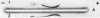Abstract
Haptoglobin binding to haemoglobin and its isolated alpha- and beta-chains was studied by use of a highly sensitive solid-phase radiometric assay. As expected, adsorbents of haemoglobin bound 125I-labelled haptoglobin more efficiently than did adsorbents of the alpha-chain. However, unexpectedly, adsorbents of the beta-chain were found to be essentially identical with those of the alpha-chain in their ability to bind haptoglobin. These results demonstrate, unequivocally, the ability of beta-chains to bind to haptoglobin, and indicate that this assay is particularly convenient and useful for studying haptoglobin interactions with haemoglobin and its alpha- and beta-chains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Chemical studies on haemoglobins A1 and A0. Biochem J. 1964 Oct;93(1):189–197. doi: 10.1042/bj0930189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Sakata S., Kazim A. L. Localization and verification by synthesis of five antigenic sites of bovine serum albumin. Biochem J. 1979 May 1;179(2):327–331. doi: 10.1042/bj1790327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiancone E., Alfsen A., Ioppolo C., Vecchini P., Agrò A. F., Wyman J., Antonini E. Studies on the reaction of haptoglobin with haemoglobin and haemoglobin chains. I. Stoichiometry and affinity. J Mol Biol. 1968 Jul 14;34(2):347–356. doi: 10.1016/0022-2836(68)90258-1. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Gray G. D., Mickelson M. M., Crim J. A. The demonstration of two gamma-globulin subclasses in the goat. Immunochemistry. 1969 Sep;6(5):641–644. doi: 10.1016/0019-2791(67)90128-0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Prediction and conformation by synthesis of two antigenic sites in human haemoglobin by extrapolation from the known antigenic structure of sperm-whale myoglobin. Biochem J. 1977 Oct 1;167(1):275–278. doi: 10.1042/bj1670275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Accurate definition of the antigenic site around the disulphide bridge 30-115 (site 3) by 'surface-simulation' synthesis. Biochem J. 1977 Dec 1;167(3):571–581. doi: 10.1042/bj1670571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. XIX. Definition of the direction and conformational restrictions of the antigenic site around the disulfide bonds 64-80 and 76-94 (site 2) by 'surface-simulation' synthesis. Biochim Biophys Acta. 1977 Dec 20;495(2):354–368. doi: 10.1016/0005-2795(77)90391-9. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Waks M., Alfsen A. Structural studies of haptoglobins. I. Hydrogen ion equilibria and optical rotatory dispersion of Hp 1-1 and Hp 2-2. Arch Biochem Biophys. 1966 Feb;113(2):304–314. doi: 10.1016/0003-9861(66)90191-3. [DOI] [PubMed] [Google Scholar]