Abstract
OBJECTIVE:
In uterine artery embolization (UAE) for the treatment of fibroids, nondegradable particles permanently occlude the uterine artery (UA). These particles remain in the vessels and can cause secondary undesirable effects, such as severe pain after embolization and fertility issues. In this prospective experimental study, we aimed to evaluate the angiographic recanalization, local and systemic reactions, and uterine damage occurring after performing UAE with newly developed degradable starch microspheres (DSMs) in sheep.
MATERIALS AND METHODS:
Under general anesthesia, eight nonpregnant sheep underwent bilateral UAE using DSMs to achieve stasis. Angiographic evaluation was performed on days 1, 3 and 7 after embolization to assess in vivo recanalization. In addition, the angiographic series were scored via a modified embolization score. A postmortem tissue examination was performed to determine whether DSMs and foreign body inflammatory reactions were present and to assess uterine necrosis.
RESULTS:
Complete bilateral embolization of the UA and cervicovaginal branches was achieved in all treated animals. Recanalization of the occluded arteries was evident in 25 of 27 arteries during the angiographic evaluation. In all sheep, there were multifocal areas of uterine necrosis, and some uterine vessels contained intraluminal material consistent with DSMs. The average weight of both uterine horns was significantly correlated with both the number of microspheres needed for complete embolization (r = 0.69, ρ<0.01) and the average percentage of necrosis in both uterine horns (r = 0.64, ρ<0.05).
CONCLUSIONS:
Our findings demonstrated the efficacy of vascular embolization with DSM by inducing ischemic changes in the uterus and subsequent recanalization of previously occluded arteries.
Keywords: Uterine artery embolization, degradable starch microspheres, animal models, angiography, leiomyoma
1. Introduction
Fibroids are the most common benign genital tumors in women of childbearing age. Clinical symptoms can include pelvic/abdominal pain, abnormal uterine bleeding and anemia [1]. Fibroids also appear to increase the likelihood of complications during pregnancy, labor, and delivery [2]. Fibroid treatment is based on clinical symptoms as well as other factors, such as fibroid size and location [3]. The currently available treatment strategies can involve both surgical and nonsurgical procedures.
Selective embolization of the uterine arteries (UAE) was first described in 1995 [4]. Bilateral occlusion of the uterine artery (UA) produces fibroid ischemia, which decreases the lesion size, consequently diminishing any associated symptoms, while collateral circulation maintains the vascular integrity of the other parts of the uterus. UAE is associated with lower morbidity and cost than surgical methods [5], with its ability to provide symptom relief being comparable to methods such as myomectomy [6] and hysterectomy [7–9]. Although unilateral embolization is performed in cases where the contralateral UA does not allow for selective catheter embolization, it usually results in a poor clinical response, with almost 60% of patients requiring additional intervention [10] or subsequent hysterectomy [11].
Moderate-to-severe pain after UAE is the primary adverse effect of this therapy [12, 13]. The most intense pain typically subsides after 24 hours [14, 15], but in 3–4% of patients, it may last for more than 2 weeks [16]; this pain has been attributed mostly to myometrial ischemia rather than fibroid ischemia [17]. Up to one-third of women have fever and leukocytosis, a condition known as postembolization syndrome (PES) [18]. Furthermore, with UAE, nondegradable particles remain in the uterus and generate an inflammatory response that can last for months [19, 20]. This foreign body giant cell chronic inflammatory reaction can occlude the uterine vessel lumen [19] and affect fertility in women [21]. Even though pregnancy after UAE is possible [22], the risk of miscarriage and other complications increases [23].
Currently available studies have reported image-based correlates for myometrial and fibroid ischemia at various time points after embolization. For example, immediately after UAE, myometrial perfusion on MR imaging was shown to decrease from 110% to 26% ME90 (maximal contrast enhancement above baseline at 90 seconds), whereas fibroid perfusion was zero [24]. Additionally, Kroenke et al. reported complete or almost complete infarction, defined as the absence of contrast enhancement on 80% of fibroids on follow-up T1-weighted MR images 24–72 hours after UAE compared with baseline images [25], and Katsumori et al. reported a more than 90% infarction of all fibroids one week after UAE [26].
Notably, these studies and procedures have all used nondegradable embolic agents. However, degradable embolic agents such as starch microspheres (DSMs) are now available. They are designed to be biodegraded by serum α-amylase within one week of embolization to reduce inflammation and foreign body reactions during mid- and long-term follow-up, as demonstrated in a porcine renal model [27]. The rationale for temporary embolization is to avoid short- and long-term effects caused by residual particles lingering after the treatment period. Ideally, an embolization agent should occlude the UA for more than 72 hours so that sufficient fibroid necrosis can occur; then, the agent should degrade to prevent unnecessary pain due to myometrial ischemia as well as to prevent the development of a chronic inflammatory response that could permanently alter vessels and healthy tissue. To our knowledge, there are no published studies using degradable microspheres for bilateral UA embolization.
Therefore, the purpose of this prospective experimental study was to evaluate angiographic recanalization, local and systemic reactions and uterine damage in sheep after UAE with newly developed degradable starch microspheres (DSMs). This animal model was chosen because of the similarities between sheep and humans with respect to plasma α-amylase levels [28, 29] and uterine vascular anatomy [30]. While similar studies have been published recently, they have either only performed unilateral embolization [31, 32] or only conducted one evaluation on the 7th day after bilateral embolization [33]. In this study, bilateral embolization with DSMs was performed. To determine whether and when particle degradation occurs, angiographic occlusion was assessed at several time points after UAE. Uterine damage was assessed seven days after UAE.
2. Materials and methods
2.1. Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with institutional guidelines for animal experimentation. Our study population was composed of 8 merino-ewes (Merinofleischschaf) that were 3–7 years of age, with an average weight of 80±9.2 kg and a history of at least one pregnancy before the study began. The ewes were housed at the outpatient-obstetric veterinary clinic barn of the University of Leipzig and received standard medical care from a group of veterinary and technical professionals. Blood samples were taken before anesthesia to monitor overall health as well as liver (AST = aspartate aminotransferase, ALT = alanine aminotransferase) and kidney (creatinine, urea) function. The animals underwent analgesia with buprenorphine (0.01 mg/kg) for 4 days and antibiotic treatment with enrofloxacin (5 mg/kg) for 5 days after the embolization. After the last angiography, the sheep were euthanized under anesthesia with pentobarbital (100 mg/kg).
