Abstract
BACKGROUND
Incidental pulmonary nodules are an increasingly common finding on computed tomography (CT) scans of the thorax due to the exponential rise in CT examinations in everyday practice. The majority of incidental pulmonary nodules are benign and correctly identifying the small number of malignant nodules is challenging. Ultra-low-dose CT (ULDCT) has been shown to be effective in diagnosis of respiratory pathology in comparison with traditional standard dose techniques. Our hypothesis was that ULDCT chest combined with model-based iterative reconstruction (MBIR) is comparable to standard dose CT (SDCT) chest in the analysis of pulmonary nodules with significant reduction in radiation dose.
AIM
To prospectively compare ULDCT chest combined with MBIR with SDCT chest in the analysis of solid pulmonary nodules.
METHODS
A prospective cohort study was conducted on adult patients (n = 30) attending a respiratory medicine outpatient clinic in a tertiary referral university hospital for surveillance of previously detected indeterminate pulmonary nodules on SDCT chest. This study involved the acquisition of a reference SDCT chest followed immediately by an ULDCT chest. Nodule identification, nodule characterisation, nodule measurement, objective and subjective image quality and radiation dose were compared between ULDCT with MBIR and SDCT chest.
RESULTS
One hundred solid nodules were detected on ULDCT chest and 98 on SDCT chest. There was no significant difference in the characteristics of correctly identified nodules when comparing SDCT chest to ULDCT chest protocols. Signal-to-noise ratio was significantly increased in the ULDCT chest in all areas except in the paraspinal muscle at the maximum cardiac diameter level (P < 0.001). The mean subjective image quality score for overall diagnostic acceptability was 8.9/10. The mean dose length product, computed tomography volume dose index and effective dose for the ULDCT chest protocol were 5.592 mGy.cm, 0.16 mGy and 0.08 mSv respectively. These were significantly less than the SDCT chest protocol (P < 0.001) and represent a radiation dose reduction of 97.6%.
CONCLUSION
ULDCT chest combined with MBIR is non-inferior to SDCT chest in the analysis of previously identified solid pulmonary nodules and facilitates a large reduction in radiation dose.
Keywords: Ultra-low dose computed tomography, Solid pulmonary nodules, Computed tomography methods, Radiation dosage, Adult human
Core Tip: Recent advancements in computed tomography (CT) hardware and software have facilitated the development of ultra-low-dose imaging protocols that have the potential to significantly reduce radiation dose while, crucially, maintaining image quality and diagnostic integrity. Previously identified indeterminate solid pulmonary nodules may be effectively monitored with ultra-low-dose CT chest with the added benefit of a large reduction in radiation dose.
INTRODUCTION
Incidental pulmonary nodules are an increasingly common finding in routine patient care secondary to the exponential rise in utilisation of chest computed tomography (CT)[1,2]. The majority of incidental pulmonary nodules are benign and correctly identifying malignant nodules poses a diagnostic challenge[3]. Based on various morphological nodule metrics, indeterminate solid pulmonary nodules are frequently followed with serial chest CT to monitor for changes that may represent malignancy. Typical solid pulmonary nodule features that correlate with likelihood of malignancy include size, internal features (e.g. cavitation), border characteristics (e.g. smooth, spiculated) and perinodular surround characteristics (e.g. pleural tethering)[4-6]. Hendrix et al[7] report that in a cohort of almost 75000 patients between 2008 and 2019 the percentage of patients with pulmonary nodules increased from 38% to 50% and the proportion of stage 1 lung cancers doubled. These findings highlight the paramount importance of correctly identifying and characterising pulmonary nodules.
The concept of low-dose CT (LDCT) chest imaging was proposed by Naidich et al[8] in 1990. The National Lung Screening Trial, a randomised control trial in 2011, demonstrated a 20% reduction in mortality when LDCT chest was utilised in favour of traditional chest radiography in screening an asymptomatic high risk population[9]. LDCT has been utilised for over a decade in screening programmes of high risk patients for lung malignancy and its ability to characterise solid pulmonary nodules is well established[10]. However, the use of serial ionising radiation examinations in an asymptomatic population raises concern regarding cumulative effective dose (ED) and subsequent carcinogenesis[11]. Ultra-LDCT (ULDCT) chest imaging protocols have been developed in an effort to further reduce this ionising radiation burden. Inadequate signal to noise ratio (SNR) has typically been the most common limitation of ULDCT techniques and recent advances in hardware and software in a range of clinical settings, have shown potential in surmounting this limitation. Model-based iterative reconstruction (MBIR) takes advantage of statistical algorithm techniques to model x-ray production, tissue attenuation and sources of noise in a CT examination which allows large reductions in noise in the reconstructed images[12].
ULDCT chest imaging protocols with MBIR have been shown to facilitate both a large reduction in radiation dose and produce CT images capable of providing comparable diagnostic accuracy of respiratory pathology in comparison with the more traditional filtered back projection reconstruction technique[13-15]. Maintaining diagnostic integrity is essential when adjusting imaging protocols. The ability of ULDCT chest protocols to detect and characterise pulmonary nodules has been demonstrated in numerous in vitro studies[16-19]. Currently, the clinical utility of ULDCT chest in the assessment of pulmonary nodules is uncertain.
