroglobulin immunohistochemistry ×200 magnification: Neoplastic struma ovarii cells show positive membrane staining.
At her initial Gynecologic Oncology consultation, she was at 16 weeks of gestational age. Investigations were ordered including bloodwork and ultrasounds of the thyroid, abdomen and pelvis. These confirmed normal thyroid and pelvic anatomy and a live intrauterine pregnancy. Laboratory results showed a normal TSH, elevated T3 and T4 levels, and a thyroglobulin (TG) level of 232 ng/ml. She was started on TSH suppression therapy with 75 mcg of levothyroxine orally daily.
Given the patient’s solitary high-risk feature – an intraoperative cyst rupture – and absence of other high-risk factors, such as presence of surface adhesions, tumor size greater than 5–10 cm, ascites greater than 1 L, or extra-capsular extension (Leuștean et al., 2022), close monitoring through serial ultrasound imaging of the thyroid and pelvis was recommended after discussion at our institutional multidisciplinary case conference. These investigations remained unremarkable throughout her otherwise normal pregnancy.
At 38 weeks and 1 day of gestational age, she underwent an elective repeat caesarean-section with concurrent ipsilateral salpingo-oophorectomy, washings and omentectomy. She delivered a healthy infant. Surgical pathology revealed that the peritoneal washings, omentum and fallopian tube were benign. The ovary contained a residual focus of papillary carcinoma measuring 1.7 mm in greatest dimension.
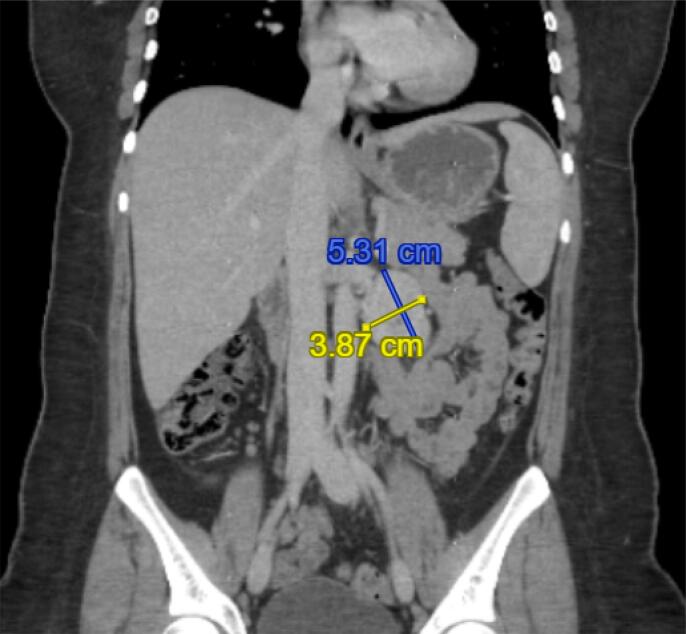
The patient had persistently elevated TG levels post-operatively (1,900 ng/mL 1 month postoperatively, from 311-322 ng/mL throughout pregnancy), prompting further imaging. A CT scan of the chest, abdomen and pelvis revealed a well-defined 5.3 × 3.9 cm left-sided retroperitoneal mass in the para-aortic region and an additional 1.3 × 1.1 cm left para-aortic lymph node (Fig. 2). She thus underwent a cytoreductive surgery at 4 months postpartum to resect the retroperitoneal mass and the para-aortic, paracaval and common iliac lymph nodes, as well as a total thyroidectomy and paratracheal node sampling. She tolerated the procedure well.
Fig. 2.
Radiologic findings at the time of recurrence. Well-defined 5.3 cm x 3.9 cm hypervascular left-sided retroperitoneal mass/lymph node in the para-aortic location, anteromedial to the left kidney with central focus of nonenhancement.
