Abstract
The effect of human cytomegalovirus (HCMV) infection on cellular mRNA accumulation was analyzed by gene chip technology. During a 48-h time course after infection of human diploid fibroblasts, 1,425 cellular mRNAs were found to be up-regulated or down-regulated by threefold or greater in at least two consecutive time points. Several classes of genes were prominently affected, including interferon response genes, cell cycle regulators, apoptosis regulators, inflammatory pathway genes, and immune regulators. The number of mRNAs that were up-regulated or down-regulated were roughly equal over the complete time course. However, for the first 8 h after infection, the number of up-regulated mRNAs was significantly less than the number of down-regulated mRNAs. By analyzing the mRNA expression profile of cells infected in the presence of cycloheximide, it was found that a minimum of 25 mRNAs were modulated by HCMV in the absence of protein synthesis. These included mRNAs encoded by a small number of interferon-responsive genes, as well as beta interferon itself. Cellular mRNA levels in cytomegalovirus-infected cells were compared to the levels in cells infected with UV-inactivated virus. The inactivated virus caused the up-regulation of a much greater number of mRNAs, many of which encoded proteins with antiviral roles, such as interferon-responsive genes and proinflammatory cytokines. These data argue that one or more newly synthesized viral gene products block the induction of antiviral pathways that are triggered by HCMV binding and entry.
Human cytomegalovirus (HCMV) is a ubiquitous member of the Herpesviridae family that causes disease and mortality in immunocompromised individuals. The virus expresses its genes in a cascade fashion with immediate-early genes being transcribed to high levels by 4 h postinfection (hpi) in cultured fibroblasts (17). The immediate-early genes act as transcriptional activators for the early genes, many of which code for proteins involved in the replication of the viral genome. Following the onset of viral DNA replication at about 24 hpi, transcription of the late genes begins, and many of these genes code for structural components of the virion (16).
The majority of studies investigating the replication of HCMV have used human fibroblasts. In this cell type, the virus replication cycle spans 48 to 72 h, ultimately resulting in profound morphological alterations and cell death. Many events in infected fibroblasts that would be expected to alter cellular mRNA levels have been described. Binding and entry of HCMV are known to cause Ca2+ influx, inositol triphosphate, and cyclic AMP (cAMP) synthesis (11, 12, 37) as well as the activation of the transcription factors NF-κB and Sp1 (20, 40), and mitogen-activated protein kinase is also activated in a process that depends on viral protein synthesis (31). HCMV also modulates cell cycle progression, inducing infected cells to enter the cell cycle but blocking them at the G1/S transition (5, 9, 23). A number of reports have shown that apoptotic pathways are suppressed (15, 41). In vivo, HCMV infects a wide variety of cell types, including fibroblasts, monocytes, and endothelial cells. It is likely that the details of HCMV's interaction with cellular machinery differs somewhat from cell type to cell type, but many conserved pathways are also likely to exist.
Identification of the cellular transcription pathways that are activated and repressed during viral infection could lead to a better understanding of the virus-host interaction and to the identification of targets for antiviral therapy. A virus can potentially modulate the level of a cellular mRNA by a number of different mechanisms. Binding of the viral surface glycoproteins to cellular receptors can activate signal transduction pathways that lead to the activation of cellular transcription factors (3, 40). Components of the virion and immediate-early proteins are known to act as transcriptional regulators with the potential to directly affect the expression of cellular genes. Cellular transcription changes could also be caused by the activation of immune defense pathways used by the cell to limit viral replication. Furthermore, it is likely that there would be secondary effects on gene expression resulting from increased expression of cellular transcription factors, factors that affect the stability of mRNAs, or signaling molecules that activate specific transcriptional programs in cells.
A previous study from our laboratory investigated the effect of HCMV replication on cellular RNA levels at 0.66, 8, and 24 hpi using a microarray with 6,500 probe sets, and 258 genes were identified whose mRNA levels were regulated by a factor of 4 or more by the virus (43). Other viruses that have been studied in a similar manner include human immunodeficiency virus (13), poliovirus (19), echovirus (30), human T-cell leukemia virus type 1 (8), herpes simplex virus type 1 (28), and influenza virus (14). While each of these studies clearly demonstrates changes in cellular gene expression resulting from infection, it is not yet clear which changes are deliberately instigated by the virus to facilitate replication, which are part of the host cell antiviral response, and which are bystanders that do not play a specific role in the infection process.
In this study, we studied the expression profile of cellular mRNAs in HCMV-infected cells using a larger array (12,626 unique probe sets) and with a much greater density of samples (13 time points). We identified 1,425 mRNAs that are up-regulated or down-regulated (threefold or greater) during infection. Many of these genes have potentially significant roles in either the viral replication cycle or pathogenesis. A clustering program was used to organize the genes according to the time after infection at which their mRNA levels change, and distinct classes of expression profiles were observed. For the first 8 h after infection, the number of up-regulated mRNAs was significantly lower than the number of down-regulated mRNAs. By analyzing cells infected with UV-inactivated HCMV (UV-HCMV), we found that this was due to the synthesis of one or more viral proteins during the initial stage of infection that prevents the up-regulation of cellular mRNAs, some of whose products play roles in antiviral pathways, such as interferon response genes and inflammatory cytokines.
MATERIALS AND METHODS
Cells and viruses.
The AD169 strain of HCMV was used. Virus particles were partially purified by centrifugation through a sorbitol cushion, resuspended in phosphate-buffered saline, and stored at −80°C. Primary human foreskin fibroblasts (HFFs) at passage 13 to 15 were maintained in medium supplemented with 10% fetal calf serum. Plates (10-cm diameter) of cells that had been confluent for at least 3 days were infected at a multiplicity of infection of 6 PFU/cell with purified virions in conditioned medium. After adsorption, additional conditioned medium was added to the cells. At the time points indicated, the medium was removed and the cells were washed once with phosphate-buffered saline and lysed with 8 ml of TRIZOL reagent (Gibco-BRL). For infections in the presence of cycloheximide, the cells were pretreated for 1 h with 100 μg of cycloheximide per ml in the conditioned medium before infection, and cycloheximide was maintained in the medium throughout the infection. For UV inactivation of viruses, purified virions were suspended in a total of 250 μl of phosphate-buffered saline and placed in a well of a six-well dish. The uncovered suspension was irradiated in a Stratagene UV-crosslinker for a period of time that was sufficient to reduce the infectivity of the virions by >105-fold. For the mixed infection of HCMV with UV-HCMV, a purified virus suspension was divided in two, and one half was irradiated and then combined with the competent virus for an infection at 4 PFU/cell.
Sample preparation and hybridization to gene chips.
Frozen TRIZOL suspensions were thawed, and total RNA was purified. Five micrograms of the sample was used as a template for cDNA synthesis in a reaction that was primed with an oligonucleotide containing an oligo(dT) and a T7 RNA polymerase promoter. The cDNA was used to make biotin-labeled cRNA probes with an RNA transcript labeling kit (ENZO). The cRNA was purified to remove unincorporated ribonuclotides, and 15 μg was fragmented at 95°C for 30 min in fragmentation buffer (40 mM Tris-acetate [pH 8.1], 100 mM potassium acetate, 30 mM magnesium acetate). The fragmented cRNA was hybridized to HG-U95A arrays for 16 h at 45°C. The arrays were washed and stained according to the Affymetrix antibody amplification protocol (Affymetix Expression Analysis technical manual, 2000). Scanning was performed using an Agilent GeneChip scanner.
Data analysis.
Scanned chip data sets were analyzed using the Affymetrix GeneChip analysis software. Data from the time course experiment were analyzed using mock-infected cell data as a baseline. For each time point, we extracted the fold change and difference call for each probe set and compiled it in a spreadsheet. To generate a filtered data set of genes that were considered to be significantly regulated by HCMV, a Perl script that extracted only genes that had a fold change of at least ±3 at two consecutive time points and had a difference call of I (increased), MI (marginally increased), D (decreased), or MD (marginally decreased) was used. Clustering analysis was performed using CLUSTER (10), a hierarchical clustering program obtained from the Eisen Laboratory at the University of California at Berkeley. Before clustering, the data were normalized by gene. For the time course experiment, a gene was considered to be significantly altered if it changed by threefold or greater in two sequential samples and had a difference call of I, MI, D, or MD. For the experiments performed at 6 hpi, duplicate or triplicate samples were analyzed, and a gene was considered to be significantly altered if it changed by threefold or greater in two replicates and had a difference call of I, MI, D, or MD. Data from infections in the presence of cycloheximide were analyzed using data from mock-infected, cycloheximide-treated cells as a baseline. To assign genes in the data sets to specific functional groups, a Perl script that retrieved information on the molecular function, cellular location, and biological process for each gene from the GeneOntology database (2) was used.
RESULTS AND DISCUSSION
To study cellular mRNA accumulation in HCMV-infected cells, human fibroblasts were infected and RNA was harvested at different times over a 48-h time course. RNA samples were analyzed using the Affymetrix HG-U95A array, which contains probe sets designed to detect 12,626 unique human transcripts, and the results were quantified using Affymetrix software. The GeneChip software calculates a fold change as well as a difference call for each gene; the fold change and difference call are reported as MI, D, or MD. Mock-infected cells were used as a baseline. A mRNA was considered to be significantly regulated if (i) its fold change was threefold or greater and (ii) the difference call was I, D, MI, or MD for at least two consecutive time points. It was recently demonstrated that these criteria are sufficient to keep the number of false positives at an acceptably low level (29).
Levels of numerous cellular RNAs change in response to HCMV infection.
