ABSTRACT
Kidney transplant ultrasonography is an important diagnostic tool in the care of transplant recipients. This modality of nonradiation‐based imaging allows for precise and expedient reporting of allograft architecture, which can inform clinical decision‐making. However, as with any diagnostic tool, overuse may lead to unnecessary interventions and costs on the healthcare system. To better understand the use of ultrasonography in hospitalized kidney transplant recipients and outcomes of subsequent interventions, we conducted a single‐center retrospective study at a large transplant program in Ontario, Canada. We noted that over 30% of admissions resulted in a ultrasonographic survey within the first 24 h of presentation; however, most of these did not change clinical management or lead to a subsequent procedural intervention. Using multivariable logistic regression, we identified predictors for receiving an ultrasound, including time from transplantation, elevated serum creatinine and infectious diagnosis. Procedural interventions (e.g., drain or biopsy) resulted from less than 20% of all ultrasound investigations, with patients closer to the time of index transplant or with elevated serum creatinine values more likely to receive an intervention. In conducting a cost analysis, we estimated that approximately $80 000 CAD per year could be saved with more selective decisions on ultrasound requisitions. Overall, our results indicate that despite being an informative tool, the broad use of ultrasonography in the kidney transplant population may not yield significant changes to transplant care.
Keywords: kidney transplant, quality improvement, ultrasonography
Abbreviations
- CAD
Canadian dollar
- ED
emergency department
- ESKD
end‐stage kidney disease
- IQR
interquartile range
- OHIP
Ontario Health Insurance Plan
- ROC
receiver operating characteristic
- SD
standard deviation
- UHN
University Health Network
- USD
United States dollar
1. Introduction
Kidney transplant is the treatment of choice for eligible patients with end‐stage kidney disease (ESKD), offering improved long‐term survival, quality of life, and cost benefits compared to remaining on dialysis [1]. The rate of kidney transplantation in Canada has risen ∼23% over the last decade, with improved patient and allograft survival, creating a cohort of kidney transplant recipients requiring comprehensive longitudinal care [2]. Recipients also often have complex comorbidities accrued from prior chronic kidney disease and amplified further with chronic immunosuppression after transplant, leading to increased risks of infection, malignancy, and cardiovascular disease [3].
It is therefore not surprising that recipients are subjected to a higher burden of healthcare resource utilization [1, 4] including clinic visits, diagnostic investigations, interventions, emergency department (ED) visits, and hospitalizations [5]. Reported incidence of hospitalizations, and reasons for admission, among kidney transplant recipients is variable. Few small observational studies have largely focused on the early posttransplant period and suggest re‐hospitalization rates may be as high as 80% in the first 6 months [6, 7, 8, 9]. Ultrasonography is routinely performed among kidney transplant recipients who present to hospital, even in the absence of genitourinary symptoms or other clinical indications. Hospitalizations after kidney transplantation can occur in up to 80% of patients in the first 6‐month posttransplant. As a result, ultrasounds (US) routinely performed as part of the hospital admission may have substantial implications for hospital resource utilization. Duplex ultrasound is the first‐line imaging modality of choice in evaluating the kidney allograft due to its broad availability, noninvasive method and lack of exposure to radiation or nephrotoxic contrast dye [10, 11]. US can quickly identify numerous causes of allograft dysfunction, including vascular compromise, obstruction, and perinephric collections, with high sensitivity and specificity [12, 13]. Resistive indices from sonographic investigations have also been associated with cardiovascular events [14]. Over‐reliance on diagnostic investigations could also lead to inappropriate treatment decisions, particularly in the absence of clinical symptoms [10]. The relationship between ultrasound use and admission characteristics, radiographic findings or subsequent interventions remains unclear. There is a need to establish evidence‐based guidance for clinicians to ensure effective patient care and appropriate use of finite healthcare resources.
To better understand these associations, we utilized a cohort of adult kidney transplant recipients at the largest multi‐organ transplant center in Ontario, Canada, to examine the rates and characteristics of hospitalizations, frequency and predictors of ultrasound usage and resulting interventions.