In this study, we aimed to prospectively compare ULDCT chest combined with MBIR with standard dose CT (SDCT) chest in the detection, measurement and characterisation of solid pulmonary nodules. A secondary aim was to compare the radiation dose between the two protocols.
MATERIALS AND METHODS
Study design and population
Following institutional ethical board approval (Clinical Research Ethics Committee reference number: ECM4(g)1/3/16 & ECM3(nnnn)9/3/21) a prospective cohort study was conducted. The study population consisted of adult patients (n = 30) attending respiratory outpatient clinic for surveillance of previously detected indeterminate solid pulmonary nodules on SDCT chest.
Inclusion criteria for the study were as follows: > 35 years of age, ability to provide informed written consent, and current or former smoker. Exclusion criteria were as follows: Unable to give informed consent, active malignancy, pregnancy or any condition, or ailment precluding the ability to lie flat for the duration of the scan.
Each potential participant was given information in simple language about the objective, methods, and risks of study participation. The study procedure which involved the acquisition of reference SDCT chest followed by an ULDCT chest was explained to all subjects. If aggregable to participation, written informed consent was obtained.
CT imaging technique
A SDCT and an ULDCT chest without intravenous contrast were acquired with a 64-row multi-detector CT system (Discovery CT750 HD; GE Healthcare, Waukesha, WI, United States). Our previously published MBIR ULDCT chest protocol was utilised in all ULDCT acquisitions[14]. Briefly, this involved a tube voltage: 80 Kv; tube current: 20 mA; gantry rotation time: 0.4 seconds; pitch factor: 1.375; and FOV of 32 cm. Scanning was performed at end-inspiration from lung apices to bases. No additional expiratory phase imaging was performed. Images were acquired at slice thickness of 0.625 mm.
Nodule identification, measurement and characterisation
Nodules were detected, measured and characterised, according to the Fleischner Society Guidelines, independently on review of the PACS system on a dedicated workstation (Advantage Workstation VolumeShare 2, Version 4.4, GE Medical Systems, Milwaukee, WI, United States) by two consultant radiologists with a subspeciality interest in chest radiology[4].
Objective image quality
Objective image quality analysis was performed independently on a dedicated workstation (Advantage Workstation VolumeShare 2, Version 4.4, GE Medical Systems, Milwaukee, WI, United States) by two readers in line with our previously published work[20]. The readers were blinded to the scanning protocol used and the order of the datasets was randomized. Briefly, attenuation values were measured in Hounsfield units (HU) at three levels: Aortic arch, carina, and the maximum cardiac diameter. Measurements were recorded by placing circle histograms of equal size (diameter, 10 mm) in the descending aorta and paraspinal muscles of the posterior chest wall at each level. These regions of interest (ROIs) were placed in a homogenous area, taking care to avoid fat planes and blood vessels. The standard deviation of the mean attenuation in the ROI served as an objective measure of image noise. The SNR of each ROI was calculated by dividing the mean HU by its standard deviation. Measurements were taken three times by each operator to reduce error and the mean recorded. The mean of both readers’ measurements was used for analysis.
Subjective image quality
The ability to identify and characterise the pulmonary nodules on the ULDCT chest protocol was subjectively scored by two readers on a scale of 1-10, with 1 being unacceptable, 5 acceptable and 10 excellent. The ULDCT chest was also subjectively rated for overall diagnostic acceptability on the same scale. The mean of both readers’ measurements was used for analysis. The subjective image quality of the ULDCT chest with MBIR was assessed independently and not directly compared with the corresponding SDCT chest for each patient. SDCT chest is our institution’s ‘gold standard’ for the assessment of lung nodules and is considered 10/10 on subjective image quality assessment.
Radiation dose analysis
Radiation dose data were taken from individual institutional reports. The CT dose index volume (CTDIvol) in mGy and dose length product (DLP) in mGy.cm were recorded. The ED was calculated using a conversion factor of 0.014 as per validated literature[21].
Statistical analysis
Statistical analysis was carried out using SPSS version 28 (IBM SPSS Inc., Chicago, Il). Data were exported into SPSS from Microsoft Office Excel (Microsoft Corporation, CA, United States) for statistical analysis. Descriptive statistics were utilised for patient demographics. Following Shapiro-Wilk normality testing, a paired t-test was utilised to compare the ULDCT and SDCT protocols. Intraclass correlation coefficient (ICC) was used to evaluate interrater reliability. A P value of < 0.05 was considered statistically significant. Data are presented as median and standard deviation unless otherwise specified in the text.
RESULTS
Thirty patients (13 female; 17 male) (mean age 64 ± 12.3 years) were included. Each patient underwent both SDCT chest and ULDCT chest with MBIR examinations and these findings were compared with previously performed index imaging to establish a baseline nodule burden. The mean duration between index and follow up examinations was 217 days.