The levels of 1,425 cellular mRNAs were significantly altered during the 48-h time period. This data set is available at the website http://www.molbio.princeton.edu/labs/shenk/browneetal2001/. Of these mRNAs, 702 were increased and 723 were decreased. A portion of the genes have been previously reported to be regulated by HCMV, such as several interferon response genes, gas-1, COX-2, and adrenomedullin (43), confirming the reliability of our data set.
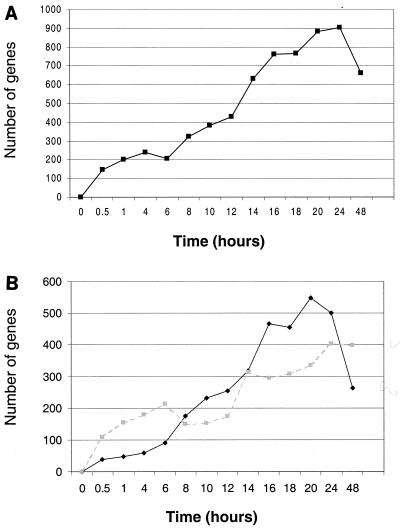
By plotting the number of genes from the filtered set whose mRNA levels have changed at each time point, we observed a roughly constant increase in the number of altered genes up to 24 hpi (Fig. 1A). When up-regulated and down-regulated mRNAs are plotted separately, they exhibit markedly different profiles (Fig. 1B). From 0 to 8 hpi, the number of down-regulated mRNAs greatly exceeds the number of up-regulated mRNAs, whereas from 8 to 20 h, the up-regulated mRNAs are more numerous.
FIG. 1.
HCMV alters the mRNA level of many cellular genes over a 48-h time course. A set of 1,425 genes was identified as being altered significantly (threefold or greater) during an HCMV time course. The numbers of genes from this set that were significantly up-regulated or down-regulated (change of threefold or greater and a difference call of I, MI, D, or MD) at each time point were calculated and plotted (A). The numbers of genes up-regulated (black diamonds) and down-regulated (shaded squares) were also plotted separately (B).
Certain classes of genes were prominently affected. Consistent with previous reports (42, 43), several interferon-responsive mRNAs were strongly up-regulated by 4 hpi. It is interesting to note that the mRNA encoding the 58-kDa DNAJ-C3 protein, which inhibits the activity of the interferon-inducible kinase PKR (21), was induced by a factor of 8.6. This could be a means by which HCMV is able to bypass the block to protein synthesis caused by the activation of PKR. Indeed, influenza virus is thought to induce this protein to evade PKR activity as well (21).
Ninety-seven mRNAs encoding proteins with likely roles in cell cycle regulation and oncogenesis were modulated by the virus (Table 1). HCMV infection activates both proliferative and antiproliferative signals in infected cells. HCMV infection is known to induce quiescent (G0) cells to enter the cell cycle (5, 18), and this is reflected by the rapid down-regulation of transcription for G0-specific transcripts such as Gas-1 and p27 (44) (Table 1). Transcription of a number of the cyclins is also affected. Consistent with a previous report (18), cyclin E2 mRNA is strongly induced (6.2-fold at 20 h). Cyclin A1 and D3 mRNAs are also induced, although somewhat less strongly. Cyclins T2 and B1 mRNAs are transiently repressed early in infection, while cyclin G1 mRNA was weakly repressed late in infection. The oncogenic transcription factor c-fos and the growth repressive factor junB were rapidly and strongly, but transiently, induced (10- and 5.7-fold, respectively, at 2 h). Several tumor suppressor mRNAs, such as AIM1, DLC3, and ST5, were down-regulated during the time course, while the ets2 repressor factor was up-regulated.
TABLE 1.
Cell cycle regulators or oncogenes
| Gene | Nuc IDa | Max fold changeb | Time (h) |
|---|---|---|---|
| 37-kDa leucine-rich repeat (LRR) protein | U32907 | ∼−4.6 | 24 |
| Absent in melanoma 1 (AIM1) | AI800499 | −7.4 | 20 |
| Activator of S-phase kinase | AB028069 | ∼9.2 | 18 |
| Acute myeloid leukemia (AML1) | M83215 | ∼−6 | 18 |
| Aryl hydrocarbon receptor | L19872 | 5 | 14 |
| AXL receptor tyrosine kinase | M76125 | −4.8 | 24 |
| Baculoviral IAP repeat-containing 5 (survivin) | U75285 | ∼−10.5 | 4 |
| BRCA1-associated protein 1 | AF045581 | ∼21.4 | 24 |
| BRCA2 region, mRNA sequence CG018 | U50527 | ∼6.2 | 8 |
| BTG family, member 3 | D64110 | 3.9 | 24 |
| Butyrate response factor 2 (epidermal growth factor response factor 2) | U07802 | −3.7 | 20 |
| cdc25 | S78187 | −4.6 | 16 |
| cdc2-related protein kinase mRNA | M68520 | ∼24 | 48 |
| CDC6 (cell division cycle 6, Saccharomyces cerevisiae) homolog | U77949 | ∼5.9 | 20 |
| Cdc6-related protein (HsCDC6) | U77949 | ∼5.9 | 20 |
| Cell division cycle 27 | AA166687 | ∼3.1 | 10 |
| Cell growth regulatory with ring finger domain | U66469 | 5.4 | 16 |
| c-fos | K00650 | ∼44.8 | 0.5 |
| Chediak-Higashi syndrome 1 | U67615 | −5.5 | 4 |
| c-kit | X06182 | ∼−3.2 | 48 |
| Coagulation factor II (thrombin) receptor | M62424 | −4.5 | 24 |
| Cyclin A1 | U66838 | 6.3 | 20 |
| Cyclin B | M25753 | ∼−15.4 | 1 |
| Cyclin D3 | M92287 | 4.2 | 24 |
| Cyclin E2 | AF091433 | 6.2 | 20 |
| Cyclin G1 | X77794 | −3.4 | 48 |
| Cyclin T2 | AF048731 | ∼−4.3 | 4 |
| Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | AI304854 | −7 | 8 |
| Cyclin-selective ubiquitin carrier protein | U73379 | ∼−14.5 | 16 |
| Deleted in liver cancer 1 (dlc1) | AF035119 | −4.7 | 24 |
| Diphtheria toxin receptor | M60278 | ∼23.2 | 14 |
| Discs, large (Drosophila) homolog 1 | U13896 | ∼5.7 | 18 |
| E2F transcription factor 5, p130-binding | U31556 | ∼13.7 | 24 |
| E2F-5 | U31556 | 3.5 | 24 |
| EphB2 | AF025304 | 4.4 | 48 |
| Epithelial membrane protein 1 | Y07909 | −5.2 | 14 |
| ets variant gene 1 | U17163 | ∼−5.0 | 6 |
| Ets2 repressor factor | U15655 | 5.7 | 14 |
| Exostoses (multiple) 1 | S79639 | −7.1 | 48 |
| Fatty acid-binding protein 3, muscle and heart | AI041520 | ∼−4.6 | 18 |
| FBJ murine osteosarcoma viral oncogene homolog B | L49169 | 4.1 | 10 |
| Fibroblast growth factor 7 (keratinocyte growth factor) | M60828 | ∼−9.7 | 48 |
| Fibroblast growth factor receptor 3 | M64347 | ∼9.1 | 20 |
| Four and a half LIM domains 2 | U29332 | −5.4 | 24 |
| Gonadotropin-releasing hormone 1 | X01059 | ∼−6.4 | 6 |
| Growth arrest and DNA damage-inducible protein (gadd45) | M60974 | −3.3 | 6 |
| Growth arrest-specific 1 | L13698 | −3.8 | 6 |
| highly expressed in cancer, rich in leucine heptad repeats | AF017790 | −3.3 | 20 |
| homeo box A9 | U41813 | ∼−7.0 | 4 |
| IGF-II mRNA-binding protein 3 | U97188 | −3.7 | 14 |
| Interferon regulatory factor 4 | U52682 | ∼3.8 | 8 |
| Kinesin-like 1 | U37426 | −5.1 | 10 |
| Loss of heterozygosity, 11, chromosomal region 2, gene A | AI632589 | ∼−19.0 | 48 |
| MAD (mothers against decapentaplegic, Drosophila) homolog 3 | U68019 | −4.2 | 12 |
| Mad4 homolog | AF040963 | ∼−12.3 | 48 |
| Matrix metalloproteinase 11 (stromelysin 3) | X17610 | ∼−37.6 | 48 |
| MAX-binding protein | X96401 | −4.6 | 10 |
| MAX protein | AI693307 | ∼10.0 | 8 |
| Meis1 (mouse) homolog | U85707 | −9.1 | 48 |
| Melanoma antigen, family A, 2 | L18920 | ∼−10.4 | 1 |
| Menage a trois 1 (CAK assembly factor) | X87843 | ∼−5.0 | 4 |
| Neuropilin 1 | AF016050 | ∼−9.2 | 18 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 (p49/p100) | X61498 | ∼9.4 | 24 |
| Oncogene Ret/Ptc, fusion activated | HG4679– HT5104 | ∼10.1 | 48 |
| Oncogene TC21 | AI365215 | −3.7 | 48 |
| p53 activated fragment 1 (WAF1) | U03106 | −4.4 | 48 |
| Peripheral myelin protein 22 | D11428 | −4 | 14 |
| Phorbol-12-myristate-13-acetate-induced protein 1 | D90070 | 17.2 | 10 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Maximum (Max) fold change. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
CMV is able to inhibit apoptosis (7, 15, 41). We found that 25 mRNAs, encoding proapoptotic and antiapoptotic proteins, were significantly regulated by the virus (Table 2). The proapoptotic caspase 1 enzyme is down-regulated by 14 h into infection, reaching a 10.8-fold reduction by 48 hpi. The proapoptotic cytokine tumor necrosis factor alpha (TNF-α), which has roles in the proliferation of T lymphocytes and activation-induced cell death (1), is greatly up-regulated (up to 130-fold) beginning at 6 hpi—presumably as part of an antiviral response. The APO-1 gene, which encodes a receptor for the apoptosis-inducing FAS protein (35) is strongly down-regulated (6.9-fold), possibly protecting infected cells from cytotoxic T-cell killing.