2. Methods
2.1. Study Population
The University Health Network (UHN) is the largest multi‐organ transplant program in Canada and conducts over 200 kidney transplants annually. In this single‐center observational retrospective cohort study, all adult patients with a functioning kidney transplant, including multiorgan transplant recipients, followed by the UHN transplant program were eligible to be included in this study. Hospital admissions to a UHN‐affiliated hospital from January 1, 2022 to December 31, 2022, were studied and, for the purposes of this study, admission to hospital was defined as a presentation to a hospital where the kidney transplant service was involved in their care (as an attending or consultative service). The study was submitted for, and received approval by the UHN Quality Improvement Review Committee. We excluded patients who had a previously failed kidney allograft and were on kidney replacement therapy, and patients aged 18 or younger at the time of admission. Patients who presented to hospital and were discharged from the emergency department without involvement of the kidney transplant service were also excluded (e.g., left without being seen). Finally, data on UHN kidney transplant patients presenting to a nonaffiliated peripheral hospital were not available for study.
2.2. Data Sources
Data regarding hospital admissions were obtained from the local electronic health record. This included demographics, transplant history, length of hospital stay, discharge summaries, and radiographic and laboratory data.
2.3. Outcomes
The study's primary outcome was whether a recipient had an ultrasound (focused on the kidney allograft) within 24 h of admission to hospital. Secondary outcomes included the ultrasound findings, incidence of subsequent interventions, and cost. We defined follow‐up interventions as surgical or interventional radiology procedures (e.g., kidney allograft biopsy or placement of a drain) occurring within the same admission using hospital radiographic/procedural records, and discharge summaries. Patients could also receive more than one intervention during the same admission. Individual chart reviews were conducted to ensure the procedure was directly associated with the findings from the ultrasound study. We also narratively described costs associated with ultrasound scans and subsequent interventions. Cost approximations were obtained with the assistance of the UHN Joint Department of Medical Imaging and the Ontario Health Insurance Plan (OHIP) physician billing codes for the 2023 financial year.
2.4. Statistical Analysis
Descriptive statistics were presented as mean (± standard deviation, SD) for normally distributed continuous variables (e.g., age); as median (interquartile range, IQR) for skewed distributed continuous variables (e.g., time from transplant to admission); and as frequencies and percentages for categorical variables (e.g., organ type). Where appropriate, the Student t‐test and chi‐square test of independence were performed to examine differences in means and proportions, respectively. Standardized mean differences were used to measure the distance in the distribution of baseline characteristics between ultrasound versus no ultrasound groups.
Univariable and multivariable logistic regression models were fitted to examine the association of baseline characteristics on the likelihood of ultrasound within 24 h of hospital admission. For both analyses, we utilized factors and investigations that would be available at time of presentation to hospital. Receiver operating characteristic (ROC) curve analysis evaluated the ability of the logistic regression model to discriminate patients undergoing versus not undergoing ultrasound. Bootstrap ROC curves with 1000 times of resampling were generated for internal validation of the model. Missing values were imputed using multiple imputation.
Logistic regression models were also fitted to predict intervention among patients who had ultrasound within 24 h of admission. Continuous predictors were transformed such that the predictors and predicted logit had better linear relationship. The Lasso method was used to select predictors in the final model. The performance of the model was assessed using calibration plots, in which the closer the apparent curve or bias‐corrected curve to the diagonal (e.g., the ideal line), the better the model performance. Bootstrap method with 1000 times resampling was used to internally validate the model.
Our analyses were completed using Stata/MP 17.0 and R version 4.0.3. A two‐sided p value of <0.05 was considered statistically significant.
3. Results
3.1. Hospital Admissions
Over the duration of the study period, there were exactly 1000 hospital visits that resulted in hospital admissions for kidney transplant recipients (Figure 1). Of these, 217 (21.7%) were admissions for patients undergoing kidney transplantation, and 783 (78.3%) admissions occurred in patients who had previously received a kidney transplant, which comprised of the patient cohort included for analyses. On average, there were approximately 65 hospitalizations per month from patients with kidney transplants during the study period.
FIGURE 1.