Nodule identification, measurement and characterisation
One hundred and twenty-two nodules (100 solid nodules) were detected on ULDCT chest (mean of 4.1 nodules), and 116 nodules (98 solid nodules) were detected on SDCT chest (mean of 3.9 nodules; Table 1).
Table 1.
Comparison of nodule identification
|
Identified nodules
|
Index SDCT
|
Study SDCT
|
ULDCT
|
| Solid nodules | 94 | 98 | 100 |
| Ground glass opacities | 5 | 4 | 5 |
| Calcified granulomas | 10 | 11 | 14 |
| Part solid nodules | 4 | 3 | 3 |
| Total | 113 | 116 | 122 |
Comparison of nodule identification between the index standard dose computed tomography (SDCT), study SDCT and ultra-low dose computed tomography (ULDCT) chest imaging protocols. This table demonstrates minimal variability between protocols with ULDCT identifying slightly more solid pulmonary nodules than the SDCT. This discrepancy did not reach statistical significance. SDCT: Standard dose computed tomography; ULDCT: Ultra-low dose computed tomography.
The number of solid pulmonary nodules on SDCT chest in comparison with index CT were similar with the expected interval resolution and subsequent development of a small number of pulmonary nodules.
There was no significant change in size of the solid pulmonary nodules detected between ULDCT chest with MBIR and SDCT chest protocols. The mean pulmonary nodule size for ULDCT chest was 4.51 ± 2.47 mm and for SDCT chest was 4.47 ± 2.53 mm (P = 0.328; Figure 1). ICC as an indication of interrater agreement for nodule size on ULDCT was r = 0.961 and on SDCT, r = 0.933 (excellent reliability).
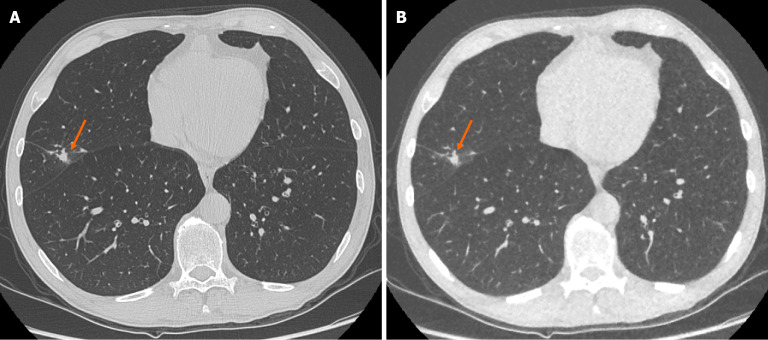
Figure 1.
Representative images demonstrating image quality and ability to identify pulmonary nodules on both standard dose computed tomography chest and ultra-low-dose computed tomography chest with model-based iterative reconstruction imaging protocols. A: Selected axial slice of a standard dose computed tomography (CT) chest presented in lung windows with a solid pulmonary nodule with spiculation and pleural tethering in the lateral segment of the middle lobe (arrow); B: Selected axial slice of an ultra-low dose CT chest in the same patient at the same level presented in lung windows with the same correctly identified pulmonary nodule in the middle lobe (arrow). These images demonstrate the ability of ultra-low-dose CT chest with model-based iterative reconstruction to adequately maintain diagnostic accuracy with regard to solid pulmonary nodules.
There was no significant difference in the characteristics of correctly identified nodules when comparing SDCT chest to ULDCT chest with MBIR protocols (P = 0.09). For example, there was no significant difference in the ability to characterise lesions as cavitating or spiculated (Figure 2).
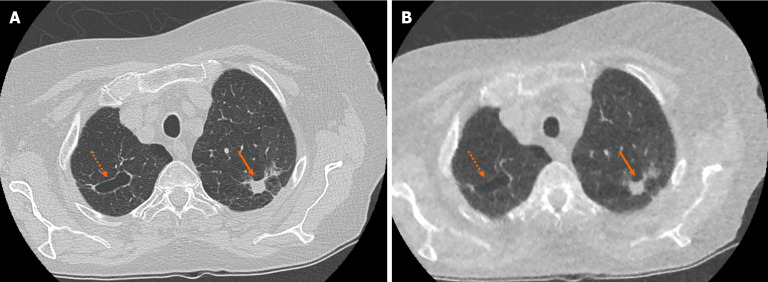
Figure 2.
Example of accurate pulmonary nodule characterisation on ultra-low-dose computed tomography chest. A: Selected axial slice of a standard dose computed tomography (CT) chest presented in lung windows with a spiculated solid pulmonary nodule with pleural tethering in the apico-posterior segment of the left upper lobe (solid arrow) and a parenchymal cyst in the apical segment of the right upper lobe (dashed arrow); B: Selected axial slice in the same patient presented in lung windows at the same level highlights the ability of ultra-low-dose CT chest with model-based iterative reconstruction to correctly characterise pulmonary nodule features such as spiculation, tethering and cavitation.