2.2. Embolization particles
The DSMs developed by PharmaCept GmbH (PharmaCept, Berlin, Germany) were used for the treatment of uterine fibroids (prototype name: Myospheres). Specifically, the DSMs consisted of hydrolyzed potato starch that was chemically crosslinked and substituted with glycerol ether groups, with a ∼1% potential for deformation of and ∼300–800 μm diameters. These particles are enzymatically degraded by serum α-amylase in vitro within 54–72 h, as described in a previous publication [27].
2.3. Embolization procedure
The embolization procedure and the angiographic evaluations were performed in the Radiology Department of the University Hospital in Leipzig, Germany by a team of interventional radiologists and health care professionals certified to perform animal testing. All procedures were performed under general anesthesia induced intravenously with ketamine (4.4–6.0 mg/kg) and xylazine (0.05–0.1 mg/kg) and deepened with midazolam (0.1–0.3 mg/kg). The animal’s airways were secured with a cuffed endotracheal tube of size 8 or 10. During transportation, we used propofol (5–10 mg/kg per bolus). In the operating theater, the sheep were maintained under gas anesthesia with isoflurane and oxygen with a Dräger Fabius® MRI system. After the embolization procedure was completed, isoflurane and oxygen were reduced until the sheep exhibited self-sufficient respiration and regained a stable state. The pulse, respiration and temperature of the animals were monitored during anesthesia by a veterinarian. The intervention was performed under fluoroscopy with the animal in the supine position. After sterile preparation and covering of the inguinal area, the right common femoral artery was punctured using the Seldinger technique, and the aorta was reached with a long 5F sheath (Super Arrow Flex® lock, 45 cm). An aortogram was performed with manual injection of 5 ml of Imeron® 350 mg Iod/ml (Bracco Imaging Deutschland GmbH) to identify the pelvic arterial anatomy.
Using a microcatheter (Terumo Progreat® 2.7 Fr, 130 cm), selective bilateral embolization of the UA and the cervicovaginal branch (CV) was performed with the Myosphere particles. Owing to the absence of fibroids in the model organism used, both the UA and CV arteries were occluded to examine necrotic changes and to determine whether the occlusion caused by the DSMs was significant. Bilateral embolization was performed as the standard practice for fibroid treatment [34]. Digital subtraction angiography (DSA) was performed before and after embolization, and the procedure was deemed successful when complete stasis of uterine parenchymal perfusion was achieved. The amount of DSMs used on each artery and the duration of the procedure (from arterial puncture until the last postembolization scan = embolization time) were recorded.
After embolization, the 5F sheath was left in situ for use in subsequent evaluation angiographies to avoid multiple punctures of the femoral artery. It was blocked with 1000 IU of heparin, fixed with sutures, and covered with gauze and bandages.
2.4. Angiographic evaluation
Transportation, anesthesia and preparation were similar to those described on the day of the embolization procedure. In all cases, the persistent femoral artery sheath was reused for catheterization. Angiographic evaluations of the previously embolized vessels were performed in the same manner immediately after embolization in all animals, and follow-up evaluations were performed on the 1st, 3rd and 7th days after embolization. A DSA examination was performed with a selective injection of contrast agent delivered at a constant rate in each UA and CV branch of every sheep, and 80 images were recorded with a sampling rate of two images per second.
2.5. Embolization score
To evaluate vessel occlusion and recanalization of each individual artery, a dedicated embolization score (“Leipzig score”) was established for subsequent angiographic evaluations (Table 1). This score was derived from that described by Stampfl et al. [35] in a swine kidney model and takes a 0–4 range, where a score of 4 indicates the occlusion of additional adjacent nontarget branches in the organ. Since our objective was complete organ embolization, we focused on the loss of parenchymal staining (scores 0–3). By comparing the evaluation images against the preembolization images, scores could be assigned. To ensure consistent results, it was important to assess all images at the same time point (in seconds) after the application of the contrast agent into the artery. Parenchymal staining of the organ was evaluated approximately 10 seconds after contrast agent injection into the selected artery, and the images obtained before embolization were used as reference images. All the images were then reviewed by two experienced interventional radiologists (Observers A and B) who were blinded and determined the aforementioned “Leipzig score”.
Table 1.
Leipzig embolization score
| 0 | Complete perfusion after less than 10 sec (complete recovery of homogeneous parenchyma blush in less than 10 sec after contrast injection with no or minimal reflux to adjacent vessels) |
| 1 | Partial reduction of parenchymal staining after ∼10 sec. |
| 2 | Almost complete reduction of parenchymal staining after ∼10 sec. |
| 3 | Complete reduction of parenchymal staining/complete stasis |
2.6. Pathomorphological examinations
Sheep genital tracts were examined at the Institute of Veterinary Pathology by a board-certified veterinary pathologist via the following standardized procedure. Reproductive organs (uterus, vagina, vulva, both fallopian tubes and both ovaries) were assessed macroscopically and photographed. Individual parts of the uterus (cervix, uterine body, and right and left uterine horns) were separated and weighed. Representative samples of both ovaries, uterine tubes, and uterine arteries were fixed in 10% buffered formalin and in 96% ethanol for 24 hours.
The cervix, uterine body, and each uterine horn were cut transversely into approximately 1 cm thick slices that each consisted of the centrally located lumen surround by the inner endometrium, myometrium and outer perimetrium. Each tissue slice was subsequently divided into two evenly sized parts while preserving the described anatomical orientation; the cranial part was fixed in 10% buffered formalin, and the caudal part was fixed in 96% ethanol for 24 h. Comparison of the formalin- and ethanol-fixed parts of the uterine slices of sheep No. 1 revealed that ethanol fixation allowed better macroscopic distinction between normal and necrotic tissue than formalin fixation. Therefore, the caudal site of the ethanol-fixed part of each slice was photographed to determine the uterine necrosis score as described below. All formalin- and ethanol-fixed tissue slices were subjected to histological examination.
Pathomorphological examinations of the remaining tissues/organs involved macroscopic assessment, including assessment of lamellations; histological examination of altered tissue areas; and standardized localization of the heart, liver, kidneys, lungs, brain, lumbar spinal cord and skeletal muscle. Representative samples were fixed in 10% buffered formalin and 96% ethanol for 24 h.
For some samples, formalin fixation was used since it is routinely applied for histopathological examinations of hematoxylin–eosin-stained tissue sections. For other samples, ethanol fixation was used since it is considered more suitable for the detection of glycogen or starch via the periodic acid–Schiff (PAS) reaction.
Fixed tissue samples were routinely processed, embedded in paraffin and sectioned at 2 μm. Sections of formalin-fixed tissues were stained with hematoxylin-eosin; tissue sections of ethanol-fixed tissues were examined via the PAS reaction to better visualize starch containing DMS.