TABLE 2.
Apoptosis regulators
| Gene | Nuc IDa | Max fold changeb | Time (h) |
|---|---|---|---|
| APG5 (autophagy 5, S. cerevisiae)-like | Y11588 | ∼−11.7 | 48 |
| aryl hydrocarbon receptor | L19872 | −5 | 14 |
| Baculoviral IAP repeat-containing 5 (survivin) | U75285 | ∼−10.5 | 4 |
| Bruton agammaglobulinemia tyrosine kinase | U10087 | 7.4 | 48 |
| Caspase 1, apoptosis-related cysteine protease (interleukin 1, beta, convertase) | U13697 | ∼−10.8 | 48 |
| Caspase 2 | U13021 | ∼6.7 | 18 |
| Chromobox homolog 4 (Drosophila Pc class) | AF013956 | ∼10.4 | 12 |
| Coagulation factor II (thrombin) receptor | M62424 | −4.5 | 24 |
| Fas/Apo-1 | X83490 | −4.3 | 24 |
| Fragile X mental retardation, autosomal homolog 1 | U25165 | 4.3 | 24 |
| Kruppel-type zinc finger (C2H2) | AB011414 | 6.8 | 16 |
| Modulator of apoptosis 1 | AF305550 | 4.1 | 20 |
| Myxovirus resistance 1, homolog of murine (interferon-inducible protein p78) | M33882 | 17.4 | 12 |
| Neurotrophin 3 | X53655 | −3 | 6 |
| RIP protein kinase | U50062 | 10.6 | 8 |
| Serine (or cysteine) proteinase inhibitor, clade B (ovalbumin), member 2 | Y00630 | −7.7 | 48 |
| Serine/threonine kinase 17a (apoptosis-inducing) | AB011420 | −4.3 | 14 |
| STAT-induced STAT inhibitor 2 | AF037989 | 10.6 | 24 |
| TIA1 cytotoxic granule-associated RNA-binding protein-like 1 | D64015 | 4.9 | 14 |
| TNF-related apoptosis inducing ligand TRAIL | U37518 | ∼25.8 | 12 |
| TNFRSF1A-associated via death domain | AI439047 | ∼9.8 | 1 |
| Toll-like receptor 2 | AF051152 | ∼18.1 | 24 |
| Tumor necrosis factor (ligand) superfamily, member 9 | U03398 | ∼129.9 | 24 |
| Tumor necrosis factor receptor superfamily, member 6 | X63717 | −7.4 | 16 |
| v-raf murine sarcoma viral oncogene homolog B1 | M95712 | ∼5.1 | 18 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Maximum (Max) fold change. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
HCMV is well-known for its ability to evade normal immune response pathways such as antigen presentation (22, 25, 26, 38). Many genes with a role in the immune system were perturbed by viral infection (Table 3). Consistent with our previous study (43), several mRNAs encoding proteins with roles in inflammation were up-regulated, including COX2 and phospholipase A2. Proinflammatory cytokine mRNAs such as RANTES and interleukin 8 were also moderately induced. Strikingly, interleukin 11 mRNA was massively up-regulated (up to 78-fold) from very early times after infection. Interleukin 11 is an antiinflammatory cytokine that interacts with CD4+ lymphocytes and inhibits the development of a cell-based Th1 response in favor of an antibody-based Th2 response (4). This might be an HCMV strategy to subvert the cell-mediated Th1 response. Infection also up-regulated mRNA encoding the interleukin 1 receptor antagonist IL1RN (17.7-fold), possibly preventing this cytokine from performing its roles in inflammation and immunity. A transcription factor, TCF8, known to repress IL-2 transcription (39), was highly up-regulated (43-fold). Il-2 is secreted by activated Th cells and functions to enhance Th cell proliferation and to stimulate Tc cells to become activated cytotoxic lymphocytes (36). Although there is no evidence that HCMV replicates in lymphocytes, it is possible that virions can either contact a receptor on the cell surface or enter these cells and elicit the up-regulation of this gene. HCMV might hinder Th cell function even without undergoing productive replication.
TABLE 3.
Immune system regulators
| Gene | Nuc IDa | Max fold changeb | Time (h) |
|---|---|---|---|
| Adenosine A2b receptor | X68487 | ∼−6.5 | 6 |
| ATP-binding cassette, subfamily B (MDR/TAP), member 2 | X57522 | 4.6 | 24 |
| Chediak-Higashi syndrome 1 | U67615 | −5.5 | 4 |
| Class I cytokine receptor | AF053004 | ∼10.8 | 20 |
| Coagulation factor III (thromboplastin, tissue factor) | J02931 | −5.9 | 6 |
| Collectin subfamily member 10 (C-type lectin) | AB002631 | ∼−7.2 | 0.5 |
| Complement component 4-binding protein, beta | L11245 | ∼5.7 | 6 |
| Cyclooxygenase-2 (COX2) | U04636 | 5.4 | 10 |
| DnaJ (Hsp40) homolog, subfamily C, member 3 | U28424 | 8.6 | 20 |
| Dual-specificity phosphatase 10 | AB026436 | −7.4 | 6 |
| Fibroblast growth factor receptor 3 (achondroplasia, thanatophoric dwarfism) | M64347 | ∼11.9 | 1 |
| Glutathione reductase | X15722 | 3.3 | 24 |
| GRO2 oncogene | M36820 | 16.2 | 2 |
| Guanylate-binding protein 2, interferon-inducible | M55543 | 4.2 | 12 |
| Interferon regulatory factor 7 | U53831 | 18.2 | 12 |
| Interferon-induced, hepatitis C-associated microtubular aggregate protein (44 kDa) | D28915 | 9.4 | 24 |
| Interleukin 1 receptor accessory protein | AB006537 | ∼13.8 | 20 |
| Interleukin 1 receptor antagonist | X52015 | 17.7 | 48 |
| Interleukin 11 | X58377 | ∼78.2 | 14 |
| Interleukin 6 (interferon, beta 2) | X04430 | 5 | 12 |
| Interleukin 6 signal transducer (gp130, oncostatin M receptor) | M57230 | 4.3 | 8 |
| Interleukin 8 | M17017 | 5 | 2 |
| JAK-binding protein | AB000734 | ∼−25.7 | 1 |
| Major histocompatibility complex, class I, F | AL022723 | 9.6 | 48 |
| Major histocompatibility complex, class II, DP beta 1 | M83664 | 10.3 | 24 |
| Mitogen-activated protein kinase 8 interacting protein 2 | U62317 | −27.4 | 48 |
| Monokine induced by gamma interferon | X72755 | ∼−5.5 | 16 |
| Myxovirus resistance 1, homolog of murine (interferon-inducible protein p78) | M33882 | 17.4 | 12 |
| Neutrophil cytosolic factor 2 (65 kDa, chronic granulomatous disease, autosomal 2) | M32011 | −3.9 | 12 |
| Nuclear receptor subfamily 4, group A, member 2 | X75918 | ∼32.4 | 8 |
| Opioid receptor, kappa 1 | L37362 | ∼−14.5 | 48 |
| Phospholipase A2, group IVA (cytosolic, calcium-dependent) | AL022147 | 13.9 | 48 |
| Prostaglandin D2 synthase gene, exon 7 | M98539 | 5.1 | 2 |
| Regulatory factor X, 1 (influences HLA class II expression) | X58964 | ∼−10.8 | 48 |
| Small inducible cytokine subfamily B (Cys-X-Cys), member 11 | AF030514 | ∼7.6 | 12 |
| Small inducible cytokine subfamily C, member 2 | D63789 | ∼−6.6 | 0.5 |
| STAT-induced STAT inhibitor 2 | AF037989 | 10.6 | 24 |
| Stromal cell-derived factor 1 | L36033 | −11.9 | 24 |
| TIA1 cytotoxic granule-associated RNA-binding protein-like 1 | D64015 | 4.9 | 14 |
| Toll-like receptor 2 | AF051152 | ∼18.1 | 24 |
| Transcription factor 8 (represses interleukin 2 expression) | D15050 | ∼43.1 | 14 |
| Tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | AB008822 | −17.3 | 48 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Maximum (Max) fold change. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
Modest correlation between time clusters and function-based groupings of cellular mRNAs.