The ULDCT chest protocol demonstrated a small number of false positive and false negative pulmonary nodules when compared to the traditional SDCT chest protocol (Figure 3 and Figure 4 respectively). These minor discrepancies did not reach statistical significance.
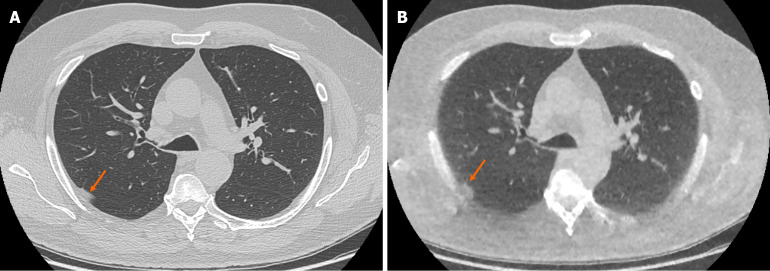
Figure 3.
Example of false positive pulmonary nodule identification on ultra-low-dose computed tomography chest. A: Selected axial slice of a standard dose computed tomography (CT) chest presented in lung windows with a small focus of peripheral atelectasis in the posterior segment of the right upper lobe (arrow); B: Selected axial slice of an ultra-low dose CT chest with model-based iterative reconstruction presented in lung windows at the same level in the same patient with the focus of soft tissue attenuation in the posterior segment of the right upper lobe incorrectly identified as a solid pulmonary nodule (arrow). The incidence of false positive solid nodule identification was minimal and did not reach statistical significance.
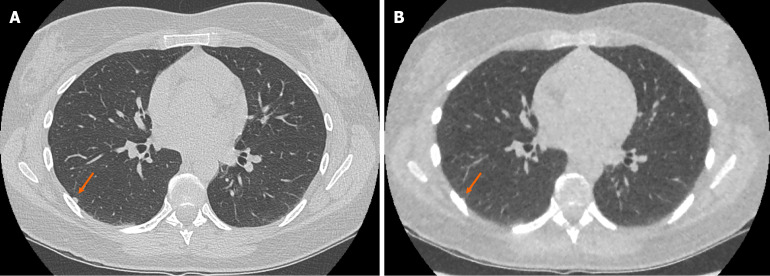
Figure 4.
Example of false negative pulmonary nodule identification on ultra-low-dose computed tomography chest. A: Selected axial slice of a standard dose computed tomography (CT) chest presented in lung windows with a solid pulmonary nodule abutting the pleura in the lateral segment of the right lower lobe (arrow); B: Selected axial slice of an ultra-low dose CT chest with model-based iterative reconstruction in the same patient at the same level presented in lung windows demonstrating the less conspicuous pulmonary nodule that was not identified (arrow). The incidence of false negative solid nodule identification was minimal and did not reach statistical significance.
Objective image quality
Objective noise and SNR were measured in the aortic lumen and paraspinal muscles at the level of the aortic arch, carina and largest cardiac diameter (Table 2). Noise was significantly reduced in the paraspinal muscles at the level of the aortic arch and carina in the ULDCT chest protocol (reconstructed with MBIR) in comparison with the SDCT chest protocol (P < 0.001). SNR was significantly increased in the ULDCT chest with MBIR in comparison to SDCT chest in all areas except in the paraspinal muscle at the maximum cardiac diameter level (P < 0.001). The remainder of the results did not reach statistical significance.
Table 2.
Noise and signal-to-noise ratio
| Level | 10 mm ROI |
Noise (HU)
|
SNR
|
||||
|
ULDCT
|
SDCT |
P value
|
ULDCT
|
SDCT
|
P value
|
||
| Aortic arch | Aortic lumen | 22.28 ± 3.6 | 21.06 ± 2.9 | 0.149 | 2.86 ± 0.8 | 1.53 ± 0.3 | < 0.001 |
| Paraspinal muscles | 20.86 ± 3.6 | 27.29 ± 4 | < 0.001 | 2.84 ± 0.7 | 1.89 ± 0.4 | < 0.001 | |
| Carina | Aortic lumen | 23.8 ± 12.4 | 22.98 ± 4.2 | 0.359 | 2.63 ± 0.9 | 1.57 ± 0.6 | < 0.001 |
| Paraspinal muscles | 22.33 ± 4.4 | 28.28 ± 4.9 | < 0.001 | 2.35 ± 1.1 | 1.72 ± 0.5 | < 0.001 | |
| Max. cardiac diameter | Aortic lumen | 23.14 ± 7.9 | 27.7 ± 12.9 | 0.061 | 2.14 ± 0.9 | 1.17 ± 0.6 | < 0.001 |
| Paraspinal muscles | 23.44 ± 3.2 | 23.37 ± 4 | 0.472 | 1.66 ± 0.6 | 1.61 ± 0.6 | 0.318 | |
Bold face P value indicates statistical significance.