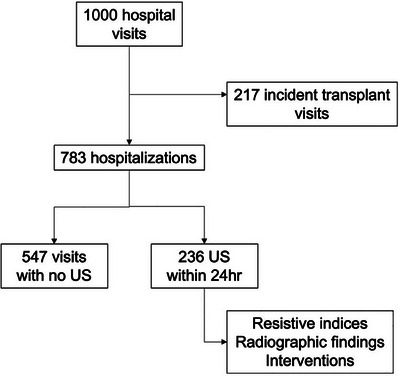
Study flow diagram demonstrating kidney transplant patients admitted to hospital and receiving ultrasound investigations.
Ultrasound imaging of the kidney allograft occurred in 236 (30.1%) patients within the first 24 h of hospital admission. Between patients who did or did not receive ultrasounds within the first 24 h of admission, there was a similar distribution in biological sex (49.2% vs. 53.6% men) and age (56 vs. 53.4 years) (Table 1). Patients who received ultrasound imaging had a shorter time from transplantation compared to those who did not (2.4 vs. 6.2 years). There was no difference in the length of admission between groups (4 vs. 5 days).
TABLE 1.
Kidney transplant recipient characteristics by ultrasound obtained within 24 h of admission.
| Variable | Overall (N = 783) | Ultrasound within 24 h (N = 783) | ||
|---|---|---|---|---|
| No (n = 547) | Yes (n = 236) | Standardized mean difference | ||
| Mean age at admission (± SD a ) (years) | 55.2 (± 15.0) | 56.0 (± 14.9) | 53.4 (± 15.1) | 0.18 |
| Male (%) | 409 (52.2%) | 293 (53.6%) | 116 (49.2%) | −0.09 |
| Median time from transplant to admission (IQR b ) (years) | 5.3 (0.9, 11.5) | 6.2 (1.8, 12.1) | 2.4 (0.3, 9.7) | 0.30 |
| Median length of stay (IQR b ) (days) | 4 (2, 8) | 4 (1, 7) | 5 (3, 9) | −0.08 |
| Organ type (%) | ||||
| Kidney alone | 688 (87.9%) | 480 (87.8%) | 208 (88.1%) | 0.01 |
| Combined | 95 (12.1%) | 67 (12.3%) | 28 (11.9%) | |
| Donor type (%) | ||||
| Deceased | 519 (66.3%) | 356 (65.1%) | 163 (69.1%) | 0.08 |
| Living | 264 (33.7%) | 191 (34.9%) | 73 (30.9%) | |
| Median serum creatinine at admission (IQR b ) (mL/min) | 144 (107, 208) | 132 (102, 191) | 168 (126, 250.5) | −0.38 |
| Mean white blood cell (WBC) count at admission (± SD a ) (×109/L) | 8.8 (± 4.8) | 8.5 (± 4.5) | 9.5 (± 5.2) | −0.21 |
Standard deviation.
Interquartile range.
Patients with a history of kidney transplant alone made up the largest proportion of patients in our study population (87.9%), with the remainder being dual organ transplants (e.g., heart/kidney, liver/kidney, and lung/kidney). Deceased donor transplants comprised the majority (66.3%) of organs transplanted (compared to living donor). Both serum creatinine (168 vs. 132 umol/L) and leukocyte count (9.5 vs. 8.5 × 109/L) trended higher in patients who received ultrasounds within 24 h of admission, though were not significantly different.
3.2. Reasons for Admission and Ultrasonography Findings
Reasons for hospital admissions were reviewed and categorized based on affected systems (Table S1). For the analysis, admission diagnoses were dichotomized as infectious (357 of 783, 45%) versus noninfectious (55%) (Table 2). The number of infections pertaining to the genitourinary system (92) was one‐third of that of nongenitourinary infections (265, p < 0.01). The most common noninfectious reasons for admission were related to the gastrointestinal (e.g., abdominal pain), hematological (e.g., cytopenia), and musculoskeletal systems.
TABLE 2.