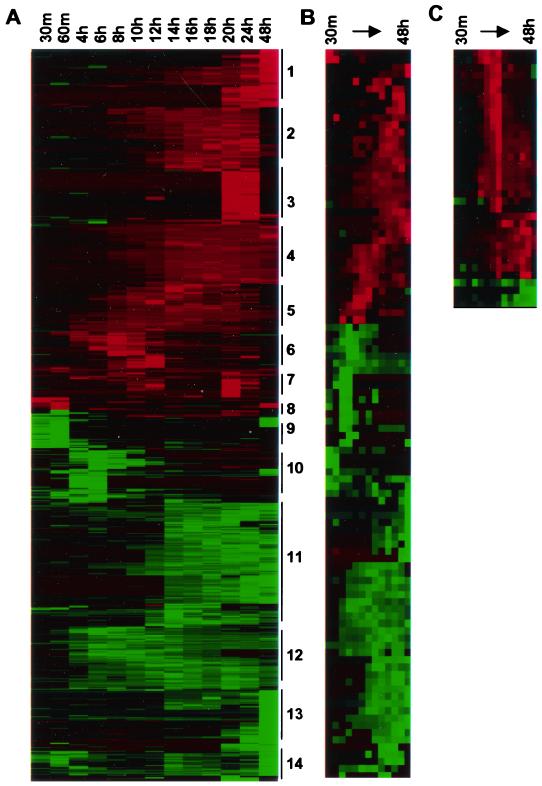
The data were imported into the hierarchical clustering program CLUSTER (10). The expression profiles were found to be complex: at least 14 distinct clusters of mRNAs whose levels changed in a coordinated fashion as a function of time were observed (Fig. 2A). We did not observe a strong tendency for particular functional groups to be associated with a specific time-based cluster. Indeed, cluster analysis of a specific functional group such as cell cycle genes (Fig. 2B) or interferon-responsive genes (Fig. 2C) highlighted the fact that even within each functional class, several different patterns of expression were discernible. However, it is important to note that functional groupings are imprecise because the functions of most genes remain incompletely understood. It is likely that many of the genes in the same time-related cluster are coregulated by common transcriptional activators.
FIG. 2.
HCMV induced altered mRNA cellular mRNA levels in infected cells. Expression data for 1,425 genes judged as being significantly altered by HCMV were normalized by gene and clustered using the hierarchical clustering program CLUSTER. The clusters were then visualized using the TREEVIEW program (A). Putative clusters are noted by numbers to the right of the image. Elevated (red) and reduced (green) mRNA levels are indicated. Genes with functions relating to cell cycle, cell proliferation, or oncogenesis were identified by a Perl program that annotated Affymetrix GeneChip data with information about the biological function of the genes using the GeneOntology database. These genes were then clustered separately and visualized using TREEVIEW (B). Alpha-interferon-sensitive genes found to be modulated by HCMV were also clustered separately (C).
Changes in cellular mRNA levels that occur independently of protein synthesis after infection.
To identify the changes in cellular mRNA levels that result directly from binding and entry of HCMV in the absence of new protein synthesis, we infected fibroblasts in the presence of cycloheximide and harvested RNA for analysis 6 h later. Cells that were mock infected in the presence of cycloheximide were used as a control. A total of 8 mRNAs were up-regulated and 17 mRNAs were down-regulated (Table 4). This is clearly an underestimate of the changes that occur in infection independently of new protein synthesis, since cycloheximide alone is known to activate inflammatory pathways that HCMV also activates, such as the transcription factor NF-κB (33). Indeed, by comparing mock-infected, cycloheximide-treated cells to mock-infected cells that were not treated with the drug, it was observed that 53 mRNAs that change significantly in HCMV infection by 6 hpi were also activated by cycloheximide alone (data not shown). Four interferon-responsive mRNAs were among those up-regulated by HCMV in the presence of cycloheximide, including beta-interferon itself. No known interferon-responsive genes were induced by cycloheximide in the absence of infection.
TABLE 4.
Genes modulated by HCMV in the presence of cycloheximid
| Gene | Nuc IDa | Fold changeb |
|---|---|---|
| Angelman syndrome gene, E6-AP ubiquitin protein ligase 3A (UBE3A) | AF002224 | ∼29.5, ∼8.1 |
| Beta nerve growth factor | X52599 | −5.4, ∼−8.4 |
| CIG49 | AF026939 | 17.9, 38.9 |
| Cryptochrome 1 (photolyase-like) | D83702 | −3.3, −5.7 |
| Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | AI304854 | −7.6, ∼−5.8 |
| Folate receptor 3 | U08471 | −3.8, ∼−3.9 |
| GRO3 oncogene | M36821 | −6.2, ∼−7.9 |
| Homo sapiens 14A6CK DNA sequence | X72882 | −33.7, ∼3.2 |
| Histone deacetylase 7B | AB018287 | −5.5, −12.3 |
| Human mRNA for proto-oncogene protein | D14497 | −4.3, ∼−8.4 |
| Hypothetical protein FLB6421 | AI133727 | 26.5, ∼31.9 |
| Inhibitor of apoptosis protein 1 | U45878 | −3.1, −3.7 |
| Interferon, beta | V00535 | ∼65.1, ∼8.1 |
| Interferon-induced 17-kDa/15-kDa protein | M13755 | 8.6, 13.4 |
| Interferon-induced 56-kDa protein | M24594 | 3.9, 4.7 |
| ISG-54K gene (interferon-stimulated gene) exon 2 | M14660 | 29.6, ∼47.1 |
| KIAA0354 gene product | AB002352 | −6, −6.4 |
| KIAA0477 protein | AB007946 | −6.8, ∼−4.3 |
| Melanoma growth stimulatory activity (MGSA) | X54489 | −3, −4.3 |
| Mitogen-activated protein kinase kinase kinase 8 | Z14138 | −5.1, ∼−4.6 |
| Novel human gene mapping to chomosome 13, similar to rat RhoGAP | AL049801 | −3.7, −3.1 |
| Protein kinase C-alpha | AF035594 | ∼−10.3, ∼−4.3 |
| rel proto-oncogene | X75042 | −4.4, ∼−4.4 |
| Transforming growth factor beta-2 | M19154 | ∼−8.3, ∼−3.5 |
| Tyrosine kinase arg gene mRNA | M35296 | −5.4, ∼−4.1 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Two fold change values were determined in independent experiments. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
It has been shown previously that binding of glycoprotein B to the surface of cells is sufficient to induce activation of interferon response genes (3) as well as NF-κB (40), suggesting that a signal transduction cascade is activated by the binding of gB or the intact virion to its fusion receptor. Alternatively, it is possible that many or most of these changes are not gB specific but rather are caused by the triggering of an innate defensive pathway that detects the attachment or entry of a variety of different virus particles and leads directly to the transcription of a small subset of interferon response genes, including beta-interferon. A similar pathway was recently reported to be activated by herpes simplex virus (28), although this report did not distinguish between genes whose transcription is directly triggered by the virus and genes that are induced as a secondary consequence of interferon synthesis. This pathway seems to be distinct from the JAK-STAT interferon signaling pathway, since a U2OS cell line that was defective for the induction of interferon response genes during infection with herpes simplex virus, was still responsive to the addition of interferon (28).
More cellular mRNAs are induced by UV-HCMV than by HCMV.
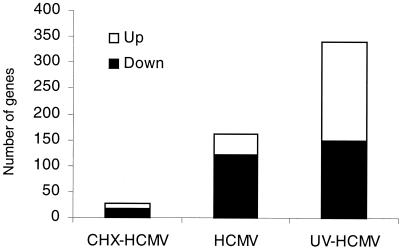
To monitor the effect of newly synthesized viral proteins on cellular gene expression, we compared the effects of infection with HCMV to UV-HCMV at 6 hpi. UV-inactivated virus delivers virion proteins to the cell, but virion DNA and mRNAs (6) are damaged and cannot be expressed. The levels of 161 mRNAs were altered by HCMV; UV-HCMV, by contrast, affected 340 (Fig. 3). The large increase in the number of mRNAs affected by UV-HCMV comes predominantly from mRNAs that are up-regulated. It is likely that the accumulation of this set of cellular mRNAs is normally blocked by a newly synthesized viral protein, a protein that is not produced in cells infected with the UV-irradiated virus.
FIG. 3.
HCMV replication represses the up-regulation of cellular genes. HFFs were infected at 6 PFU/cell with purified AD169 virus particles. For UV-irradiated HCMV, virus samples were exposed to UV radiation sufficient to reduce infectivity by 105-fold (data not shown). Infections were carried out in duplicate, and at 6 hpi, the cells were lysed and RNA samples were extracted and analyzed by Affymetrix GeneChip analysis to determine the number of up-regulated or down-regulated genes. Genes that changed by threefold or greater and had a difference call of I, MI, D, or MD in both replicates were considered to be significantly regulated. The total number of genes up and down-regulated is shown. CHX-HCMV, HCMV treated with cycloheximide.
We next identified the cellular mRNAs that changed in response to infection with one or both of the virus preparations (HCMV and UV-HCMV). The numbers of mRNAs in the subsets listed in Tables 5 to 7 are not equal to the total numbers of genes displayed in Fig. 3. This is because in many instances, an unambiguous change in a specific mRNA was evident after infection with one of the virus preparations, say HCMV, but the change was borderline and difficult to call for the other virus, i.e., UV-HCMV. Cellular mRNAs were placed into the categories of Tables 5 to 7 only when the data for both virus preparations allowed a clear determination.
TABLE 5.