Comparison of objective quantitative measures of image noise and signal-to-noise ratio at three levels from the ultra-low dose computed tomography (ULDCT) with model-based iterative reconstruction and standard dose computed tomography chest protocols. Overall, there is a statistically significant increase in signal to noise ratio in the ULDCT imaging protocol. HU: Hounsfield units, SNR: Signal to noise ratio; ROI: Region of interest; ULDCT: Ultra-low dose computed tomography; SDCT: Standard dose computed tomography.
Subjective image quality
The mean subjective image quality score, from a maximum score of 10, for the ability to identify and characterise the pulmonary nodules on ULDCT with MBIR was 7.8 ± 1.48 and overall diagnostic acceptability was 8.9 ± 0.93. The ICC for diagnostic satisfaction had an r value of 0.548 and quality of nodule visualisation r value was 0.511 (moderate reliability).
Radiation dose analysis
The mean DLP, CTDIvol and ED for the ULDCT chest with MBIR protocol were 5.592 mGy.cm, 0.16 mGy and 0.08 mSv respectively. These were significantly less than the SDCT chest protocol with mean DLP, CTDIvol and ED of 237.1 mGy.cm, 7.2 mGy and 3.21 mSv respectively (P < 0.001). This represents an overall radiation dose reduction of 97.6%.
DISCUSSION
This prospective cohort study demonstrates that ULDCT chest combined with MBIR is adequate when compared to SDCT chest in the identification, measurement and characterisation of previously identified solid pulmonary nodules while facilitating a 97.6% associated reduction in radiation dose (mean ED of 0.08 mSv vs 3.21 mSv).
Excessively long computer processing times is a typical limitation of advanced reconstruction algorithms and MBIR represents the latest attempt to overcome this and facilitate its routine inclusion in clinical practice[22]. A clear benefit of CT radiation dose reduction techniques are the potential benefits in paediatric patient cohorts in particular. MBIR has been shown to be superior to other reconstruction techniques in imaging children[23,24]. Carcinogenesis risk due to lifetime cumulative ED from medical imaging is a challenging topic without a definitive consensus. A large systematic review and dose-response meta-analysis assessing over 110 million adults over 3 continents found an inordinate increase in cancer risk in adults that positively correlated with CT radiation dose exposure[25]. Given the current data, a prudent approach is one that endeavours to reduce the radiation dose delivered to the patient without compromising diagnostic integrity. In concordance with other published work, our MBIR protocol facilitated a large reduction in radiation dose while maintaining diagnostic integrity[26].
With such a large reduction in radiation dose there are inevitable concerns regarding the potential consequences of misdiagnosis. In our prospective study of 30 patients, the ULDCT chest with MBIR protocol identified slightly more solid pulmonary nodules than the SDCT chest protocol (100 vs 98 solid pulmonary nodules respectively). When considering inherent false positive and false negative results in any diagnostic test and interobserver variability, this slight discrepancy is not unexpected. Interobserver variability in identifying pulmonary nodules is well documented and we have demonstrated no statistically significant difference between ULDCT with MBIR and SDCT chest[27].
Gheysens et al[28] report that in a prospective study of 63 patients, a scoutless fixed-dose ULDCT chest was comparable to SDCT chest in detection of pulmonary nodules > 50 mm3 in size, the volume variation in the assessed nodules was within previously reported interscan variability, and body habitus did not affect nodule detection. However, LDCT chest has been shown to underestimate the size/volume of smaller pulmonary nodules, which is an area of continued attention in this rapidly progressing field[17]. Dunning et al[29] have shown the utilisation of an ultra-sharp kernel in a modern photon-counting detector LDCT chest examination in various phantoms increases the accuracy of pulmonary nodule measurement.
In a study of 99 patients, Miller et al[30] assessed the reliability of ULDCT chest in identifying pulmonary nodules in comparison to a reference LDCT chest and, in line with our study, found excellent sensitivity and specificity with a significant radiation dose reduction. Paks et al[31] have shown ULDCT chest to be comparable to SDCT chest for solid pulmonary nodules > 2 mm in a prospective 57 patient cohort and propose its utilisation in serial imaging of known pulmonary nodules. Multiple groups have prospectively compared ULDCT chest with LDCT or SDCT chest and concluded adequate image quality and sensitivity in nodule detection[32-35].
These findings highlight the value of continued in vitro and in vivo research in ultra-low dose CT imaging and represent encouraging progress in the field.
Objective image quality was improved in our ULDCT chest with MBIR protocol with increased SNR in almost all assessed areas, substantiating recent advances in hardware and software and in particular the power of MBIR. MBIR is particularly useful in thoracic imaging given the high inherent contrast between background normal lung parenchyma and solid pulmonary nodules[36]. Iterative reconstruction methods produce less image noise than traditional filtered back projection methods[37]. Subjective image quality was more than acceptable in our study in terms of nodule identification (mean score 7.8/10) and diagnostic acceptability (mean score 8.9/10) for both readers. The ability for diagnostic radiologists to trust the objective and subjective image quality of ULDCT is essential for ongoing advancements in the field.