Kidney transplant patients stratified by admission infection versus noninfectious diagnosis and source of infection (genitourinary vs. not).
| Variable | Overall (N, %) | Ultrasound within 24 h (N, %) | |
|---|---|---|---|
| No | Yes | ||
| Infection, GU | 92 (11.75) | 28 (30.43) | 64 (69.57) |
| Infection, non‐GU | 265 (33.84) | 204 (76.98) | 61 (23.02) |
| Noninfectious, GU | 86 (10.98) | 32 (37.21) | 54 (62.79) |
| Noninfectious, non‐GU | 340 (43.42) | 283 (83.24) | 57 (16.76) |
| Total | 783 (100) | 547 (69.86) | 236 (30.14) |
Abbreviation: GU, genitourinary.
We then stratified patients who received ultrasound imaging by admission diagnosis categories. In patients admitted with infectious diagnoses, 35% (125/357) received sonographic imaging. This was compared to only 26% of patients who were admitted for noninfectious diagnoses (p < 0.01). For patients with a presumptive diagnosis related to the genitourinary system, ultrasonography was performed in 66% of admissions, compared to 19% of admissions that were not related to the genitourinary system (p < 0.01).
Of 236 ultrasounds completed, 196 had radiographic abnormalities (83.1%). The most common ultrasound findings were urothelial thickening (25.9%), followed by fluid collections and hydronephrosis. Of the 236 ultrasounds performed in the first 24 h of admission, interventions occurred in 48 patients, with renal biopsy (20), drain insertions (14), and nephrostomy tube insertions (8), being the most common procedures completed following these ultrasounds.
3.3. Predictors of Ultrasound Usage in Hospital Admissions
We fit both univariable and multivariable logistic regression models to examine the predictors of ultrasound usage in hospitalized kidney transplant patients (Tables 3 and S2). Age at the time of hospital presentation, biological sex, donor status (living vs. deceased), and organ transplanted (kidney alone or multi‐organ transplant) were not associated with whether an ultrasound was requisitioned within the first 24 h of admission. Patients who received a kidney allograft >1 year prior to admission were less likely to receive ultrasound imaging compared to those who received a transplant within 1 year prior to admission. Similarly, when comparing initial creatinine measurement at the time of presentation, higher levels were associated with an increased likelihood of receiving a sonographic investigation (Table S2). Patients who presented with higher leukocyte readings trended toward a greater likelihood of receiving ultrasounds, with the highest likelihood among patients presenting with leukocyte counts above 11.1 × 109/L (p < 0.01).
TABLE 3.
Univariable and multivariable analysis of kidney transplant recipients admitted to hospital stratified by receiving ultrasound within 24 h of admission.
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Adjusting for admission diagnosis | ||||
| O.R. (95% C.I.) | p value | O.R. (95% C.I.) | p value | |
| Age at admission (every 1 year increase) | 0.99 (0.98, 1.00) | 0.03 | 0.99 (0.98, 1.00) | 0.14 |
| Sex (female vs. male) | 1.19 (0.88, 1.62) | 0.26 | 1.02 (0.71, 1.45) | 0.93 |
| Time from transplant to admission (every 1 year increase) | 0.96 (0.94, 0.98) | <0.001 | 0.96 (0.94, 0.99)# | 0.004 |
| Organ type (combined vs. kidney alone) | 0.96 (0.60, 1.54) | 0.88 | 0.84 (0.48, 1.48) | 0.55 |
| Donor type (living vs. deceased) | 0.83 (0.60, 1.16) | 0.28 | 0.87 (0.58, 1.29) | 0.48 |
| Serum creatinine at admission (every 50 mL/min increase) | 1.14 (1.08, 1.21) | <0.001 | 1.15 (1.07, 1.23) | <0.001 |
| WBC count at admission (every 1 × 109/L increase) | 1.05 (1.02, 1.08) | 0.01 | 1.04 (1.00, 1.07) | 0.06 |
| Reason for admission | ||||
| Infection, GU vs. noninfectious, non‐GU | 11.35 (6.70, 19.23) | <0.001 | 9.80 (5.66, 16.98) | <0.001 |
| Infection, non‐GU vs. noninfectious, non‐GU | 1.48 (0.99, 2.22) | 0.06 | 1.46 (0.97, 2.22) | 0.07 |
| Non‐infectious, GU vs. noninfectious, non‐GU | 8.38 (4.97, 14.12) | <0.001 | 5.88 (3.38, 10.21) | <0.001 |
Abbreviations: GU, genitourinary; WBC, white blood cell.