Genes modulated similarly by HCMV and UV-HCMV
| Gene | Nuc IDa | HCMV fold changeb | UV-HCMV fold changeb |
|---|---|---|---|
| 2′-5′oligoadenylate synthetase-like | AJ225089 | 57.1, 26.1 | 74.7, 65.1 |
| Activating transcription factor 3 (ATF3) | L19871 | 6.5, 8.6 | 47.3, 47.9 |
| Acute myeloid leukemia protein | X90976 | −3.4, −4.1 | −3.7, −3.8 |
| ADP-ribosylation factor-like 7 | AB016811 | ∼−19.0, ∼−16.2 | −3.3, −3.5 |
| Adrenomedullin | D14874 | −11.8, −11.5 | −11, −12.1 |
| AML1 mRNA for AML1b protein (alternatively spliced product) | D43968 | −6.1, −11.5 | −3.7, −3.9 |
| Apolipoprotein AI regulatory protein (ARP-1) | M64497 | −5.1, −5.6 | −5.5, −5.5 |
| B2-bradykinin receptor 3 | X86163 | −8.2, −7.9 | −7.7, −9.1 |
| beta-transducin repeat containing protein | Y14153 | ∼5.0, ∼8.9 | ∼5.4, ∼4.6 |
| C2f | U72514 | ∼−6.9, −3.7 | −6.3, ∼−12.6 |
| cAMP-responsive element modulator | S68271 | 3.8, 4.3 | 5.3, 4.7 |
| cdc25 homolog | S78187 | −3.1, −2.3 | −8.3, −4.6 |
| Cdc42 effector protein 2 | AF001436 | −3.7, ∼−11.3 | ∼−11.4, ∼−8.8 |
| c-fos | V01512 | 11.1, 8.5 | 23, 23.7 |
| Chromosome 12 open reading frame | AF052105 | 4.1, 4.5 | 4.4, 4.4 |
| Cleavage stimulation factor, 3′ pre-RNA, subunit 1, 50 kDa | L02547 | ∼6.1, −5.4 | ∼−12.7, −7.9 |
| Core-binding factor, runt domain, alpha subunit 2; cyclin D-related | D43638 | −6, −6.9 | −3.2, −4 |
| Cyclin E2 | AF091433 | 4.6, ∼6.6 | ∼4.7, ∼6.6 |
| Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | AI304854 | ∼−16.0, −10.8 | −5.6, −8.4 |
| Cyclooxygenase 2 | U04636 | 5.7, 3.9 | 25.6, 23.3 |
| DKFZP564B0769 protein | AL080186 | ∼−8.9, ∼−5.7 | ∼−9.0, ∼−6.4 |
| DKFZP586K1520 protein | AL050153 | −4.1, −4.5 | ∼−12.4, ∼−13.5 |
| DNA sequence from clone 1175I6 on chromosome 20 | AL049538 | −3.2, −3 | −3.1, −3.2 |
| Dual-specificity phosphatase 10 | AB026436 | ∼−8.9, ∼−11.9 | ∼−12.6, −3.9 |
| EGR alpha | S81439 | −6.5, −3.6 | −3.8, −4.5 |
| ESTs | H94842 | −9, −4.6 | −5.5, −7.2 |
| Fucose-1-phosphate guanylyltransferase | AF017445 | −3.8, −7.5 | −3.4, −3.1 |
| Gamma-interferon-inducible early response gene (homology to platelet proteins) | X02530 | ∼3.0, ∼7.4 | ∼199.1, ∼179.1 |
| gas1 | L13698 | −27.1, −37.7 | −24, −19.4 |
| Growth differentiation factor 5 (cartilage-derived morphogenetic protein 1) | X80915 | ∼−24.0, ∼−24.0 | ∼−19.6, ∼−25.3 |
| GS3955 | D87119 | ∼−19.9, ∼−20.0 | −9.9, −12.6 |
| GTP cyclohydrolase 1 (dopa-responsive dystonia) | U19523 | 3.6, ∼7.2 | ∼34.9, ∼29.2 |
| homeo box A9 | U41813 | ∼−8.9, ∼−9.2 | ∼−10.9, ∼−11.9 |
| Homo sapiens cDNA FLJ13555 fis, clone PLACE1007677 | AL080210 | ∼−7.5, ∼−5.4 | ∼−6.7, ∼−7.0 |
| Homo sapiens cDNA, 5′ end | N36638 | −7.3, −8.9 | −6.4, −7.1 |
| Homo sapiens cig5 mRNA, partial sequence | AF026941 | ∼12.7, ∼18.9 | ∼326.2, ∼290.8 |
| Homo sapiens mRNA; cDNA DKFZp564G103 | AL049268 | ∼−5.7, ∼−5.0 | ∼−6.1, ∼−6.5 |
| hSIAH1 | U76247 | −3, −4.1 | −3.4, −3.7 |
| hTCF-4 | Y11306 | ∼−22.5, ∼−13.2 | ∼−18.3, ∼−17.2 |
| Hypothetical protein | AA522530 | −4.1, −3.6 | −9.8, −3.7 |
| Hypothetical protein LOC57187 | AA928996 | ∼−9.5, ∼−6.5 | ∼−4.2, ∼−5.1 |
| Hypothetical protein FLB6421 | AI133727 | 6, 5.4 | 9.4, 9 |
| Hypothetical protein FLJ10262 | AA115140 | −5.2, −6.3 | −5.7, −5 |
| Inhibitor of DNA binding 2, dominant-negative helix-loop-helix protein | D13891 | −3.9, −3 | −10.2, −6.6 |
| Interferon-induced protein with tetratricopeptide repeats 4 | AF026939 | 11.3, 8.8 | 8.6, 7.4 |
| Interleukin 11 | X58377 | ∼41.1, ∼53.5 | ∼133.9, ∼113.1 |
| ISG-54K gene (interferon-stimulated gene) | M14660 | 32.2, 16.7 | 11, 10.6 |
| KIAA0112 protein; homolog of yeast ribosome biogenesis regulatory protein RRS1 | D25218 | ∼−8.3, −5.3 | ∼−12.7, ∼−11.4 |
| KIAA0671 gene product | AB014571 | −10.1, −8.9 | −10.8, −9.6 |
| KIAA1010 protein | AB023227 | −3.4, −6.5 | −11.1, −7.9 |
| KIAA1116 protein | AB029039 | −3.9, −3.3 | −4.4, −4.6 |
| KIAA1288 protein | AL096842 | ∼−5.9, ∼−3.2 | ∼−7.2, ∼−4.5 |
| Monoclonal antibody 21 (Caenorhabditis elegans)-like 1 | U38810 | −13.9, −30.5 | −14.7, −9.7 |
| Meis1 (mouse) homolog | U85707 | −5.5, ∼−21.8 | −6.5, −5 |
| Mitogen-induced nuclear orphan receptor (MINOR) | U12767 | 8, 13.3 | 7.5, 5.7 |
| MN1 | X82209 | −21, ∼−34.0 | ∼−31.6, ∼−31.4 |
| Myxovirus resistance 1, homolog of murine (interferon-inducible protein p78) | M33882 | 5.2, 3.9 | 20.2, 17.4 |
| Novel human gene mapping to chomosome 13, similar to rat RhoGAP | AL049801 | −3.5, ∼−15.9 | −4.9, −4 |
| Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 4 | L41066 | ∼−18.6, ∼−14.3 | ∼−8.2, ∼−10.7 |
| Nuclear receptor subfamily 4, group A, member 2 | X75918 | ∼8.4, ∼9.9 | ∼9.5, ∼10.5 |
| Nuclear receptor subfamily 4, group A, member 3 | U12767 | ∼23.3, ∼41.5 | ∼29.8, ∼19.0 |
| Phorbol-12-myristate-13-acetate-induced protein 1 | D90070 | 9.3, 10.8 | 30.7, 25.7 |
| Phosphodiesterase 4B | L20971 | −11.2, −18.8 | −3.4, −4.4 |
| Pleckstrin homology, Sec7 and coiled/coil domains 1 (cytohesin 1) | M85169 | 7.7, 4.2 | 7.2, 6.7 |
| Polymerase (DNA directed), gamma 2, accessory subunit | U94703 | 4.6, ∼24.3 | ∼7.6, ∼6.6 |
| Protein phosphatase 1 (PPP1R5) | Y18207 | −7.6, −6.2 | −6.1, −5.3 |
| RAN-binding protein 6 | AF039023 | ∼−3.8, −3.4 | −3.2, −3.9 |
| ras homolog gene family, member E | S82240 | −5.9, −5.8 | −3.4, −3.6 |
| ras inhibitor | L36463 | −3.5, −5.4 | −3.2, −3.6 |
| RIP protein kinase | U50062 | 3, 3 | 8.5, 7.3 |
| runt-related transcription factor 1 (acute myeloid leukemia 1; aml1 oncogene) | D43969 | ∼−39.3, ∼−42.2 | ∼−41.6, ∼−43.1 |
| slug (chicken homolog), zinc finger protein | U69196 | −11, ∼−19.5 | −13.6, −9.2 |
| Solute carrier family 7 | M80244 | 4.5, 3.7 | 4, 4.3 |
| Stromal cell-derived factor 1 | L36033 | −4.2, −4.5 | −6.3, −9.7 |
| Thyroid receptor interactor (TRIP14) | L40387 | 4.1, 7.4 | 61.8, 53.8 |
| TR3 orphan receptor | L13740 | 6.3, 13.7 | 33.9, 26.9 |
| Transcription factor 21 | AF047419 | −3.9, ∼−23.0 | −3.2, −4 |
| v-myc avian myelocytomatosis viral oncogene homolog | V00568 | ∼−12.9, ∼−9.8 | ∼−12.9, −6.4 |
| wee1+ (S. pombe) homolog | X62048 | −5.3, −5.3 | −4.3, −3.5 |
| zinc finger protein 183 (RING finger, C3HC4 type) | X98253 | ∼−3.8, ∼−5.8 | ∼−7.9, ∼−7.5 |
| zinc finger protein 217 | AF041259 | ∼−27.9, ∼−41.2 | −8, −6.6 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Two fold change values were determined in independent experiments. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline on experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
TABLE 7.