Reduction or elimination of common CT related image artifacts becomes increasingly relevant in ULDCT with the inevitable reduction in signal as a consequence of the reduced radiation dose. Recent phantom work by Watanabe et al[38] has shown the addition of a dedicated tin filter photon shield reduced pacemaker related artefact in ULDCT and subsequently improved pulmonary nodule detectability. The ability of clinical imaging to diagnose patients at ever reducing radiation doses with adequate image quality is heavily supported by phantom and other non-clinical research which facilitates ongoing meaningful advances in imaging technique development.
The exciting new field of advanced image analysis through radiomics and deep learning algorithms has the potential to improve nodule characterisation and subsequent patient prognostication. Automatic nodule detection software in various forms have been utilised in ULDCT nodule detection and characterisation to good effect[26,39]. The limitations of heterogeneity in image acquisition, accurate data segmentation, limited reproducibility of results across different radiomics platforms and the novelty of the field have prevented advanced imaging analysis being utilised routinely in current daily practice[40].
This study had several limitations including its limited sample size of data from a single centre. Volumetric analysis is not routinely performed in our institution and therefore did not form a part of this study. However, the ability of MBIR to facilitate accurate volumetric analysis has been shown in a phantom study by Chen et al[41]. Our study did not assess the upfront identification of pulmonary nodules with ULDCT in patients without documented prior nodules or any change in pulmonary nodules over time.
CONCLUSION
ULDCT chest combined with MBIR is non-inferior to SDCT chest in the identification, measurement and characterisation of previously identified solid pulmonary nodules and facilitates a reduction in radiation dose of up to 97.6%. We propose the use of ULDCT chest in the routine follow-up of previously identified indeterminate solid pulmonary nodules.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Clinical Research and Ethics Committee Institutional Review Board (Approval No.ECM4(g)1/3/16 & ECM3(nnnn)9/3/21).
Clinical trial registration statement: This small prospective trial was registered with the local ethics institutional review board. Original approval PDF attached. It was not required to register the study with another governing body (i.e. an additional Clinical Trial Registration Statement is not applicable).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
CONSORT 2010 statement: The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Ireland
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Li Z; Yong T S-Editor: Lin C L-Editor: A P-Editor: Wang WB
Contributor Information
Patrick W O'Regan, Department of Radiology, School of Medicine, University College Cork, Cork T12 AK54, Ireland. patrickoregan@ucc.ie.
Antonia Harold-Barry, Department of Radiology, Cork University Hospital, Cork T12 DC4A, Ireland.
Alexander T O'Mahony, Department of Radiology, Cork University Hospital, Cork T12 DC4A, Ireland.
Claire Crowley, Department of Radiology, Mercy University Hospital, Cork T12WE28, Ireland.
Stella Joyce, Department of Radiology, Cork University Hospital, Cork T12 DC4A, Ireland.
Niamh Moore, Department of Radiology, School of Medicine, University College Cork, Cork T12 AK54, Ireland.
Owen J O'Connor, Department of Radiology, Cork University Hospital, Cork T12 DC4A, Ireland.
Michael T Henry, Department of Respiratory Medicine, Cork University Hospital, Cork T12 DC4A, Ireland.
David J Ryan, Department of Radiology, School of Medicine, University College Cork, Cork T12 AK54, Ireland.
Michael M Maher, Department of Radiology, School of Medicine, University College Cork, Cork T12 AK54, Ireland; Department of Radiology, Cork University Hospital, Cork T12 DC4A, Ireland.