When adjusting for the reasons for admission, infectious diagnoses related to the genitourinary system portended 9.7‐fold increased odds of receiving an ultrasound compared to admissions for noninfectious, non‐GU diagnoses (p < 0.01, 95% CI: 5.42, 17.38, Table S2). Similarly, genitourinary‐related, noninfectious diagnoses also resulted in a 6.3‐fold increase in likelihood of receiving imaging (p < 0.01, 95% CI: 3.57, 11.12). This prediction model resulted in an area under ROC curve of 0.86 after adjusting for reasons for admission (Figure S1).
3.4. Predictors of Procedural Interventions Following Ultrasonography
We also analyzed patients who received an intervention (e.g., biopsy, drain, etc.) following completion of a kidney transplant ultrasound (Table 4). Of the 236 ultrasounds completed within 24 h of admission, a total of 48 interventions were performed in 42 unique visits. The most common interventions were kidney biopsy (n = 20), followed by drain insertions (n = 14). When performing univariable and multivariable analyses, age, biological sex and organ or donor type was not associated with receiving an intervention. Having received a transplant greater than 1 year prior resulted in a decreased likelihood of receiving an intervention, and this was markedly reduced above 11.5 years posttransplant. Higher levels of serum creatinine measurements at presentation were also associated with receiving an intervention (compared to creatinine <107 umol/L) although no associations in leukocyte measurements were noted (Table S3). Lastly, when comparing reasons for admission, noninfectious genitourinary causes were most notably associated with receiving an intervention compared to noninfectious and nongenitourinary diagnoses.
TABLE 4.
Univariable and multivariable logistic regression of kidney transplant patients who received an ultrasound within 24 h of admission to hospital.
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| O.R. (95% C.I.) | p value | O.R. (95% C.I.) | p value | |
| Age at admission (every 1‐year increase) | 0.99 (0.97, 1.02) | 0.56 | 1.00 (0.97, 1.03) | 0.78 |
| Sex (female vs. male) | 0.60 (0.31, 1.18) | 0.14 | 0.71 (0.31, 1.63) | 0.42 |
| Time from transplant to admission (every 1‐year increase) | 0.83 (0.75, 0.92) | 0.001 | 0.81 (0.71, 0.91) | 0.001 |
| Organ type (combined vs. kidney alone) | 1.65 (0.65, 4.17) | 0.29 | 1.51 (0.44, 5.14) | 0.51 |
| Donor type (living vs. deceased) | 0.76 (0.36, 1.60) | 0.46 | 1.27 (0.47, 3.38) | 0.64 |
| Serum creatinine at admission (every 10 mL/min increase) | 1.02 (1.01, 1.04) | 0.003 | 1.02 (1.00, 1.04) | 0.07 |
| WBC at admission (every 1 × 109/L increase) | 0.96 (0.89, 1.03) | 0.26 | 1.00 (0.91, 1.09) | 0.97 |
| Reason for admission | ||||
| Infection, GU vs. noninfectious, non‐GU | 0.61 (0.18, 2.03) | 0.42 | 0.69 (0.20, 2.41) | 0.56 |
| Infection, non‐GU vs. noninfectious, non‐GU | 0.50 (0.14, 1.81) | 0.29 | 0.68 (0.18, 2.65) | 0.58 |
| Non‐infectious, GU vs. noninfectious, non‐GU | 6.63 (2.55, 17.22) | <0.001 | 6.35 (2.19, 18.38) | 0.001 |
Abbreviations: GU, genitourinary; WBC, white blood cell.
We generated a final predictive model for outcome of need for intervention of: ln(p/(1‐p)) = 0.643*log(years from transplant to admission) + 0.055*1/sqrt(creatinine (every 50 mL/min) at admission) + 6.471*reason for admission (if noninfection, GU, Table S4). The calibration plot for this model demonstrated that the apparent curve and the Bootstrap bias‐corrected curve both closely align with the ideal line, suggesting excellent model performance (Figure S2). Furthermore, the Bootstrap validation resulted in an area under the ROC curve of 0.82 (95% CI: 0.77, 0.88), indicating good model discrimination.