Repeat TG levels remained undetectable postoperatively. She subsequently underwent adjuvant radioactive iodine (RAI) treatment. Pathology of the thyroid gland revealed noninvasive follicular thyroid neoplasm with papillary like features (NIFTP) of 8 mm in greatest dimension and negative margins. The 4 resected paratracheal lymph nodes and parathyroid gland were negative for malignancy. Of the resected abdominal lymph nodes, 3 of 30 para-aortic and paracaval nodes were positive for metastatic ovarian papillary thyroid carcinoma with the largest metastasis measuring 53 mm. The 2 resected left common iliac nodes were negative for malignancy.
As of August 2024, 36 months after her initial presentation in 2021, imaging, tumor markers, and physical examination have revealed no evidence of recurrence.
3. Discussion
Malignant struma ovarii are rare monodermal germ cell tumors of the ovary, with fewer than 200 cases reported in the literature to date (Ayhan et al., 2021, Cui et al., 2021). The most common subtypes are papillary and follicular carcinomas, with other variants including carcinomas of mixed pattern (Cui et al., 2021). Histologically, they are almost identical to thyroid carcinomas (Devaney et al., 1993). Diagnosis is often incidental, as there are no distinct clinical or imaging features specific to MSO. The pathologic diagnostic criteria for malignant struma ovarii have historically been unclear, but it is generally accepted that the same criteria used for diagnosing thyroid carcinoma can be applied. Histologic criteria for MSO include thyroid tissue comprising greater than 50 % of the tumor, cellularity, atypia, mitotic activity, capsular invasion and vascular invasion (Devaney et al., 1993). Genetic mutations and rearrangements such as BRAF, NRAS, KRAS and KIT have been identified in MSO, although their prognostic significance remains unclear (Cui et al., 2021).
Metastasis of MSO can involve pelvic structures such as peritoneum or contralateral ovary, or distant sites such as bone, liver, lung, and mediastinum (Lager et al., 2018, Cui et al., 2021). The majority of patients present between the ages of 30 to 60 years old, with a mean age of 43, making cases during pregnancy particularly uncommon (Lager et al., 2018, Tokuda et al., 1993, Lee et al., 2012, Im et al., 2023). Table 1 summarizes previously documented cases of MSO in pregnancy. Some hypothesize that pregnancy may promote the progression or metastasis of struma ovarii due to elevated levels of estrogen and human chorionic gonadotropin (hCG). The structural similarites between hCG and thyroid-stimulating hormone (TSH) suggests that hCG could bind to and stimulate TSH receptors, thereby promoting tumor growth (Kennedy and Darne, 1991).
Table 1.
Previously documented cases of malignant struma ovarii in pregnancy.
| Author, date | Presentation | Treatment | Outcome | Follow-up |
|---|---|---|---|---|
| Lager, 2018 (Lager et al., 2018) | 30 y-o, 8 weeks gestational age with abdominal discomfort. | Left salpingo-oophorectomy during 2nd trimester, pathology revealing well differentiated papillary thyroid carcinoma. Initiation of levothyroxine. Total thyroidectomy at 2 months postpartum, pathology benign. |
Recurrence detected on iodide scan, with bony metastases, several months postpartum (exact timeline not reported). Treated with I-131 therapy, then levothyroxine. Further iodide scan revealed pulmonary and skeletal metastases (exact timeline not reported), treated with I-131 with complete response in lungs, partial response in bones. |
8 months post-treatment, continues monitoring and levothyroxine suppression. |
| Tokuda, 1993 (Tokuda et al., 1993) | 28 y-o at 26 weeks gestational age, with history of stage 1A MSO 3 years prior (treated with surgery and adjuvant fluorouracil), noticed mass in left frontal region. MRI head revealed extradural tumour. | Treated at 26 weeks gestation with complete surgical resection including cranial bone and dura, pathology revealing recurrent malignant follicular adenocarcinoma. | Uncomplicated remainder of pregnancy, delivered healthy at full term. | No long-term follow-up reported. |
| Lee, 2012 (Lee et al., 2012) | 35 y-o with incidental finding of ovarian cyst at routine ultrasound at 10 weeks of gestation. | Ovarian cystectomy and appendectomy at 10 weeks gestation, revealing follicular carcinoma arising in teratoma. |
Total thyroidectomy and RAI at 26 months postpartum. Recurrence 10 months later, treated with surgical cytoreduction of metastatic lesions in liver, adnexa and peritoneal nodules, followed by adjuvant RAI. |
No long-term follow-up reported. |
| Im, 2023 (Im et al., 2023) | 32 y-o with incidental finding of left ovarian mass during routine pregnancy ultrasound, asymptomatic, gestational age not reported. | Left ovarian cystectomy performed postpartum (timeline not reported), revealing papillary thyroid carcinoma and strumal carcinoid component. | PET-CT and MRI performed post-operatively to assess for residual disease (timeline not reported). Residual mass found on right ovary, prompting right laparoscopic oophorectomy, revealing benign pathology. | No long-term follow-up reported. |
RAI: radioactive iodine.