Data sharing statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.Hammerschlag G, Cao J, Gumm K, Irving L, Steinfort D. Prevalence of incidental pulmonary nodules on computed tomography of the thorax in trauma patients. Intern Med J. 2015;45:630–633. doi: 10.1111/imj.12755. [DOI] [PubMed] [Google Scholar]
- 3.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, van 't Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 4.MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, Van Schil PE, Bankier AA. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284:228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 5.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, Franks K, Gleeson F, Graham R, Malhotra P, Prokop M, Rodger K, Subesinghe M, Waller D, Woolhouse I British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 6.Christensen J, Prosper AE, Wu CC, Chung J, Lee E, Elicker B, Hunsaker AR, Petranovic M, Sandler KL, Stiles B, Mazzone P, Yankelevitz D, Aberle D, Chiles C, Kazerooni E. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Chest. 2024;165:738–753. doi: 10.1016/j.chest.2023.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix W, Rutten M, Hendrix N, van Ginneken B, Schaefer-Prokop C, Scholten ET, Prokop M, Jacobs C. Trends in the incidence of pulmonary nodules in chest computed tomography: 10-year results from two Dutch hospitals. Eur Radiol. 2023;33:8279–8288. doi: 10.1007/s00330-023-09826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI. Low-dose CT of the lungs: preliminary observations. Radiology. 1990;175:729–731. doi: 10.1148/radiology.175.3.2343122. [DOI] [PubMed] [Google Scholar]
- 9.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall HM, Bowman RV, Yang IA, Fong KM, Berg CD. Screening for lung cancer with low-dose computed tomography: a review of current status. J Thorac Dis. 2013;5 Suppl 5:S524–S539. doi: 10.3978/j.issn.2072-1439.2013.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo-Smith WW, Hara AK, Mahesh M, Sahani DV, Pavlicek W. How I do it: managing radiation dose in CT. Radiology. 2014;273:657–672. doi: 10.1148/radiol.14132328. [DOI] [PubMed] [Google Scholar]
- 12.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichikawa Y, Kitagawa K, Nagasawa N, Murashima S, Sakuma H. CT of the chest with model-based, fully iterative reconstruction: comparison with adaptive statistical iterative reconstruction. BMC Med Imaging. 2013;13:27. doi: 10.1186/1471-2342-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moloney F, Kavanagh RG, Ronan NJ, Grey TM, Joyce S, Ryan DJ, Moore N, O'Connor OJ, Plant BJ, Maher MM. Ultra-low-dose thoracic CT with model-based iterative reconstruction (MBIR) in cystic fibrosis patients undergoing treatment with cystic fibrosis transmembrane conductance regulators (CFTR) Clin Radiol. 2021;76:393.e9–393.e17. doi: 10.1016/j.crad.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Lin S, Lin M, Lau KK. Efficacy of model-based iterative reconstruction in cystic fibrosis assessment using CT. Clin Radiol. 2019;74:569.e19–569.e27. doi: 10.1016/j.crad.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Doo KW, Kang EY, Yong HS, Woo OH, Lee KY, Oh YW. Accuracy of lung nodule volumetry in low-dose CT with iterative reconstruction: an anthropomorphic thoracic phantom study. Br J Radiol. 2014;87:20130644. doi: 10.1259/bjr.20130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie X, Willemink MJ, de Jong PA, van Ooijen PM, Oudkerk M, Vliegenthart R, Greuter MJ. Small irregular pulmonary nodules in low-dose CT: observer detection sensitivity and volumetry accuracy. AJR Am J Roentgenol. 2014;202:W202–W209. doi: 10.2214/AJR.13.10830. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Zhang H, Jin X, Zhang X, Lu X, Han Q, Xiong X, Liu T, Feng Y, Tu W, Zhou T, Ge Y, Dong P, Liu S, Fan L. Ultra-low-dose spectral-detector computed tomography for the accurate quantification of pulmonary nodules: an anthropomorphic chest phantom study. Diagn Interv Radiol. 2023;29:691–703. doi: 10.4274/dir.2023.232233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanese G, Silva M, Frauenfelder T, Eberhard M, Sabia F, Martini C, Marchianò A, Prokop M, Sverzellati N, Pastorino U. Comparison of ultra-low dose chest CT scanning protocols for the detection of pulmonary nodules: a phantom study. Tumori. 2019;105:394–403. doi: 10.1177/0300891619847271. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor OJ, Vandeleur M, McGarrigle AM, Moore N, McWilliams SR, McSweeney SE, O'Neill M, Ni Chroinin M, Maher MM. Development of low-dose protocols for thin-section CT assessment of cystic fibrosis in pediatric patients. Radiology. 2010;257:820–829. doi: 10.1148/radiol.10100278. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JD, Balonov M, Bochud F, Martin CJ, Menzel HG, Smith-Bindman R, Ortiz-López P, Simmonds JR, Wakeford R. The use of dose quantities in radiological protection: ICRP publication 147 Ann ICRP 50(1) 2021. J Radiol Prot. 2021;41 doi: 10.1088/1361-6498/abe548. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Jinzaki M, Hosokawa T, Tanami Y, Sugiura H, Abe T, Kuribayashi S. Dose reduction in chest CT: comparison of the adaptive iterative dose reduction 3D, adaptive iterative dose reduction, and filtered back projection reconstruction techniques. Eur J Radiol. 2012;81:4185–4195. doi: 10.1016/j.ejrad.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Smith EA, Dillman JR, Goodsitt MM, Christodoulou EG, Keshavarzi N, Strouse PJ. Model-based iterative reconstruction: effect on patient radiation dose and image quality in pediatric body CT. Radiology. 