3.5. Cost Estimation of Ultrasound Usage in Hospitalized Kidney Transplant Patients
To examine the impact of ultrasounds on health resource utilization, we obtained cost estimates from both our Joint Department of Medical Imaging and physician billing codes from the Ontario Health Insurance Program (the provincial health insurer for all physician services and healthcare procedures). Material, human resources, and physician costs were assessed. In total, the estimated cost per ultrasound was $200 CAD. The material costs per ultrasound per scan, which included ultrasound gel, disposables, and maintenance were estimated to be $30 CAD per scan. Human resource costs (e.g., ultrasound technician and hospital porters) were estimated to be $66 CAD per scan. Lastly, physician billing costs were examined at approximately $104 CAD per ultrasound. Thus, the total direct costs over the 1‐year study period for ultrasonographic studies were estimated at approximately $80 000 CAD.
4. Discussion
Kidney transplantation remains the definitive therapy for patients with end‐stage kidney disease. Hospitalizations among patients with transplants remain higher than the background population and have been estimated to be up to 6‐fold higher [15], in part due to the complications from kidney failure, compounded by the immunosuppressive burden posttransplant. As the number of kidney transplant recipients continues to grow, so does the need to understand patterns of complications to provide more comprehensive care for this unique population. In this study, we examined the relationships between patient demographics, hospitalization characteristics, and the utility of ultrasounds conducted in kidney transplant recipients during routine hospital admissions at a large quaternary care center. In our cohort, up to 30% of patients with a history of kidney transplants received an ultrasonography study within 24 h of hospital admission. Of these completed studies, 83% had radiographic findings, but most findings did not change management (e.g., urothelial thickening). Interventions occurred following 42 ultrasounds (20%), markedly lower than the total number of ultrasounds completed.
Ultrasounds remain an important diagnostic tool in patients with kidney transplants, largely due to their ease of access, lack of radiation and sonographic detail. Studies to better optimize ultrasound use in native kidneys (i.e., for acute kidney injury), have demonstrated comparable rates of intervention [16], but our study is the first to examine the kidney transplant population. As with any investigative tool, underutilization risks missed diagnoses and opportunities for intervention, while overuse can have the opposite effect, with resultant increases in resource utilization. Thus, balancing the costs and benefits of ultrasound utilization remains an area of needed study. Our study findings highlight two important points: (1) that close to one‐fifth of ultrasounds do not have any findings and do not impact management, and (2) the detection of sonographic findings (e.g., fluid collections or hydronephrosis), uncommonly results in a subsequent intervention (e.g., drain or surgery). For this reason, many of these findings, likely would have resolved with conservative management. One conclusion from this could be that 80% of ultrasounds did not alter management and were unnecessary (i.e., poor resource utilization). At the same time, ultrasonography could lead to changes in clinical management in 20% of investigations, which when considering patient and system costs associated with a failed allograft and return to dialysis, this may be a palatable diagnostic resource.
In our predictive models, time from transplantation was one of the strongest predictors of receiving an ultrasound at admission. Patients greater than 1 year from transplant were less likely to receive ultrasonographic investigations. From a decision‐making standpoint, clinicians may interpret the proximity to time from transplant with the likelihood of having greater complications. This is supported by other studies showing higher early readmission rates associated with greater lengths of stay and also a signal toward increased graft failure [7, 17]. Similarly, both elevated leukocyte and creatinine values in serum were associated with higher likelihoods of receiving an ultrasonography investigation within 24 h of admission. Again, clinicians may view these as surrogates of allograft dysfunction leading to an increased probability of requisitioning imaging investigations. Lastly, based on the presumptive admission diagnoses, patients whose primary concerns were related to the genitourinary system (regardless of whether an infection was suspected or not), were more likely to receive diagnostic imaging (compared to noninfectious nongenitourinary causes).