I-131: sodium iodide.
There are no guidelines to inform the management of MSO due to their rarity. For early-stage presentations, conservative surgical approaches, including fertility-preserving options, may be considered. In more advanced cases or for patients who do not wish to preserve fertility, more extensive procedures such as hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and, if applicable, surgical cytoreduction may be required (Ma et al., 2016). Surgical decisions during pregnancy must carefully balance the risks to both patient and fetus against the potential for disease progression.
With regards to management of the thyroid, at a minimum, thyroid gland work-up with ultrasound and bloodwork to rule out concurrent thyroid carcinoma, which could represent either a concurrent primary or a metastasis site, is required. Although there is no consensus, surgical management with total thyroidectomy and adjuvant radioactive iodine (RAI) therapy may be considered for patients with metastases or recurrence, followed by thyroid replacement therapy (Leuștean et al., 2022). Some recommend prophylactic total thyroidectomy and RAI even in the absence of metastasis or recurrence, based on risk factors such as MSO size greater than 1–2 cm and aggressive histopathologic features such as extraovarian extension or non-papillary thyroid carcinoma histology (Ayhan et al., 2021).
Post-treatment surveillance for patients who have undergone a total thyroidectomy resembles that for primary thyroid malignancies, consisting of regular monitoring of TG and TG antibody levels or I-131 imaging every 6 to 12 months. Patients who have not undergone a thyroidectomy should undergo regular thyroid ultrasounds. Suppressive thyroxine therapy is often recommended for those who do not undergo total thyroidectomy, in order to reduce TSH secretion (Leuștean et al., 2022).While recurrence in the contralateral ovary are rare, those who have undergone fertility-sparing management should have regular pelvic ultrasounds to monitor for recurrence in the contralateral ovary. With active surveillance, MSO generally carries a favorable prognosis, with reported recurrence rates ranging from 7-35 %, and overall survival rates of approximately 95 % at 5 years and 89 % at 20 years (Goffredo et al., 2015, Li et al., 2021). Long-term follow-up is essential, as there are reported cases of recurrences after a median of 14 years, and even up to 26 years following diagnosis (Leuștean et al., 2022).
In conclusion, MSO are extremely uncommon ovarian neoplasms, and this is one of the very few reported cases in pregnancy. While rare, treatment with surgical intervention and post-operative monitoring yields favorable outcomes. Tailored and individualized treatment plans are recommended. The role of total thyroidectomy and radioactive iodine therapy remains controversial. Case reports and series are important, particularly in complex cases involving comorbidities or pregnancy.
Consent to publish
The manuscript has been carefully reviewed to ensure the removal of any patient identification details or figures. Written consent from the patient has been obtained and is securely held on file at our institution. A copy of the written consent is available for review upon request.
CRediT authorship contribution statement
Raveena Kapoor: Writing – original draft, Data curation. Monalisa Sur: Writing – review & editing, Data curation. Julie M.V. Nguyen: Writing – original draft, Supervision, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
JMVN acknowledges research funding from Hamilton Health Sciences (including the Early Career Research Award and the New Investigator Fund) and the Juravinski Hospital and Cancer Centre Foundation.