2.7. Scoring of the degree of uterine necrosis
The calculation of the necrosis score was performed using photographs of the ethanol-fixed part of each uterine slice according to the method described by Pelage et al. 2002 [36]. Tissue necrosis was indicated by abnormal brownish areas (Fig. 1). The presence of tissue necrosis and the distinction between the endo- and myometrium were confirmed by microscopic examination of the corresponding tissue sections (Fig. 1). For very few uterine slices of sheep No. 1, the determination of the necrosis score was exclusively performed on the microscopic sections. To score the degree of necrosis, each uterine slice was divided into 8 equal segments consisting of the inner uterine lumen, endometrium, myometrium and perimetrium. For each segment, the endometrium and myometrium were evaluated separately. Thus, a total of 16 segments were assessed per uterine slice, i.e., 8 in the endometrium and 8 in the myometrium.
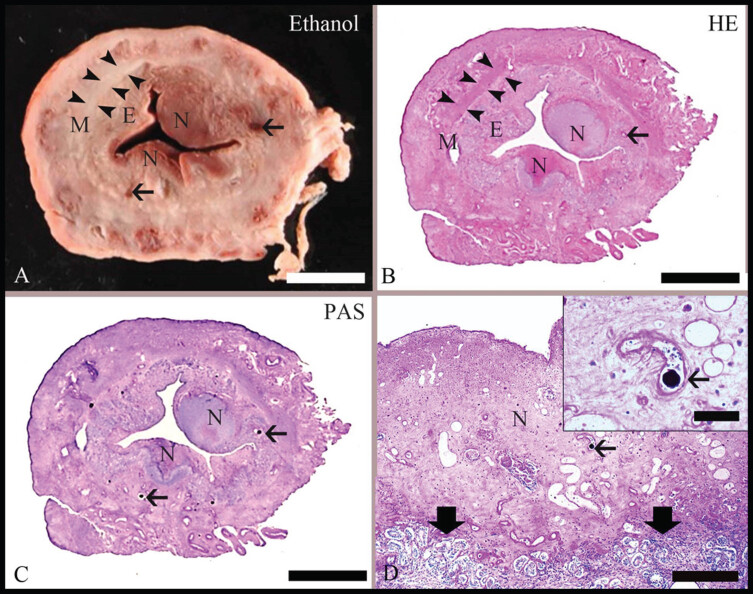
Fig. 1.
Pathomorphological findings in the uterus after embolization with degradable starch microspheres (DSMs). (A): Representative slice from a uterine horn with ethanol fixation (Ethanol-Fix.) used for the scoring of uterine necrosis. On gross examination, the inner endometrium [E] can be distinguished from the surrounding myometrium [M, with segments labeled by arrowheads]; areas of endometrial necrosis [N] are characterized by brownish discoloration. The thin arrows indicate blood vessels. Bar = 0.5 cm. (B): Microscopic hematoxylin–eosin (HE)-stained section corresponding to the formalin-fixed part of the uterine slice illustrated in (A). By microscopic examination, the distinction between E and M as well as the presence of N was confirmed. Bar = 0.5 cm. (C): Microscopic section stained with the periodic acid–Schiff reaction [PAS] and corresponding to the ethanol-fixed part of the slice illustrated in (A). A few vessels contain PAS-positive globular material [arrows], which is consistent with the DSMs. Bar = 0.5 cm. (D): A representative PAS-stained section at a higher magnification. The area of necrosis [N] is characterized by loss of normal tissue histology. It is distinct [thick arrowheads] from the unaffected endometrium. An intralesional vessel with intraluminal PAS-positive globular material is labeled with an arrow. Bar = 200 μm. Inset: Close-up of an intralesional vessel that contains PAS-positive DSM [arrow]. Bar = 50 μm.
2.8. Statistical analysis
The IBM SPSS statistical package (version 24) was used for all the statistical analyses. To test the associations between the amount of embolization agent (mL) injected, uterine weight (g), and uterine necrosis (%), myometrial necrosis (%) and endometrial necrosis (%) scores, we performed Pearson’s correlation between the variables. To evaluate the agreement between the scores given by the observers, we used Cohen’s kappa statistic. All the images were evaluated by two interventional radiologists and rated with an embolization score from 0 to 3 (details in the “Embolization score” section). Arterial blood flow before embolization was compared to later evaluation images and assigned a score depending on the degree of uterine parenchymal staining. This rating was validated through an interobserver agreement test. The kappa coefficient was evaluated as follows:<0, no agreement; 0.41 to 0.6 as moderate agreement; 0.61 to 0.8 as substantial agreement; and 0.81 to 1.00 as almost perfect agreement [37].
3. Results
3.1. Animals
The study originally included eight animals. Sheep No. 5 died before the first embolization could be performed, whereas sheep No. 8 died during the final angiographic evaluation, both due to respiratory complications during anesthesia. The angiographic evaluation after 48 hours was removed from the protocol per veterinary advice after the first two animals exhibited respiratory distress. For the remainder of the study, the sheep exhibited no signs of pain or discomfort. Laboratory diagnostics are not fully available for some sheep because no laboratory services were available either due to weekends or holidays. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) increased significantly at follow-up but remained within the reference range. Creatinine levels remained below the upper reference value during the study. The other hematological and clinical chemistry tests did not show significant changes. A summary of the results is presented inTable 2.
Table 2.