From our study, we also analyzed predictors of receiving an intervention based on ultrasound findings. Our data would suggest that most sonographic investigations do not yield an intervention. Time from transplantation was associated with a significantly less likelihood of receiving a subsequent intervention an effect seen as early as 1‐year posttransplant. As the majority of interventions pertain to postsurgical complications, this finding is not surprising. This is supported by the fact that noninfectious, genitourinary‐related diagnoses (e.g., acute kidney injury, hematoma and fluid collection), are almost 8‐times more likely to receive an intervention compared to noninfectious and nongenitourinary related admissions. Infectious genitourinary diagnoses were not associated with higher rates of interventions. Rather, most of these patients’ conditions would have resolved with antibiotics and conservative management without needing ultrasonographic imaging.
The finding that noninfectious, genitourinary‐related diagnoses resulted in more interventions implies that the highest yield for sonographic investigations lies with this population. Patients presenting with unexplained acute allograft dysfunction or symptoms of obstruction, for example, have a reasonable likelihood of requiring a follow‐up biopsy or drain. That said, our study did not examine outside of the current admission whether these interventions yield significant impact on long‐term medical management (e.g., biopsy results leading to changes in immunosuppression).
Our study also corroborates literature surrounding hospitalizations posttransplant. In our population, we observed that kidney transplant patients remain at higher risk of infectious and noninfectious complications. Hospitalizations due to infections (357 in our cohort) comprised of almost half of all admissions, yet only one‐third of infections involved the genitourinary system. This rate is higher than reported in limited literature findings, where Khan et al., reported infections as the cause of 18% hospitalizations in the first 3 years posttransplant [8]. A Spanish cohort also cited infectious causes as approximately 20% of reasons for emergency department visits among kidney transplant recipients [18]. In this latter cohort, 40% of infectious causes were associated to the genitourinary source, slightly higher than observed in our population.
From a health resource perspective, we estimate that a single ultrasound contributes $200 CAD toward the total direct cost of the patient's hospitalization. While this may seem nominal, at our single center, this accounts for almost $80 000 CAD annually when considering material and human resource costs. What this cannot account for, are ‘intangible’ costs attributable to patient care, such as delays to care or discharge (as a result of ordering investigations) and increased demands on the ultrasound department. Furthermore, not assessed in our study are the number of patients who then have follow‐up sonographic investigations (perhaps as an outpatient), to ensure either resolution or stability of findings. These additional tests can significantly impact the health care resources. These impacts are more challenging to ascertain, but undoubtedly decrease the efficiency of care delivery for transplant and nontransplant patients alike. Point of care ultrasound (POCUS) has allowed for rapid, bedside assessments in acute care settings. However, remains challenged by inter‐operator variability and resource availability. This would be of particular concern with more advanced image capture (such as grading of hydronephrosis, renal artery Dopplers, and resistive indices) requiring more advanced training. This could ultimately lead to inaccurate diagnoses or delays in care.
Our study adds to the knowledge base in this area by using hospitalization data to predict ultrasound usage, its impact on clinical management, and its implications on health resource utilization. Limitations of this study include its potential limited generalizability beyond a single center, the use of administrative data collected for purposes other than the study question, and lack of long‐term follow up. Since this was a retrospective study assessing clinical decisions around ultrasound usage, it is impossible to fully reconstruct the clinical rationale that went into the decision to order ultrasounds.
Despite these limitations, we believe that our study highlights an important aspect of care as it applies to the transplant population. Understanding these baseline characteristics which influence admission characteristics and ultrasound requisitions may be helpful in improving health care efficiency and reducing unnecessary investigations. Additionally, identifying factors which may help predict kidney transplant recipients at higher likelihood of receiving procedural interventions may help efficiently streamline imaging studies and resources associated with these investigations. The predictive model derived from this cohort will require external validation before clinical deployment but may offer some data‐driven guidance on the appropriate use of ultrasounds in newly hospitalized kidney transplant recipients.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting Information
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Koto P., Tennankore K., Vinson A., et al., “What Are the Short‐Term Annual Cost Savings Associated With Kidney Transplantation?,” Cost Effectiveness and Resource Allocation 20, no. 1 (2022): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organ Replacement in Canada: CORR Annual Statistics. CIHI [Internet]. [cited 2023 Nov 27]., https://www.cihi.ca/en/organ‐replacement‐in‐canada‐corr‐annual‐statistics.