References
- Addley S., Mihai R., Alazzam M., Dhar S., Soleymani M.H. Malignant struma ovarii: surgical, histopathological and survival outcomes for thyroid-type carcinoma of struma ovarii with recommendations for standardising multi-modal management. A retrospective case series sharing the experience of a single institution over 10 years. Arch. Gynecol. Obstet. 2021;303(4):863–870. doi: 10.1007/s00404-021-05969-0. [DOI] [PubMed] [Google Scholar]
- Ayhan S., Kilic F., Ersak B., Aytekin O., Akar S., Turkmen O., et al. Malignant struma ovarii: From case to analysis. J. Obstet. Gynaecol. Res. 2021;47(9):3339–3351. doi: 10.1111/jog.14902. [DOI] [PubMed] [Google Scholar]
- Cathcart A.M., Nezhat F.R., Emerson J., Pejovic T., Nezhat C.H., Nezhat C.R. Adnexal masses during pregnancy: diagnosis, treatment, and prognosis. Am. J. Obstet. Gynecol. 2023;228(6):601–612. doi: 10.1016/j.ajog.2022.11.1291. [DOI] [PubMed] [Google Scholar]
- Cui Y., Yao J., Wang S., Zhao J., Dong J., Liao L. The clinical and pathological characteristics of malignant Struma Ovarii: an analysis of 144 published patients. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.645156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney K., Snyder R., Norris H.J., Tavassoli F.A. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int. J. Gynecol. Pathol. 1993;12(4):333–343. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- Eskander R., Berman M., Keder L. Evaluation and management of adnexal masses. Obstetr. Gynecol. (New York 1953) 2016;128(5):e210–e226. doi: 10.1097/AOG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- Goffredo P., Sawka A.M., Pura J., Adam M.A., Roman S.A., Sosa J.A. Malignant struma ovarii: a population-level analysis of a large series of 68 patients. Thyroid. 2015;25(2):211–215. doi: 10.1089/thy.2014.0328. [DOI] [PubMed] [Google Scholar]
- Im M., Kim D., Ryang S., Kim B.H. Coexistence of Papillary Thyroid Carcinoma and Strumal Carcinoid Arising from Struma Ovarii in Pregnant Women: a Case Report and Review. Int. J. Thyroidol. 2023;16(1):134–138. [Google Scholar]
- Kennedy R.L., Darne J. The role of hCG in regulation of the thyroid gland in normal and abnormal pregnancy. Obstet. Gynecol. 1991;78(2):298–307. [PubMed] [Google Scholar]
- Lager C.J., Koenig R.J., Lieberman R.W., Avram A.M. Rare Clinical Entity: Metastatic malignant struma ovarii diagnosed during pregnancy – Lessons for management. Clin. Diabetes Endocrinol. 2018;4(1):13. doi: 10.1186/s40842-018-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Yi N.J., Kim H., Choi Y., Park M., Hong G., et al. Metastatic follicular struma ovarii complicating pregnancy: a case report and review of the literature. Kor. J. Hepatobiliary Pancreat Surg. 2012;16(3):123–127. doi: 10.14701/kjhbps.2012.16.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuștean L., Ungureanu M.C., Preda C., Bilha S.C., Obrocea F., Dănilă R., et al. Management of malignant struma ovarii: is aggressive therapy justified? Case report and literature review. Thyroid Res. 2022;15(1):14. doi: 10.1186/s13044-022-00132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yang T., Xiang Y., Li X., Zhang L., Deng S. Clinical characteristics and survival outcomes of malignant struma ovarii confined to the ovary. BMC Cancer. 2021;21(1):383. doi: 10.1186/s12885-021-08118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Guseva N.V., Dahmoush L., Robinson R.A. Struma Ovarii with malignant transformation and germline KIT mutation: a case report with review of the literature. Int. J. Gynecol. Pathol. 2016;35(5):442–447. doi: 10.1097/PGP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Tokuda Y., Hatayama T., Sakoda K. Metastasis of malignant struma ovarii to the cranial vault during pregnancy. Neurosurgery. 1993;33(3):515–518. doi: 10.1227/00006123-199309000-00025. [DOI] [PubMed] [Google Scholar]