Experimental results of the analyzed animals
| Individual animals | Mean | ||||||||
| Sheep 1 | Sheep 2 | Sheep 3 | Sheep 4 | Sheep 5* | Sheep 6 | Sheep 7 | Sheep 8 | ||
| Laboratory findings | AST↑ ALT↑ | AST↑ ALT↑ | AST↑ ALT↑ | AST↑ ALT↑ | platelet count↓ | AST↑ ALT↑ | AST↑ ALT↑ | AST↑ ALT↑ | n/a |
| Weight | |||||||||
| Animal [kg] | 94.8 | 78.8 | 87.6 | 69.8 | 79.8 | 83 | 76.6 | 66.4 | 80±9.2 |
| Cervix [g] | 32.5 | 31.1 | 5.9 | 42.3 | 12.5 | 20.1 | 39.6 | 30.9 | 27±13 |
| Corpus [g] | 18.1 | 8.7 | 4.3 | 23.6 | 8.3 | 13.2 | 19.2 | 21.4 | 15±7 |
| R uterine horn [g] | 26.7 | 22.7 | 14.6 | 48 | 18.2 | 46.5 | 44.7 | 40.8 | 33±14 |
| L uterine horn [g] | 33 | 26.7 | 13.5 | 35 | 17.7 | 64.7 | 42 | 25.7 | 32±16 |
| Entire uterus [g] | 110.3 | 89.2 | 38.3 | 148.9 | 56.7 | 144.5 | 145.5 | 118.8 | 107±42 |
| Necrosis (%) | |||||||||
| Uterus | 21.87 | 12.85 | 16.96 | 37.17 | n/a | 30.30 | 37.04 | 27.31 | 26±9.5 |
| Uterus E/M | 14.93/6.94 | 10.07/2.78 | 12.50/4.46 | 27.30/9.87 | n/a | 23.48/6.82 | 26.85/10.19 | 20.83/6.48 | 19±7/ |
| 6.8±2.7 | |||||||||
| Uterine body | 18.75 | 25.00 | n/a | 31.25 | n/a | 75.00 | 68.75 | 37.5 | 43±24 |
| Body E/M | 18.75/n/a | 25.00/n/a | n/a | 27.08/4.17 | n/a | 43.75/31.25 | 50.00/18.75 | 20.83/16.67 | 31±13/ |
| 18±11 | |||||||||
| R uterine horn | 13.75 | 13.54 | 41.25 | 61.46 | n/a | 36.36 | 58.93 | 59.38 | 41±21 |
| R horn E/M | 10.00/3.75 | 10.42/3.12 | 28.75/12.50 | 43.75/17.71 | n/a | 29.54/6.82 | 42.86/16.07 | 46.88/12.5 | 30±15/ |
| 10±5.8 | |||||||||
| L uterine horn | 57.50 | 20.83 | 6.25 | 40.63 | n/a | 45.00 | 56.25 | 18.75 | 35±20 |
| L horn E/M | 36.25/21.25 | 15.63/5.20 | 6.25/n/a | 29.17/11.46 | n/a | 36.25/8.75 | 40.62/15.63 | 15.63/3.13 | 26±13/ |
| 11±6.7 | |||||||||
AST = aspartate aminotransferase; ALT = alanine aminotransferase; R = right; L = left; E = endometrium; M = myometrium; * = died before embolization; n/a = not applicable/available.
3.2. Embolization procedure
Bilateral UAE was successful in seven animals. The desired angiographic endpoint (score 3) was obtained for 14 UAs and 13 CVs (Fig. 2). The mean procedure time was 89±24 min per animal. Mean microsphere volumes of 7.26±3.9 mL per UA and 5.72±4.1 mL per CV were injected. There was a positive correlation between uterine horn weight and the amount of embolic agent used on the ipsilateral side (r = 0.69, p = 0.006) (Table 3).
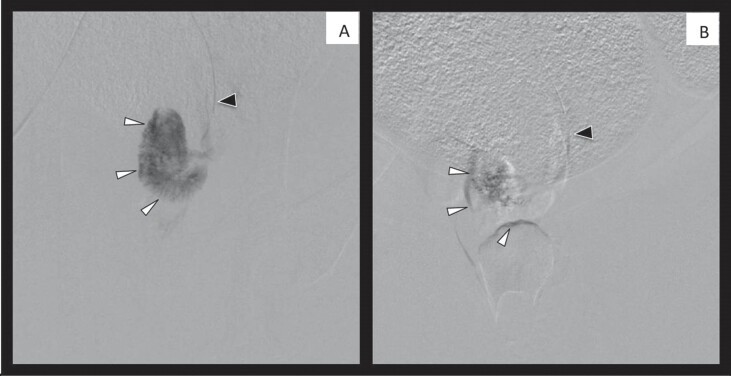
Fig. 2.
Examples of the uterine artery before (A) and after (B) embolization. Black arrow = catheter; white arrow = outline of the uterine parenchyma.
Table 3.
Pearson correlation matrix
| Total necrosis (%) | Endometrium necrosis (%) | Myometrium necrosis (%) | Particle amount (ml) | |
| Horn weight | 0.644* | 0.71** | 0.259 | 0.694** |
| Total necrosis (%) | 0.986** | 0.859** | 0.112 | |
| Endometrium necrosis (%) | 0.783** | 0.23 | ||
| Myometrium necrosis (%) | 0.368 |
*p < 0.05; **p < 0.01.
3.3. Angiographic follow-up/recanalization
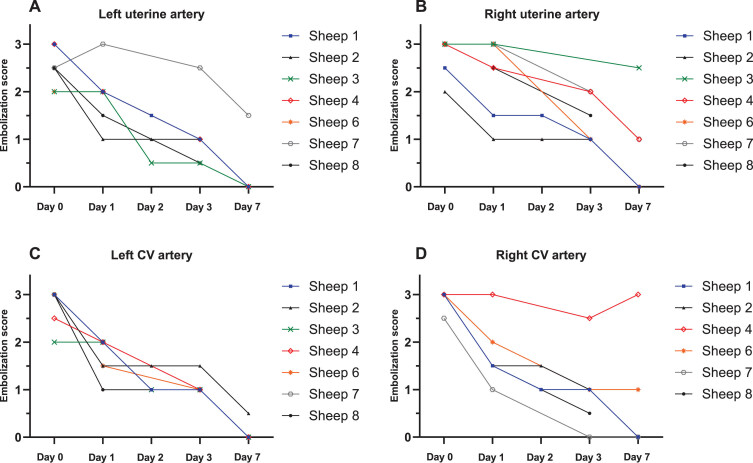
The interobserver agreement test indicated a significant match between the observers’ scores (Kappa value = 0.63; substantial agreement). The right UA of sheep No. 3 and the right CV of sheep No. 4 appeared to have been dissected during the procedure and thus remained unchanged during each evaluation. Recanalization (i.e., a decrease in score) was evident in all other arteries after 24 hours, and complete (score = 0) or almost complete (score = 1) recanalization was achieved by day 7 (Table 4, Fig. 3). An example of the angiographic evaluation is shown in Fig. 4.
Table 4.