Genes modulated by UV-HCMV but not HCMV
| Gene | Nuc IDa | HCMV fold changeb | UV-HCMV fold changeb |
|---|---|---|---|
| 37-kDa leucine-rich repeat (LRR) protein | U32907 | 1.2, −1.5 | ∼−8.4, ∼−8.5 |
| ATPase, class V, type 10D | AB025254 | 1.5, 1.5 | 9.1, 8.1 |
| ATP-binding cassette, subfamily A (ABC1), member 6 | AI651024 | 1.1, 1.1 | ∼−6.5, ∼−7.1 |
| ATP-binding cassette, subfamily B (MDR/TAP), member 2 | X57522 | 1.7, 1.3 | 7.9, 7.5 |
| Basic fibroblast growth factor | M27968 | −1.1, −1.4 | 3.6, 3.3 |
| B-cell CLL/lymphoma 7A | X89984 | −1.3, −1 | ∼−8.2, ∼−8.3 |
| BCR/ABL | M15025 | ∼1.8, 1.4 | ∼−7.9, ∼−6.6 |
| BRCA2 | U43746 | ∼−2.1, ∼1.8 | ∼5.3, ∼5.1 |
| CACCC box-binding protein | L04282 | −1.5, −1.5 | −3.2, −3.9 |
| CD38 antigen (p45) | D84276 | −1, ∼1.2 | ∼9.1, ∼8.5 |
| Cell cycle-related kinase | AF035013 | ∼−4.5, ∼2.8 | ∼13.2, ∼10.4 |
| Cell division cycle 2-like 1 (PITSLRE proteins) | M37712 | −1.4, −1.3 | −3.9, −3.1 |
| Chromobox homolog 6 | H10776 | −2.6, 3 | ∼−12.0, ∼−12.4 |
| Chromosome 21 open reading frame 5 | AJ237839 | 1.5, 1.4 | 3.6, 4.4 |
| Collagen, type XV, alpha 1 | L25286 | 1, 1.2 | 3.3, 3.2 |
| Cyclin B | M25753 | ∼−1.6, 1.9 | 4.1, 3.9 |
| Cyclin B1 | M25753 | 1.5, 1.5 | 3.5, 3.9 |
| Cyclin D2 | D13639 | −1.1, 1.7 | 4.1, 4.3 |
| Dermatopontin | AL049798 | −1.4, 2.2 | 7.4, 6.4 |
| Disintegrin-like and metalloprotease (reprolysin type) | AB002364 | 1.1, 2.5 | 3.5, 4.6 |
| DKFZP564A032 protein | AL050267 | ∼−2.2, ∼2.4 | ∼7.6, ∼7.2 |
| DKFZP564O0823 protein | AL080121 | ∼1.7, ∼−2.6 | ∼−8.6, ∼−8.5 |
| DNA sequence from clone 71L16 on chromosome Xp11 | AL022165 | ∼1.3, −2.7 | ∼−4.0, ∼−3.6 |
| E74-like factor 1 (ets domain transcription factor) | M82882 | −1.1, −1.4 | 3.2, 3.3 |
| Early growth response 2 (Krox-20 [Drosophila] homolog) | J04076 | ∼1.2, ∼2.1 | ∼12.8, ∼15.4 |
| Elastin (supravalvular aortic stenosis, Williams-Beuren syndrome) | X52896 | ∼−8.5, 1.9 | ∼52.0, 6.7 |
| Epidermal growth factor receptor | X00588 | −1.4, 1.2 | 7.7, 10.5 |
| ERCC5 excision repair protein | L20046 | −1.2, −1.2 | −3.1, −13.8 |
| Erythropoietin receptor | M60459 | 1.3, −1.2 | ∼−11.7, −3.4 |
| Formyl peptide receptor-like 1 | M84562 | −1, −1.6 | −5.4, ∼−9.9 |
| Gamma-interferon-inducible protein (IP-30) | J03909 | 1.1, 1.3 | 4.1, 3.3 |
| GTP-binding protein (RAB4) | M28211 | 1.1, −1.1 | −6, −5.7 |
| GTP-binding protein overexpressed in skeletal muscle | U10550 | −8.2, −5.8 | 4.1, 3.5 |
| Guanine nucleotide exchange factor | L13857 | ∼1.3, ∼2.9 | ∼6.0, ∼7.0 |
| H3 histone family, member B | N35832 | ∼−1.1, ∼1.8 | ∼6.7, ∼6.8 |
| Hepatitis B virus-associated factor (XAP4) | U67322 | 1.2, 1.1 | 10.6, 10 |
| Heat shock protein 3, 27 kDa | Y17782 | −2.9, −1.5 | −3, −3.1 |
| Histidyl-tRNA synthetase | U18937 | −1.6, −1.6 | −4.2, −3.2 |
| Homo sapiens BAC clone CTB-60N22 from 7q21 | AI825798 | 1.2, ∼2.5 | ∼3.9, ∼6.2 |
| Homo sapiens cDNA | W27604 | ∼−3.9, ∼2.1 | ∼3.1, ∼3.5 |
| Homo sapiens cDNA | AA160708 | 1.1, −1 | 3.3, 3.1 |
| Homo sapiens cDNA | AA203476 | 1.1, 2.5 | 4.2, 3.6 |
| Homo sapiens cDNA, 3′ end | AI887421 | −2.4, −1.8 | ∼−10.1, ∼−7.6 |
| Homo sapiens cDNA, 3′ end | AA156240 | −1.6, −2 | −7.4, ∼−21.6 |
| Homo sapiens cDNA, 3′ end | AI093511 | −1.4, −2.1 | −3.3, −4.4 |
| Homo sapiens cDNA, 3′ end | AI246894 | 1.5, ∼1.8 | ∼3.4, ∼4.5 |
| Homo sapiens clone 24438 mRNA sequence | AF070647 | ∼−1.8, ∼−2.3 | ∼−4.6, ∼−4.1 |
| MAD-3 | M69043 | −2.1, −2 | 3.5, 3.4 |
| MADS box transcription enhancer factor 2, polypeptide C (myocyte enhancer factor 2C) | S57212 | ∼1.2, ∼2.5 | −9.5, ∼7.9 |
| Major histocompatibility complex, class I, F | AL022723 | 1.1, −1.2 | 3.4, 3.2 |
| Matrilin 2 | U69263 | −1.1, −1.5 | ∼−5.7, ∼−7.0 |
| Microtubule-associated tau protein | X14474 | ∼2.0, ∼2.8 | ∼7.8, ∼5.8 |
| MIP-1a | D90144 | 1.3, 1.1 | 6.9, 6.5 |
| MIP-3b | AB000887 | ∼−2.5, ∼1.5 | ∼6.7, ∼8.2 |
| Monokine induced by gamma interferon | X72755 | ∼−2.5, ∼1.5 | ∼11.8, ∼12.6 |
| Msh (Drosophila) homeo box homolog 1 (formerly homeo box 7) | M97676 | 2, 1.8 | 6.1, 7.9 |
| Myeloid differentiation primary response gene (88 kDa) | U70451 | 1.1, 1.2 | 4, 3.4 |
| Nuclear antigen Sp100 | U36501 | −1, 1.9 | 5.7, 6.6 |
| P55pik | D88532 | −1.2, 2.1 | 6.2, 4.7 |
| Pancreatic polypeptide receptor 1 | U42387 | ∼8.2, −1 | ∼−5.8, ∼−7.3 |
| Phosphodiesterase 1A, calmodulin-dependent | U40370 | 3, 1.4 | 4.5, 5.2 |
| Phosphoinositide-3-kinase, regulatory subunit, polypeptide 3 (p55, gamma) | U90907 | 1.3, 1.2 | 3.3, 3 |
| Phosphorylase kinase, beta | L19314 | 1.1, ∼2.8 | ∼7.5, ∼6.6 |
| Plasminogen activator inhibitor-1 | J03764 | −1, ∼−4.9 | 14, 9.6 |
| Protein kinase C, nu | AB015982 | −1.1, −1.7 | ∼−6.5, −6.1 |
| Protein kinase Chk2 | AF086904 | ∼−3.0, −1.1 | ∼13.1, ∼11.2 |
| Protein tyrosine phosphatase (PAC-1) | L11329 | −1.6, ∼−1.5 | ∼34.4, ∼27.8 |
| Protein-tyrosine kinase (HEK7) | L36644 | ∼1.0, ∼−1.4 | ∼4.4, ∼3.9 |
| Putative DNA/chromatin binding motif | AJ132440 | −1.8, −1.4 | −5.4, −15 |
| RANTES | M21121 | 2.3, 1.2 | 76.6, 74.6 |
| Ras inhibitor Inf | HG511-HT511 | 1.2, 1.1 | −3.8, −5.5 |
| Receptor-interacting serine-threonine kinase 2 | AF117829 | −1.8, 1.4 | 3.4, 3.2 |
| Regulator of G-protein signaling 6 | AF073920 | 1.2, −1.2 | 5.5, 4.9 |
| Retinitis pigmentosa 2 (X-linked recessive) | AJ007590 | −1, 1.5 | 4.2, 4.7 |
| Retinoic acid receptor responder (tazarotene induced) 3 | AF060228 | 1.4, 1.3 | 4.6, 4.4 |
| Serine protease | AF015287 | −1.1, 1.3 | 3.3, 3.1 |
| Serine/threonine kinase 15 | AF011468 | −1.2, −1.8 | ∼−22.3, ∼−13.1 |
| Sjogren syndrome antigen A1 (52 kDa, ribonucleoprotein autoantigen SS-A/Ro) | M62800 | 2, 1.7 | 5.9, 5.5 |
| Solute carrier family 1 (glial high-affinity glutamate transporter), member 3 | D26443 | 1, 1.1 | 5.6, 5 |
| Steroid receptor (TR2-11) | M29960 | −1.7, −2.1 | ∼−6.2, −3.4 |
| Tachykinin, precursor 1 | U37529 | ∼−1.8, ∼2.0 | ∼4.9, ∼4.3 |
| Three prime repair exonuclease 1 | AJ243797 | −1.5, ∼1.9 | ∼25.4, −30.8 |
| Tissue inhibitor of metalloproteinases-2 (TIMP-2) | U44385 | −1.5, −1.2 | −4.4, −4.5 |
| TNF-related apoptosis inducing ligand TRAIL | U37518 | 1.5, 1.2 | 20.8, 18.2 |
| Toll-like receptor 3 | U88879 | ∼1.1, 1 | 4.5, 4.7 |
| Transcobalamin I (vitamin B12-binding protein, R binder family) | J05068 | −1.3, ∼1.3 | −4.7, ∼7.2 |
| Transcriptional unit N143 | AJ002572 | ∼−2.2, −2.8 | ∼−4.2, −7.2 |
| Tumor necrosis factor alpha-inducible protein A20 | M59465 | −1.3, −1.6 | 3.7, 3.1 |
| Tyrosine kinase-type receptor (HER2) | M12036 | ∼−2.8, −1.1 | ∼−11.6, ∼−10.9 |
| XIAP-associated factor 1 | X99699 | ∼−4.2, ∼−2.1 | ∼−10.1, ∼9.6 |
| zinc finger protein homologous to Zip-36 in mouse | M92843 | −6.6, −3 | 3.7, 3.2 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Two fold change values were determined in independent experiments. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
Eighty-two mRNAs (Table 5) changed by a factor of at least 3 after infection with both HCMV and UV-HCMV. Presumably, this set of changes occurs as a result of binding and entry of virus particles and/or the action of virion proteins within the infected cell. This set included mRNAs encoding cell cycle regulators that change early in HCMV infection, such as cyclin E, gas-1, and p27. This indicates that the ability of HCMV to cause G0 cells to reenter the cell cycle is probably mediated by virus binding or a tegument protein. Consistent with this hypothesis, the tegument protein pp71 can induce quiescent cells to reenter the cell cycle (R. Kalejita, J. Bechtel, and T. Shenk, submitted for publication).