- 3. Kalil R. S., Carpenter M. A., Ivanova A., et al., “Impact of Hyperuricemia on Long‐Term Outcomes of Kidney Transplantation: Analysis of the FAVORIT Study,” American Journal of Kidney Diseases 70, no. 6 (2017): 762–769. [DOI] [PubMed] [Google Scholar]
- 4. Łabuś A., Niemczyk M., Czyżewski Ł., et al., “Costs of Long‐Term Post‐Transplantation Care in Kidney Transplant Recipients,” Annals of Transplantation 24 (2019): 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schold J. D., Elfadawy N., Buccini L. D., et al., “Emergency Department Visits After Kidney Transplantation,” Clinical Journal of the American Society of Nephrology CJASN 11, no. 4 (2016): 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harhay M., Lin E., Pai A., et al., “Early Rehospitalization After Kidney Transplantation: Assessing Preventability and Prognosis,” American Journal of Transplantation 13, no. 12 (2013): 3164–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dashti S., Dhrolia M., Nasir K., Qureshi R., and Ahmad A., “Re‐Hospitalization in First Six Months After Live Related Renal Transplantation: Risk Factors, Burden, Causes and Outcomes,” Cureus 14, no. 2 (2022): e22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan S., Tighiouart H., Kalra A., Raman G., Rohrer R. J., and Pereira B. J. G., “Resource Utilization Among Kidney Transplant Recipients,” Kidney International 64, no. 2 (2003): 657–664. [DOI] [PubMed] [Google Scholar]
- 9. Famure O., Kim E. D., Au M., et al., “What Are the Burden, Causes, and Costs of Early Hospital Readmissions After Kidney Transplantation?,” Progress in Transplantation (Aliso Viejo, Calif.) 31, no. 2 (2021): 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharfuddin A., “Imaging Evaluation of Kidney Transplant Recipients,” Seminars in Nephrology 31, no. 3 (2011): 259–271. [DOI] [PubMed] [Google Scholar]
- 11. Sugi M. D., Joshi G., Maddu K. K., Dahiya N., and Menias C. O., “Imaging of Renal Transplant Complications Throughout the Life of the Allograft: Comprehensive Multimodality Review,” RadioGraphics [Internet] 39, no. 5 (2019): 1327–1355. [cited 2024 Feb 7];, https://pubs.rsna.org/doi/10.1148/rg.2019190096. [DOI] [PubMed] [Google Scholar]
- 12. Pappas P., Zavos G., Kaza S., et al., “Angioplasty and Stenting of Arterial Stenosis Affecting Renal Transplant Function,” Transplantation Proceedings 40, no. 5 (2008): 1391–1396. [DOI] [PubMed] [Google Scholar]
- 13. Loubeyre P., Abidi H., Cahen R., and Tran Minh V. A., “Transplanted Renal Artery: Detection of Stenosis With Color Doppler US,” Radiology 203, no. 3 (1997): 661–665. [DOI] [PubMed] [Google Scholar]
- 14. van de Kuit A., Benjamens S., Sotomayor C. G., et al., “Postoperative Ultrasound in Kidney Transplant Recipients: Association Between Intrarenal Resistance Index and Cardiovascular Events,” Transplantation Direct 6, no. 8 (2020): e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Y., Villeneuve P. J., Schaubel D., Mao Y., Rao P., and Morrison H., “Long‐Term Follow‐Up of Kidney Transplant Recipients: Comparison of Hospitalization Rates to the General Population,” Transplantation Research 2 (2013): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly B. C., Fung R., and Fung C., “Risk Stratification Framework to Improve the Utility of Renal Ultrasound in Acute Kidney Injury,” South African Journal of Radiology 28, no. 1 (2024): 2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naylor K. L., Knoll G. A., Slater J., et al., “Risk Factors and Outcomes of Early Hospital Readmission in Canadian Kidney Transplant Recipients: A Population‐Based Multi‐Center Cohort Study,” Canadian Journal of Kidney Health and Disease 8 (2021): 20543581211060926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. del Ruiz‐Fuentes M C., Vargas‐Rivas J., de Gracia‐Guindo C., et al., “Renal Transplant Patient in Emergency Department,” Nefrología (English Edition) 35, no. 6 (2015): 591–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