Results of recanalization follow-up. The average embolization value is shown ((Observer A + Observer B)/2)
| Directly after | 24 hours | 48 hours | 72 hours | One week | ||||||||||||||||
| embolization | ||||||||||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |||||||||||
| ID | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV | UA | CV |
| 1 | 2.5 | 3 | 3 | 3 | 1.5 | 1.5 | 2 | 2 | 1.5 | 1 | 1.5 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 2 | 3 | 2.5 | 3 | 1 | 1.5 | 1 | 1.5 | 1 | 1.5 | 1 | 1.5 | 1 | 1 | 1 | 1.5 | 0 | 0 | 0 | 0.5 |
| 3 | 3* | n/a | 2 | 2 | 3* | n/a | 2 | 2 | n/a | n/a | 0.5 | 1 | n/a | n/a | 0.5 | 1 | 2.5* | n/a | 0 | 0 |
| 4 | 3 | 3* | 3 | 2.5 | 2.5 | 3* | 2 | 2 | ‡ | ‡ | ‡ | ‡ | 2 | 2.5* | 1 | 1 | 1 | 3* | 0 | 0 |
| 6 | 3 | 3 | 2 | 3 | 3 | 2 | 2 | 1.5 | ‡ | ‡ | ‡ | ‡ | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| 7 | 3 | 2.5 | 2.5 | 3 | 3 | 1 | 3 | 2 | ‡ | ‡ | ‡ | ‡ | 2 | 0 | 2.5 | 1 | 1 | 0 | 1.5 | 0 |
| 8 | 3 | 3 | 2.5 | 3 | 2.5 | 1.5 | 1.5 | 1 | ‡ | ‡ | ‡ | ‡ | 1.5 | 0.5 | 0.5 | 1 | † | † | † | † |
UA = uterine artery, CV = cervicovaginal branch. n/a not able to be selected with a microcatheter because of artery spasm or anatomical variation. † deceased. * Artery dissected during the procedure. ‡ evaluation after 48 h was removed to give the animals time to rest.
Fig. 3.
The embolization score over the observation period is shown in the graphs for the left (A) and right (B) uterine arteries and for the left (C) and right (D) cervicovaginal (CV) branches in Table 4. Sheep 3 is not shown in (D) because there are no data available; see the text for details.
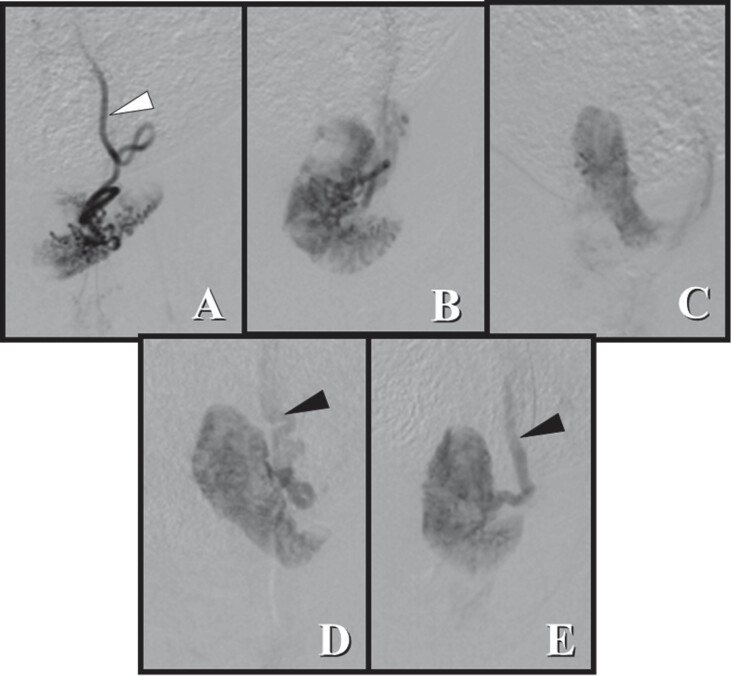
Fig. 4.
Angiographic evaluation of the uterine horn before and after embolization. (A) After 24 hours, there was an almost complete reduction in parenchymal staining. (B) After 48 hours, partial parenchymal staining was observed, but some areas were still not perfused. (C) After 72 hours, parenchymal staining was almost completely restored, and (D) after 7 days, there was complete recovery of homogenous parenchyma compared with the image before embolization. (E). The outline of the uterine parenchyma varied due to uterine horn mobility. White arrow: Uterine artery; black arrow: uterine vein.
3.4. Necrosis score
The uterine necrosis scores for each of the examined sheep are provided in Table 1. The average necrosis scores (means±SDs) were calculated from the individual necrosis scores of sheep Nos. 1–4 and Nos. 6–8 (Table 1). The average total necrosis score (TNS) of the entire uterus (26±9.5%) was the sum of the average endometrial (E) necrosis score of 19.0±7.0% and average myometrial (M) necrosis score of 6.8±2.7%. Considering the scores of both uterine horns, the average combined TNS was 38.0±20.0%, which was the sum of the average combined E score of 28.0±14.0% and the average combined M score of 11.0±6.0%. The average TNS for the uterine body was 43.0±24.0%, with an E score of 31.0±13.0% and M score of 18.0±11.0%. The cervix of all the examined sheep showed no evidence of necrosis.
The combined average weight of the right and left uterine horns was calculated from the individual weights of the right and left uterine horns of sheep Nos. 1–4 and 7–8. With respect to both uterine horns, the combined average weight was significantly correlated with the combined average TNS (r = 0.644, p = 0.013) and the combined average E necrosis score (r = 0.71, p = 0.004). However, there was no statistically significant correlation between the average combined weight of the uterine horns and the combined average M necrosis score (r = 0.259, p = 0.372) (Table 3).
3.5. Pathomorphological evaluation
The uterine body and both uterine horns presented multifocal areas of necrosis in the endometrium and, to a lesser degree, in the myometrium (Table 2). All examined cases displayed alterations in some uterine vessels corresponding to areas irrigated by the uterine artery, i.e., fibrinoid necrosis of vessel walls, vasculitis and/or perivasculitis, intraluminal thrombi and/or intraluminal globular PAS-positive structures consistent with DSMs. Very few PAS-positive particles were surrounded by a granulomatous foreign body response (sheep Nos. 1, 2 and 7). In sheep Nos. 1 and 3, the examined segment of the right uterine artery showed a partial focal occlusion by a fibrin thrombus, which contained a few PAS-positive structures consistent with the DSMs in sheep No. 3.
The skeletal muscles of the medial (sheep No. 2) and caudal thighs (sheep Nos. 3–4 and 6–8) had circumscribed areas of necrosis and histiocytic inflammation, without detectable intralesional PAS-positive material.