Fifteen mRNAs (Table 6) were found to be significantly regulated at 6 hpi by HCMV but not by UV-HCMV, presumably representing a subset of mRNAs whose regulation in infection is dependent on new viral protein synthesis, i.e., they were not modulated by entry or virion proteins. Of these, 1 was up-regulated and 14 were down-regulated.
TABLE 6.
Genes modulated by HCMV but not UV-HCMV
| Gene | Nuc IDa | HCMV fold changeb | UV-HCMV fold changeb |
|---|---|---|---|
| ArgBPIB | X95677 | ∼−5.6, ∼−4.4 | ∼−1.7, ∼−2.3 |
| Beta nerve growth factor | X52599 | ∼−9.4, −6.8 | 1.3, 1.2 |
| Brain-derived neurotrophic factor | X60201 | −3.7, −3 | −1.5, −1.2 |
| c-jun | J04111 | ∼−16.1, −5.4 | −1.4, −1.3 |
| Dickkopf-1 | AB020315 | −14.7, −16.1 | −1.2, −1.2 |
| Guanine nucleotide exchange factor 2 | HG961-HT961 | ∼−4.8, ∼−6.0 | −1.7, −2.7 |
| Homo sapiens cDNA, 3′ end | AI761647 | −4.1, −5 | −2.6, −2.8 |
| Insulin receptor substrate 1 | S62539 | ∼−4.8, ∼−5.6 | −2.5, −1.7 |
| KIAA0459 protein | AB007928 | ∼10.9, 7 | ∼−2.9, 1.3 |
| KIAA0628 protein | AB014528 | ∼−5.1, ∼−3.1 | ∼−2.9, −2.2 |
| KIAA0989 protein | AB023206 | −3.8, −4.4 | −1.1, 1.1 |
| NF-IL6-beta | M83667 | −4.7, −5.7 | −1.2, −1.2 |
| Ovarian cancer downregulated myosin heavy-chain homolog (Doc1) | U53445 | −3, −3 | −1, 1 |
| p82 (ST5) | U15780 | −3.1, −5 | −1.8, −1.5 |
| SIX1 | X91868 | −12.9, −6.5 | −1.1, −1.2 |
Nuc ID, nucleotide sequence identification or sequence accession number.
Two fold change values were determined in independent experiments. A ∼ symbol in front of a fold change value indicates that the transcript was scored as absent in either the baseline or experimental sample and that the fold change was then calculated using the background noise value as the intensity for that comparison. The complete data set with difference calls can be accessed at http://www.molbio.princeton.edu/labs/shenk/browneetal2001/.
One hundred seventeen mRNAs (Table 7) were modulated by UV-HCMV but not HCMV. The majority (82 genes) were up-regulated, representing a set of mRNAs whose up-regulation is normally prevented by a newly synthesized viral protein. This list included many known antiviral genes, including members of the interferon response pathway and proinflammatory cytokines.
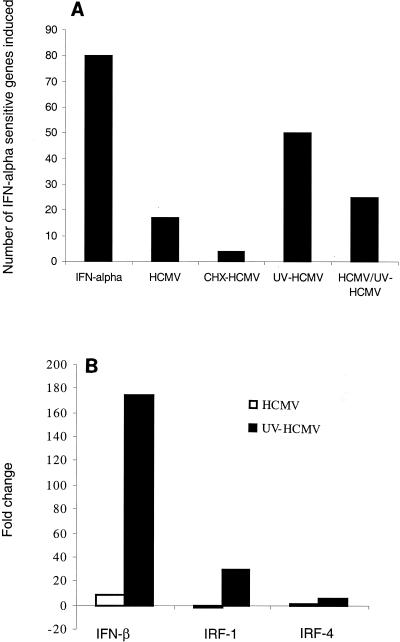
We next wanted to determine whether HCMV modulated the expression of all or a subset of the interferon-responsive genes on the 12,626 gene array. To identify the full set of interferon-responsive genes on the array, uninfected human fibroblasts were treated with alpha-interferon for 6 h, and RNA was prepared and analyzed. Interferon induced 79 mRNAs (Fig. 4A) and reduced the level of only one. Of the 161 genes that change in HCMV-infected cells by 6 hpi, only 17 (Fig. 4A) of these overlap with the set that are activated by alpha-interferon. However, when fibroblasts were infected with UV-HCMV, a much larger subset, 50 genes (Fig. 4A) of the interferon-sensitive genes were found to be significantly up-regulated. To rule out the possibility that the interferon response pathway is being activated by the presence of UV-damaged DNA in infected cells, we carried out a mixed infection of HCMV with UV-HCMV. If interferon-sensitive genes are induced by damaged viral DNA, then the inclusion of functional virus will not block their induction. However, the mixed infection led to the up-regulation of only 25 interferon response genes at 6 hpi (Fig. 4A), and the genes that continued to score were induced to a lesser extent. Since the inclusion of HCMV substantially blocked the induction of interferon-responsive mRNAs, we conclude that the lack of a viral gene product, rather than the presence of damaged DNA, accounts for the larger number of interferon response genes up-regulated by UV-HCMV. It has been reported previously that HCMV infection causes a block in the interferon signaling pathway by inducing degradation of JAK1 and p48 (27). However, this was not observed until 48 hpi. Since the interferon response is substantially blocked at 6 hpi, it is likely that a different mechanism is involved, a possibility that is consistent with our observation that HCMV synthesizes one or more gene products that inhibit cellular mRNA accumulation. Interferon regulatory factor 1 (IRF-1) and IRF-4, two proteins that play a role in beta-interferon transcription (24), were much more greatly up-regulated after infection with UV-HCMV than HCMV (Fig. 4B). Consequently, it is possible that the early block to the accumulation of interferon-responsive mRNAs results primarily from a block to transcription of the beta-interferon gene—indeed, beta-interferon transcription was much more highly induced by UV-HCMV than by HCMV (Fig. 4B). A specific subset of interferon-responsive mRNAs continues to accumulate to high levels in the presence of this block. However, many of these mRNAs appear to be expressed at substantially lower levels during infection with HCMV than with UV-HCMV as well.
FIG. 4.
HCMV replication represses activation of the interferon response pathway. Human foreskin fibroblast cells were treated with 5,000 U of alpha-interferon per ml for 6 h. The cells were then lysed, and RNA was analyzed by Affymetrix GeneChip analysis. Seventy-nine genes were found be up-regulated by interferon in duplicate, and the proportion of these genes that were also up-regulated by HCMV, by HCMV in the presence of cycloheximide (CHX), and by UV-HCMV at 6 hpi were counted and graphed (A). The fold inductions for beta-interferon, IRF1, and IRF4 were averaged for two replicates and then graphed (B). IFN, interferon.
Recently it was reported that the changes in mRNA levels occurring in HCMV-infected fibroblasts at 8 or 24 hpi are not significantly different from those that occur following treatment with interferon or a recombinant soluble form of the HCMV glycoprotein B (34). By contrast, our data reveal that at 6 hpi, many interferon-responsive mRNAs fail to accumulate due to active suppression by the virus (Fig. 4A). Furthermore, many mRNAs that change in infection are not responsive to interferon. We cannot yet explain the difference between the two data sets.