2014;270:526–534. doi: 10.1148/radiol.13130362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst CW, Hulstaert TL, Belsack D, Buls N, Van Gompel G, Nieboer KH, Buyl R, Verhelle F, De Maeseneer M, de Mey J. Dedicated sub 0.1 mSv 3DCT using MBIR in children with suspected craniosynostosis: quality assessment. Eur Radiol. 2016;26:892–899. doi: 10.1007/s00330-015-3870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao CF, Ma KL, Shan H, Liu TF, Zhao SQ, Wan Y, Jun-Zhang, Wang HQ. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer. 2022;22:1238. doi: 10.1186/s12885-022-10310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Liu H, Han J, Xu S, Zhang G, Wang Q, Du Y, Yang F, Zhao X, Shi G. Ultra-low-dose CT lung screening with artificial intelligence iterative reconstruction: evaluation via automatic nodule-detection software. Clin Radiol. 2023;78:525–531. doi: 10.1016/j.crad.2023.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Wataya T, Yanagawa M, Tsubamoto M, Sato T, Nishigaki D, Kita K, Yamagata K, Suzuki Y, Hata A, Kido S, Tomiyama N Osaka University Reading Team. Radiologists with and without deep learning-based computer-aided diagnosis: comparison of performance and interobserver agreement for characterizing and diagnosing pulmonary nodules/masses. Eur Radiol. 2023;33:348–359. doi: 10.1007/s00330-022-08948-4. [DOI] [PubMed] [Google Scholar]
- 28.Gheysens G, De Wever W, Cockmartin L, Bosmans H, Coudyzer W, De Vuysere S, Lefere M. Detection of pulmonary nodules with scoutless fixed-dose ultra-low-dose CT: a prospective study. Eur Radiol. 2022;32:4437–4445. doi: 10.1007/s00330-022-08584-y. [DOI] [PubMed] [Google Scholar]
- 29.Dunning CAS, Marsh JF Jr, Winfree T, Rajendran K, Leng S, Levin DL, Johnson TF, Fletcher JG, McCollough CH, Yu L. Accuracy of Nodule Volume and Airway Wall Thickness Measurement Using Low-Dose Chest CT on a Photon-Counting Detector CT Scanner. Invest Radiol. 2023;58:283–292. doi: 10.1097/RLI.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AR, Jackson D, Hui C, Deshpande S, Kuo E, Hamilton GS, Lau KK. Lung nodules are reliably detectable on ultra-low-dose CT utilising model-based iterative reconstruction with radiation equivalent to plain radiography. Clin Radiol. 2019;74:409.e17–409.e22. doi: 10.1016/j.crad.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Paks M, Leong P, Einsiedel P, Irving LB, Steinfort DP, Pascoe DM. Ultralow dose CT for follow-up of solid pulmonary nodules: A pilot single-center study using Bland-Altman analysis. Medicine (Baltimore) 2018;97:e12019. doi: 10.1097/MD.0000000000012019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messerli M, Kluckert T, Knitel M, Wälti S, Desbiolles L, Rengier F, Warschkow R, Bauer RW, Alkadhi H, Leschka S, Wildermuth S. Ultralow dose CT for pulmonary nodule detection with chest x-ray equivalent dose - a prospective intra-individual comparative study. Eur Radiol. 2017;27:3290–3299. doi: 10.1007/s00330-017-4739-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Kim YK, Lee BE, Lee SJ, Ryu YJ, Lee JH, Chang JH. Ultra-Low-Dose CT of the Thorax Using Iterative Reconstruction: Evaluation of Image Quality and Radiation Dose Reduction. AJR Am J Roentgenol. 2015;204:1197–1202. doi: 10.2214/AJR.14.13629. [DOI] [PubMed] [Google Scholar]
- 34.Sui X, Meinel FG, Song W, Xu X, Wang Z, Wang Y, Jin Z, Chen J, Vliegenthart R, Schoepf UJ. Detection and size measurements of pulmonary nodules in ultra-low-dose CT with iterative reconstruction compared to low dose CT. Eur J Radiol. 2016;85:564–570. doi: 10.1016/j.ejrad.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Jin S, Zhang B, Zhang L, Li S, Li S, Li P. Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg. 2018;8:480–490. doi: 10.21037/qims.2018.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neroladaki A, Botsikas D, Boudabbous S, Becker CD, Montet X. Computed tomography of the chest with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination: preliminary observations. Eur Radiol. 2013;23:360–366. doi: 10.1007/s00330-012-2627-7. [DOI] [PubMed] [Google Scholar]
- 37.Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521–2526. doi: 10.1007/s00330-011-2227-y. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe S, Urikura A, Ohashi K, Kitera N, Tsuchiya T, Kasai H, Kawai T, Hiwatashi A. Artifact reduction in low and ultra-low dose chest computed tomography for patients with pacemaker: A phantom study. Radiography (Lond) 2024;30:770–775. doi: 10.1016/j.radi.2024.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Ma G, Dou Y, Dang S, Yu N, Guo Y, Han D, Fan Q. Improving Image Quality and Nodule Characterization in Ultra-low-dose Lung CT with Deep Learning Image Reconstruction. Acad Radiol. 2024;31:2944–2952. doi: 10.1016/j.acra.2024.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Prosper AE, Kammer MN, Maldonado F, Aberle DR, Hsu W. Expanding Role of Advanced Image Analysis in CT-detected Indeterminate Pulmonary Nodules and Early Lung Cancer Characterization. Radiology. 2023;309:e222904. doi: 10.1148/radiol.222904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B, Barnhart H, Richard S, Robins M, Colsher J, Samei E. Volumetric quantification of lung nodules in CT with iterative reconstruction (ASiR and MBIR) Med Phys. 2013;40:111902. doi: 10.1118/1.4823463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.