The mucosa of the larynx (sheep Nos. 3, 4, 6 and 7) and the trachea (sheep No. 4) showed focal areas of acute necrosis. In the lungs, moderate proteinaceous edema (sheep Nos. 1–4 and 6–8) and moderate vascular cuffing by lymphocytes and plasma cells (sheep Nos. 2–3, 6 and 8) were detected. The examined areas of the liver showed mild periportal fibrosis (sheep Nos. 6–8) and mild periportal infiltration, primarily with lymphocytes and plasma cells (sheep Nos. 1 and 6–8). In sheep No. 2, the kidneys displayed mild multifocal infiltration with lymphocytes and plasma cells, and in sheep No. 3, the right and left kidneys contained two infarcts each. The observed myocardial lesions were identified as mild to moderate fibrosis (sheep No. 3), multifocal mild infiltration with lymphocytes and plasma cells (sheep No. 8) and, in a few cases, sarcosporidian cysts (sheep Nos. 3, 4, 6 and 7). The cellular infiltrates in the lungs, liver, kidneys and myocardium, the renal infarcts and the sarcosporidian cysts most likely represented incidental findings without clinical significance.
PAS-positive globular material indicative of the presence of DSM was observed only in the lumen of the uterine artery (sheep No. 3) and some uterine vessels (all sheep). It was not detected in the ovary or oviduct (sheep 1–8) or in any other organ, including the examined areas of the brain, spinal cord, liver, heart and kidneys (sheep 1–4, 6–8).
4. Discussion
The aim of this study was to evaluate in vivo occlusion, subsequent revascularization and the associated histopathological changes after UAE with a novel DSM. Embolization with nondegradable particles has been used to treat uterine fibroids for several years. However, these nondegradable particles persist in the UA and its branches, causing fibrosis due to chronic inflammation secondary to foreign-body reactions [19]. The severe pain [15, 17, 38] that occurs directly after UAE may discourage women from considering this procedure, whereas women who wish to become pregnant may have concerns about potential effects on fertility or future pregnancies [21, 39, 40]. That said, there is long-term clinical experience with the use of approved starch-based embolization materials in the liver [41]. Owing to the limited embolization time needed in liver treatments (approximately 20 minutes), these agents are not suitable for the treatment of fibroids. Nonetheless, in recent years, different biodegradable microspheres have been studied in animal models for temporary embolization to minimize damage to normal tissue [31–33, 42, 43].
For example, in 2014, Verret et al. carried out unilateral UAE on six sheep and compared resorbable embolization microspheres (ResMic; Occlugel SAS) with nonresorbable trisacryl-gelatin microspheres (TGMS) [33]. Additionally, in 2017, Bengtsson et al. reported the effects of UAE in five sheep with a DSM (Spherex TAE, Magle AB) made from cross-linked maltodextrin with an in vitro degradation time of 10–20 h [31], and in early 2018, Keussen et al. compared the same particles to nondegradable microspheres on twenty-two sheep [32]. These studies all carried out unilateral UAE only, meaning that if the other UA was not occluded, there was some arterial supply coming from the other side, which potentially affected the results. Moreover, none of the studies carried out consistent angiographic evaluations during the first week postembolization. The time between embolization and the angiographic evaluations by Bengtsson et al. varied for each animal (i.e., 19, 20, 28, 49, and 65 h). Verret et al. performed only one angiographic postembolization evaluation on day seven, and Keussen et al. performed an evaluation only on day 14. Therefore, it is not possible to determine exactly when the degradation of the nonpermanent particles began based on previous results. Thus, the aim of this study was to monitor the rate of degradation during the first week after embolization. However, as it was not possible to examine the animals on a daily basis, examinations were carried out at 24 hours, 72 hours and 7 days after embolization. Angiograms obtained immediately after embolization (Fig. 2) revealed the interruption of arterial flow achieved with the DSM, whereas progressive revascularization due to particle degradation was demonstrated throughout the following evaluations (Table 4).
The purpose of developing DSMs for the treatment of fibroids is to occlude blood flow long enough to produce adequate ischemia on the fibroid, while ensuring the complete resolution of the embolic material to prevent side effects related to foreign-body reactions and long-term effects in normal tissue [44]. Several studies have described fibroid necrosis occurring during the first week after UAE [26]; therefore, it is important to focus on the days immediately following UAE with new degradable particles. According to the manufacturer’s “in vitro” testing, the degradation of the microspheres with a continuous source of α-amylase is achieved within 54–72 h. However, “in vivo” degradation varies slightly across test subjects; therefore, we planned angiographic evaluations around this period. Previous studies have confirmed that degradable particles can induce ischemic changes in uterine tissue and could therefore also be used to treat fibroids [31–33] and that the effects produced are comparable to those of permanent particles [32, 33]. This study confirms these results regarding the ischemic effects produced by biodegradable particles. Complete recanalization was observed after seven days but the process started within 24 hours after embolization and advanced progressively (Table 4).
The macropathological results revealed relevant necrotic changes in the uterus after embolization; these changes were more pronounced in the endometrium, with limited necrosis in the myometrium. The types of necrosis were distributed similarly to those described by Verret et al. when using other types of degradable particles: 23.5% endometrium and 8.2% myometrium necrosis [33]. Similarly, Bengtsson et al. and Keussen et al. described macroscopic changes in the endometrium with other types of degradable microspheres but did not provide an objective description of the changes in terms of a necrotic score [31, 32]. Myometrial ischemia has been associated with the severity of pain experienced after UAE [17]; therefore, it can be assumed that, by reducing the damage inflicted on the myometrium, the severity of the pain following UAE will also be reduced. Furthermore, the macropathological results revealed circumscribed necrosis and inflammation of the thigh muscles of sheep Nos. 2–4 and 6–8, which could be attributed to the sheep-specific connection of the cervicovaginal vessels with the pelvic muscles. The described laryngeal and tracheal focal necrosis most likely resulted from multiple intubations necessary for anesthesia, even though the 48 h follow-up was not conducted to allow the animals some rest in between evaluations. An increase in AST and ALT levels was noted during the study but the levels remained within the normal range. Bengtsson et al. described the increase in hepatocellular markers caused by cell damage due to ischemia [31].
The small sample size and absence of fibroids are major limitations of this study and previous studies. Owing to the lack of fibroids in the model sheep, both the UA and CV arteries were occluded to study necrotic changes and thus determine if the occlusion produced by the DSM was significant. It is likely that the results of the necrotic score in this study would have been lower if the myometrium and endometrium had been able to receive collateral supply from the CV arteries.
Nevertheless, we can conclude that the DSMs used in this study complied with the requirements of an effective nonpermanent embolization particle. These DSMs can effectively occlude vessels long enough to cause necrotic changes but also gradually degrade leading to complete revascularization after 7 days and thereby allowing tissue regeneration. The complete dissolution of embolic particles may avoid side effects related to foreign-body reactions, bioincompatibility, and long-term ischemia in normal tissue caused by current methods [45, 46]. Further research is needed to fully investigate the potential short- and long-term benefits of these DSMs in humans. However, it is reasonable to assume that these particles could be present a promising therapeutic option for the embolization of uterine fibroids if they are used in larger, prospective trials in humans.