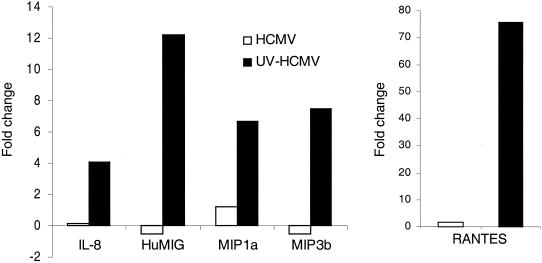
Accumulation of several proinflammatory chemokine mRNAs was also prevented by one or more newly synthesized viral gene products at 6 hpi (Fig. 5). RANTES, interleukin 8, HuMIG, MIP1a, and MIP3b were all strongly induced by UV-HCMV but not HCMV. This repression of inflammatory chemokine expression would conceivably prevent the recruitment of immune cells such as lymphocytes and macrophages to the site of infection.
FIG. 5.
HCMV replication represses activation of proinflammatory chemokines. Human foreskin fibroblast cells were infected for 6 h with HCMV or UV-HCMV at 6 PFU/cell, before RNA was extracted and used for Affymetrix GeneChip analysis. The fold inductions for the genes shown above were averaged for two replicates. IL-8, interleukin 8; HuMIG, human inducer of gamma interferon; MIP1a and MIP3b, macrophage inflammatory protein 1a and 3b, respectively.
HCMV also prevents the accumulation of mRNAs that could potentially block cell cycle progression. The transcription factor junB, which negatively regulates the growth-promoting activities of c-jun (32), is rapidly induced in the infection time course but returns to background levels by 4 to 6 hpi. In cells infected with UV-HCMV, however, junB up-regulation remains high at 6 hpi.
Conclusion.
Our array analysis has revealed an active, virus-mediated repression of cellular mRNA accumulation taking place during the first 6 h of the infection. During this time period, the number of up-regulated mRNAs lags significantly behind the number of down-regulated mRNAs (Fig. 1B). The repression is not evident at 6 h after infection of cells with UV-inactivated virus (Fig. 3), i.e., more cellular mRNAs accumulate in UV-HCMV-infected cells than in HCMV-infected cells, allowing us to conclude that the repression results from a newly synthesized viral gene product. This gene product blocks the accumulation of a variety of cellular mRNAs including those encoding antiviral proteins. The number of up-regulated mRNAs rises dramatically from 10 to 16 hpi, presumably preparing the cell for the start of viral DNA replication at about 24 hpi. A similar repressive effect of herpes simplex virus on interferon-responsive genes has been reported recently (28).
The identity of the HCMV protein or proteins that inhibit accumulation of cellular mRNAs with antiviral activities, and the mechanism by which they act remain unknown. Since repression occurs very rapidly after infection, it must result from the activity of an immediate-early gene product or an early gene product that is expressed very rapidly after infection. The viral product might function as a transcriptional repressor, directly affecting the transcription of cellular genes; it could act indirectly, up-regulating a cellular repressor; or it could act posttranscriptionally to influence mRNA levels.
ACKNOWLEDGMENT
This work was supported by a grant from the National Cancer Institute (CA87661).
REFERENCES
- 1.Aggarwal B B. Tumour necrosis factor receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-κB. Ann Rheum Dis. 2000;59(Suppl. 1):i6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner M, Ball C A, Blake J A, Botstein D, Butler H, Cherry J M, Davis A P, Dolinski K, Dwight S S, Eppig J T, Harris M A, Hill D P, Issel-Tarver L, Kasarskis A, Lewis S, Matese J C, Richardson J E, Ringwald M, Rubin G M, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle K A, Pietropaolo R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozza M, Bliss J L, Dorner A J, Trepicchio W L. Interleukin-11 modulates Th1/Th2 cytokine production from activated CD4+ T cells. J Interferon Cytokine Res. 2001;21:21–30. doi: 10.1089/107999001459123. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan W A, Bolldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan W A, Shenk T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science. 2000;288:2373–2376. doi: 10.1126/science.288.5475.2373. [DOI] [PubMed] [Google Scholar]
- 7.Brune W, Menard C, Heesemann J, Koszinowski U H. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291:303–305. doi: 10.1126/science.291.5502.303. [DOI] [PubMed] [Google Scholar]
- 8.de La Fuente C, Deng L, Santiago F, Arce L, Wang L, Kashanchi F. Gene expression array of HTLV type 1-infected T cells: up-regulation of transcription factors and cell cycle genes. AIDS Res Hum Retroviruses. 2000;16:1695–1700. doi: 10.1089/08892220050193164. [DOI] [PubMed] [Google Scholar]
- 9.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen M B, Spellman P T, Brown P O, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortunato E A, McElroy A K, Sanchez V, Spector D. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–119. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 12.Garnett H M. Increased ability of human embryonic fibroblasts to accumulate Ca2+ due to cytomegalovirus infection. Cytobios. 1981;31:107–116. [PubMed] [Google Scholar]
- 13.Geiss G K, Bumgarner R E, An M C, Agy M B, van't Wout A B, Hammersmark E, Carter V S, Upchurch D, Mullins J I, Katze M G. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology. 2000;266:8–16. doi: 10.1006/viro.1999.0044. [DOI] [PubMed] [Google Scholar]
- 14.Geiss G K, An M C, Bumgarner R E, Hammersmark E, Cunningham D, Katze M G. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J Virol. 2001;75:4321–4331. doi: 10.1128/JVI.75.9.4321-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J W, Lutz R J, Watanabe S, Cahir McFarland E D, Kieff E D, Mocarski E S, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang E S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975;16:1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahn G, Knust E, Schmolla H, Sarre T, Nelson J A, McDougall J K, Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984;49:363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jault F M, Jault J M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johannes G, Carter M S, Eisen M B, Brown P O, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc Natl Acad Sci USA. 1999;96:13118–13123. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E S. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee T G, Tang N, Thompson S, Miller J, Katze M G. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loenen W A, Bruggeman C A, Wiertz E J. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin Immunol. 2001;13:41–49. doi: 10.1006/smim.2001.0294. [DOI] [PubMed] [Google Scholar]
- 23.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marecki S, Riendeau C J, Liang M D, Fenton M J. Pu.1 and multiple ifn regulatory factor proteins synergize to mediate transcriptional activation of the human il-1β gene. J Immunol. 2001;166:6829–6838. doi: 10.4049/jimmunol.166.11.6829. [DOI] [PubMed] [Google Scholar]
- 25.Mocarski E S, Courcelle C T. Cytomegaloviruses and their replication. In: Knipe D M, Howley P M, Griffin D E, Lamb R A, Martin M A, Roizman B, Strauss S E, editors. Fields virology. 4th ed. Philadelphia, Pa: Lippincott-Raven; 2001. [Google Scholar]
- 26.Michelson S. Human cytomegalovirus escape from immune detection. Intervirology. 1999;42:301–307. doi: 10.1159/000053964. [DOI] [PubMed] [Google Scholar]
- 27.Miller D M, Zhang Y, Rahill B M, Waldman W J, Sedmak D D. Human cytomegalovirus inhibits IFN-α-stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-α signal transduction. J Immunol. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 28.Mossman K L, Macgregor P F, Rozmus J J, Goryachev A B, Edwards A M, Smiley J R. Herpes simplex virus triggers and then disarms a host antiviral response. J Virol. 2001;75:750–758. doi: 10.1128/JVI.75.2.750-758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller H, Bracken A P, Vernell R, Moroni M C, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner J D, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–285. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietiainen V, Huttunen P, Hyypia T. Effects of echovirus 1 infection on cellular gene expression. Virology. 2000;276:243–250. doi: 10.1006/viro.2000.0551. [DOI] [PubMed] [Google Scholar]
- 31.Rodems S M, Spector D H. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J Virol. 1998;72:9173–9180. doi: 10.1128/jvi.72.11.9173-9180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutte J, Viallet J, Nau M, Segal S, Fedorko J, Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989;59:987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- 33.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 34.Simmen K A, Singh J, Luukkonen B G, Lopper M, Bittner A, Miller N E, Jackson M R, Compton T, Fruh K. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc Natl Acad Sci USA. 2001;98:7140–7145. doi: 10.1073/pnas.121177598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth, M. J., J. M. Kelly, V. R. Sutton, J. E. Davis, K. A. Browne, T. J. Sayers, and J. A. Trapani. Unlocking the secrets of cytotoxic granule proteins. J. Leukoc. Biol. 70:18–29. [PubMed]
- 36.Swain S L. CD4 T cell development and cytokine polarization: an overview. J Leukoc Biol. 1995;57:795–798. doi: 10.1002/jlb.57.5.795. [DOI] [PubMed] [Google Scholar]
- 37.Valyi-Nagy T, Bandi Z, Boldogh J, Albrecht T. Hydrolysis of inositol lipids: an early signal of human cytomegalovirus infection. Arch Virol. 1988;101:199–207. doi: 10.1007/BF01311001. [DOI] [PubMed] [Google Scholar]
- 38.Wiertz E, Hill A, Tortorella D, Ploegh H. Cytomegaloviruses use multiple mechanisms to elude the host immune response. Immunol Lett. 1997;57:213–216. doi: 10.1016/s0165-2478(97)00073-4. [DOI] [PubMed] [Google Scholar]
- 39.Williams T M, Montoya G, Wu Y, Eddy R L, Byers M G, Shows T B. The TCF8 gene encoding a zinc finger protein (Nil-2-a) resides on human chromosome 10p11.2. Genomics. 1992;14:194–196. doi: 10.1016/s0888-7543(05)80307-6. [DOI] [PubMed] [Google Scholar]
- 40.Yurochko A D, Mayo M W, Poma E E, Baldwin A S, Jr, Huang E S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H, Cong J P, Shenk T. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular mRNAs: induction of interferon-responsive RNAs. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Cong J P, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]