Author contributions
CAH and MR wrote the manuscript. CAH, JF, HB and MM analyzed the data. MM and JF designed the study. MM, JF, CAH, CS, AD, AK and HB contributed to the study design and data collection. MR, CS, JF, CRB, PW, HB, TD and MM contributed to the interpretations of the findings and commented on and edited the drafts. MM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CAH, JF, HB and MM contributed equally to this work.
Funding
The project was funded by the German Federal Ministry for Economic Affairs and Energy (BMWi) (ZIM ZF4148703SK6).
References
- [1]. Moroni RM, Vieira CS, Ferriani RA, Reis RM, Nogueira AA, Brito LG. Presentation and treatment of uterine leiomyoma in adolescence: a systematic review. BMC Womens Health. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Coronado GD, Marshall LM, Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a population-based study. Obstet Gynecol. 2000;95(5):764–9. [DOI] [PubMed] [Google Scholar]
- [3]. Lefebvre G, Vilos G, Allaire C, Jeffrey J, Arneja J, Birch C, Others. The management of uterine leiomyomas. J Obstet Gynaecol Can. J Obstet Gynaecol Can. 2003;25(5):396–418. [PubMed] [Google Scholar]
- [4]. Ravina JH, Herbreteau D, Ciraru-Vigneron N, Bouret JM, Houdart E, Aymard A, Merland JJ. Arterial embolisation to treat uterine myomata. Lancet. 1995;346(8976):671–2. [DOI] [PubMed] [Google Scholar]
- [5]. Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, Maresh M, Nicholson A, McPherson K. A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess. 2008;12(5):1–248, iii. [DOI] [PubMed] [Google Scholar]
- [6]. Manyonda IT, Bratby M, Horst JS, Banu N, Gorti M, Belli AM. Uterine artery embolization versus myomectomy: impact on quality of life–results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc Intervent Radiol. 2012;35(3):530–6. [DOI] [PubMed] [Google Scholar]
- [7]. Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, Murray GD, Committee of the Randomized Trial of Embolization versus Surgical Treatment for F. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–70. [DOI] [PubMed] [Google Scholar]
- [8]. Hehenkamp WJ, Volkers NA, Birnie E, Reekers JA, Ankum WM. Symptomatic uterine fibroids: treatment with uterine artery embolization or hysterectomy–results from the randomized clinical Embolisation versus Hysterectomy (EMMY) Trial. Radiology. 2008;246(3):823–32. [DOI] [PubMed] [Google Scholar]
- [9]. de Bruijn AM, Ankum WM, Reekers JA, Birnie E, van der Kooij SM, Volkers NA, Hehenkamp WJ. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am J Obstet Gynecol. 2016;215(6):745 e1–e12. [DOI] [PubMed] [Google Scholar]
- [10]. Bratby MJ, Hussain FF, Walker WJ. Outcomes after unilateral uterine artery embolization: a retrospective review. Cardiovasc Intervent Radiol. 2008;31(2):254–9. [DOI] [PubMed] [Google Scholar]
- [11]. Gabriel-Cox K, Jacobson GF, Armstrong MA, Hung YY, Learman LA. Predictors of hysterectomy after uterine artery embolization for leiomyoma. Am J Obstet Gynecol. 2007;196(6):588.e1–6. [DOI] [PubMed] [Google Scholar]
- [12]. Laurent A, Wassef M, Namur J, Martal J, Labarre D, Pelage JP. Recanalization and particle exclusion after embolization of uterine arteries in sheep: a long-term study. Fertil Steril. 2009;91(3):884–92. [DOI] [PubMed] [Google Scholar]
- [13]. Toor SS, Jaberi A, Macdonald DB, McInnes MD, Schweitzer ME, Rasuli P. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(5):1153–63. [DOI] [PubMed] [Google Scholar]
- [14]. McLucas B, Goodwin SC, Kaminsky D. The embolised fibroid uterus. Minimally Invasive Therapy & Allied Technologies. 2009;7(3):267–71. [Google Scholar]
- [15]. Roth AR, Spies JB, Walsh SM, Wood BJ, Gomez-Jorge J, Levy EB. Pain after Uterine Artery Embolization for Leiomyomata: Can Its Severity be Predicted and Does Severity Predict Outcome? Journal of Vascular and Interventional Radiology. 2000;11(8):1047–52. [DOI] [PubMed] [Google Scholar]
- [16]. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109(11):1262–72. [DOI] [PubMed] [Google Scholar]
- [17]. Ruuskanen A, Sipola P, Hippeläinen M, Wüstefeld M, Manninen H. Pain after uterine fibroid embolisation is associated with the severity of myometrial ischaemia on magnetic resonance imaging. Eur Radiol. 2009;19(12):2977–85. [DOI] [PubMed] [Google Scholar]
- [18]. Bruno J, Sterbis K, Flick P, McCullough M, Cramp M, Murphy-Skrynarz K, Spies JB. Recovery after uterine artery embolization for leiomyomas: a detailed analysis of its duration and severity. J Vasc Interv Radiol. 2004;15(8):801–7. [DOI] [PubMed] [Google Scholar]
- [19]. Colgan TJ, Pron G, Mocarski EJ, Bennett JD, Asch MR, Common A. Pathologic features of uteri and leiomyomas following uterine artery embolization for leiomyomas. Am J Surg Pathol. 2003;27(2):167–77. [DOI] [PubMed] [Google Scholar]
- [20]. Weichert W, Denkert C, Gauruder-Burmester A, Kurzeja R, Hamm B, Dietel M, Kroencke TJ. Uterine arterial embolization with tris-acryl gelatin microspheres: a histopathologic evaluation. Am J Surg Pathol. 2005;29(7):955–61. [DOI] [PubMed] [Google Scholar]
- [21]. Torre A, Fauconnier A, Kahn V, Limot O, Bussierres L, Pelage JP. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27(7):2850–9. [DOI] [PubMed] [Google Scholar]
- [22]. Goodwin SC, Spies JB, Worthington-Kirsch R, Peterson E, Pron G, Li S, Myers ER, Fibroid Registry for Outcomes Data Registry Steering C, Core Site I. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33. [DOI] [PubMed] [Google Scholar]
- [23]. Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. deSouza NM, Williams AD. Uterine arterial embolization for leiomyomas: Perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology. 2002;222(2):367–74. [DOI] [PubMed] [Google Scholar]
- [25]. Kroencke TJ, Scheurig C, Poellinger A, Gronewold M, Hamm B. Uterine artery embolization for leiomyomas: percentage of infarction predicts clinical outcome. Radiology. 2010;255(3):834–41. [DOI] [PubMed] [Google Scholar]
- [26]. Katsumori T, Kasahara T, Kin Y, Nozaki T. Infarction of uterine fibroids after embolization: Relationship between postprocedural enhanced MRI findings and long-term clinical outcomes. Cardiovasc Intervent Radiol. 2008;31(1):66–72. [DOI] [PubMed] [Google Scholar]
- [27]. Sommer CM, Do TD, Schlett CL, Flechsig P, Gockner TL, Kuthning A, Vollherbst DF, Pereira PL, Kauczor HU, Macher-Goppinger S. In vivo characterization of a new type of biodegradable starch microsphere for transarterial embolization. J Biomater Appl. 2018;32(7):932–44. [DOI] [PubMed] [Google Scholar]
- [28]. Tschuor AC, Riond B, Braun U, Lutz H. Hämatologische und klinisch-chemische Referenzwerte für adulte Ziegen und Schafe. Schweiz Arch Tierheilkd. 2008;150(6):287–95. [DOI] [PubMed] [Google Scholar]
- [29]. Yokus B, Cakir DU, Kanay Z, Gulten T, Uysal E. Effects of seasonal and physiological variations on the serum chemistry, vitamins and thyroid hormone concentrations in sheep. J Vet Med A Physiol Pathol Clin Med. 2006;53(6):271–6. [DOI] [PubMed] [Google Scholar]
- [30]. Pelage JP, Laurent A, Bonneau M, Wassef M, Rymer R, Merland JJ. Arterial blood supply to the uterus in nonpregnant sheep: a pertinent model for clinical practice? Invest Radiol. 2001;36(12):721–5. [DOI] [PubMed] [Google Scholar]
- [31]. Bengtsson J, Cwikiel W, Sundgren PC, Karlstam E, Gavier-Widen D, Keussen I. The effects of uterine artery embolization with a new degradable microsphere in an experimental study. Acta Radiol. 2017;58(11):1334–41. [DOI] [PubMed] [Google Scholar]
- [32]. Keussen I, Bengtsson J, Gavier-Widen D, Karlstam E. Uterine artery embolization in a sheep model: biodegradable versus non-degradable microspheres. Acta Radiol. 2018;59(10):1210–7. [DOI] [PubMed] [Google Scholar]
- [33]. Verret V, Pelage JP, Wassef M, Louguet S, Servais E, Bedouet L, Beaulieu T, Moine L, Laurent A. A novel resorbable embolization microsphere for transient uterine artery occlusion: a comparative study with trisacryl-gelatin microspheres in the sheep model. J Vasc Interv Radiol. 2014;25(11):1759–66. [DOI] [PubMed] [Google Scholar]
- [34]. Spies JB. Current Role of Uterine Artery Embolization in the Management of Uterine Fibroids. Clin Obstet Gynecol. 2016;59(1):93–102. [DOI] [PubMed] [Google Scholar]
- [35]. Stampfl S, Stampfl U, Rehnitz C, Schnabel P, Satzl S, Christoph P, Henn C, Thomas F, Kauffmann GW, Richter GM. Experimental evaluation of early and long-term effects of microparticle embolization in two different mini-pig models. Part I: kidney. Cardiovasc Intervent Radiol. 2007;30(2):257–67. [DOI] [PubMed] [Google Scholar]
- [36]. Pelage JP, Laurent A, Wassef M, Bonneau M, Germain D, Rymer R, Flaud P, Martal J, Merland JJ. Uterine artery embolization in sheep: comparison of acute effects with polyvinyl alcohol particles and calibrated microspheres. Radiology. 2002;224(2):436–45. [DOI] [PubMed] [Google Scholar]
- [37]. Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33(1). [PubMed] [Google Scholar]
- [38]. Vilos AG, Vilos GA, Hollett-Caines J, Garvin G, Kozak R, Abu-Rafea B. Post-uterine artery embolization pain and clinical outcomes for symptomatic myomas using gelfoam pledgets alone versus embospheres plus gelfoam pledgets: a comparative pilot study. J Obstet Gynaecol Can. 2014;36(11):983–9. [DOI] [PubMed] [Google Scholar]
- [39]. Spies J. Uterine artery embolization for leiomyomata. Obstetrics & Gynecology. 2001;98(1):29–34. [DOI] [PubMed] [Google Scholar]
- [40]. Pelage JP, Walker WJ, Le Dref O, Laurent A, Rymer R. Treatment of uterine fibroids. Lancet. 2001;357(9267):1530. [DOI] [PubMed] [Google Scholar]
- [41]. Pieper CC, Meyer C, Vollmar B, Hauenstein K, Schild HH, Wilhelm KE. Temporary arterial embolization of liver parenchyma with degradable starch microspheres (EmboCept(R)S) in a swine model. Cardiovasc Intervent Radiol. 2015;38(2):435–41. [DOI] [PubMed] [Google Scholar]
- [42]. Maeda N, Verret V, Moine L, Bedouet L, Louguet S, Servais E, Osuga K, Tomiyama N, Wassef M, Laurent A. Targeting and recanalization after embolization with calibrated resorbable microspheres versus hand-cut gelatin sponge particles in a porcine kidney model. J Vasc Interv Radiol. 2013;24(9):1391–8. [DOI] [PubMed] [Google Scholar]
- [43]. Weng L, Seelig D, Rostamzadeh P, Golzarian J. Calibrated Bioresorbable Microspheres as an Embolic Agent: An Experimental Study in a Rabbit Renal Model. J Vasc Interv Radiol. 2015;26:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Kröncke TJ, Gauruder-Burmester A, Hamm B. Transarterielle Embolisation von Uterusmyomen – Eine neue Therapieoption bei symptomatischem Uterus myomatosus. Rofo. 2002;174(10):1227–35. [DOI] [PubMed] [Google Scholar]
- [45]. Hiebl B, Mrowietz C, Lee S, Braune S, Knaut M, Lendlein A, Franke RP, Jung F. Influence of polymeric microspheres on the myocardial oxygen partial pressure in the beating heart of pigs. Microvasc Res. 2011;82(1):52–7. [DOI] [PubMed] [Google Scholar]
- [46]. Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. 2017;105(3):927–40. [DOI] [PubMed] [Google Scholar]