Abstract
BACKGROUND:
Innovations in healthcare technologies have the potential to address challenges, including the monitoring of fluid balance.
OBJECTIVE:
This study aims to evaluate the functionality and accuracy of a digital technology compared to standard manual documentation in a real-life setting.
METHODS:
The digital technology, LICENSE, was designed to calculate fluid balance using data collected from devices measuring urine, oral and intravenous fluids. Participating patients were connected to the LICENSE system, which transmitted data wirelessly to a database. These data were compared to the nursing staff’s manual measurements documented in the electronic patient record according to their usual practice.
RESULTS:
We included 55 patients in the Urology Department needing fluid balance charting and observed them for an average of 22.9 hours. We found a mean difference of 44.2 ml in total fluid balance between the two methods. Differences ranged from 2230 ml to 2695 ml, with a divergence exceeding 500 ml in 57.4% of cases. The primary source of error was inaccurate or omitted manual documentation. However, errors were also identified in the oral LICENSE device.
CONCLUSIONS:
When used correctly, the LICENSE system performs satisfactorily in measuring urine and intravenous fluids, although the oral device requires revision due to identified errors.
Keywords: Water-electrolyte balance, monitoring, physiologic, digital technology, automation, equipment design
1. Introduction
Innovation is increasingly seen as a solution to the challenges posed by factors such as an increase in elderly and multimorbid patients and a shortage of healthcare professionals. Technological advancements have led to transformative changes in the workflows of healthcare professionals [1], improved treatment options and enabled continuous and digital monitoring.
Monitoring fluid balance is an essential nursing task in hospitals to ensure appropriate clinical interventions. Fluid balance disorders have been linked to higher morbidity and mortality rates [2, 3, 4] and prolonged hospital stays [5, 6, 7]. However, previous studies have highlighted the prevalence of inaccuracies in fluid balance charts; a study in ICU found calculation errors exceeding 500 ml in 26.1% of the charts [8], while another study reported that at least 60% of charts were incorrect and inaccurate [9]. A literature review examining the quality of fluid balance charting revealed a common occurrence of inaccuracies [10]. Achieving satisfactory compliance with completed fluid balance charts (75%) remains challenging despite various interventions, such as education, visual aids and equipment [10].
Automation has the potential to address issues related to incomplete documentation, calculation errors, and staff shortages. However, existing systems typically focus on a single aspect of fluid balance monitoring, such as urinary output [11, 12], while comprehensive monitoring requires knowledge of parameters like oral fluid intake. To address this gap, we have developed a novel monitoring device called LICENSE (LIquid balanCE moNitoring SystEm), designed to record fluid intake and output automatically [13].
The development of LICENSE stemmed from the ongoing need for accurate fluid balance monitoring and the commitment to fulfilling it. The concept of an automated solution encompassing all aspects of fluid balance evolved, leading to the creation of the initial prototype. While the innovation process can be described as linear [14], it often takes a cyclical or unplanned form with interrelated phases overlapping one another [15, 16]. The development of LICENSE involved multiple cyclical processes, integrating research and development in an interdisciplinary collaboration among engineers, doctors, and nurses [13]. The equipment underwent initial validation in a laboratory environment, focusing on crucial technological functions such as continuous data transfer and accurate data processing, reaching a Technology Readiness Level (TRL) of 3–4 [17, 18]. Subsequently, LICENSE’s precision and devices were evaluated in a relevant environment (TRL 5) under controlled conditions, demonstrating that the LICENSE system accurately measures urine output, oral intake, and intravenous intake, comparable to or surpassing standard manual methods [13].
This study aims to assess the functionality and accuracy of the digital technology LICENSE compared to standard manual documentation in routine clinical practice. The primary outcome measure is the total fluid balance measured by LICENSE and the manual procedure and the agreement between the two methods. Secondary outcomes include the agreement between LICENSE and the standard procedure for each device measuring intravenous fluids, oral fluids, and urine output, respectively.
2. Methods
This prospective observational study was conducted at a university hospital in Denmark between August 2021 and June 2022. The study received approval from the Regional Scientific Ethics Committee (ID: SJ-848) and was conducted in accordance with the principles outlined in the Declaration of Helsinki [19].
2.1. Study population
Patients were consecutively recruited during their hospital admission and selected based on the following inclusion criteria: adult patients requiring fluid balance charting and catheterized patients expected to remain hospitalized for at least one day. Patients who were unable to provide informed consent (e.g., due to cognitive impairment) or scheduled for discharge on the same day were excluded. Participating patients were provided with both oral and written information about the study and provided written informed consent.
2.2. Data collection
Data were obtained from the digital technology LICENSE and the electronic patient record to compare the standard procedure with the LICENSE measurements to determine the agreement between the two methods.
2.2.1. Digital technology
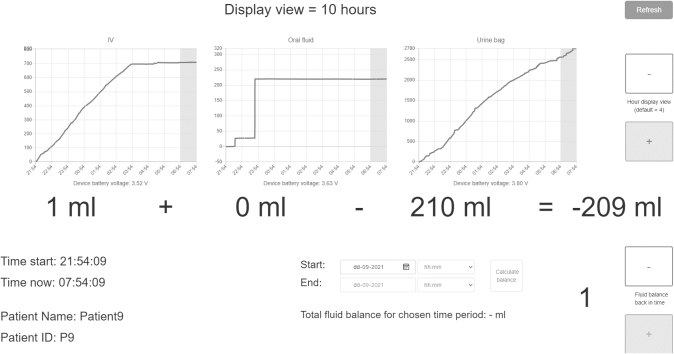
LICENSE comprises three independent measuring devices that monitor intravenous fluids, oral fluid intake, and urinary output through a catheter (as depicted in Fig. 1). The devices for intravenous fluids and urinary output are attached to a drip stand, allowing patients to move freely. Each device wirelessly collects and transmits data to a central database via Wi-Fi through the hospital’s secured medico network used for all medical devices transferring patient-related data. The database on the Microsoft Azure platform stored data and analysed it, applying software to ensure user-friendly dissemination. The results are presented to users through a user interface, displaying numerical data and graphs (as illustrated in Figs 2 and 3). The user interface includes several functions, such as graphs depicting fluid intake or output measured by each device for up to 10 hours, hourly fluid balance calculations, and total fluid balance calculations for a self-selected period. A key feature of the LICENSE technology is its ability to integrate fluid balance data from multiple sources (intravenous fluids, oral fluids, and urine output) and its high level of automation [13].
Figure 1.
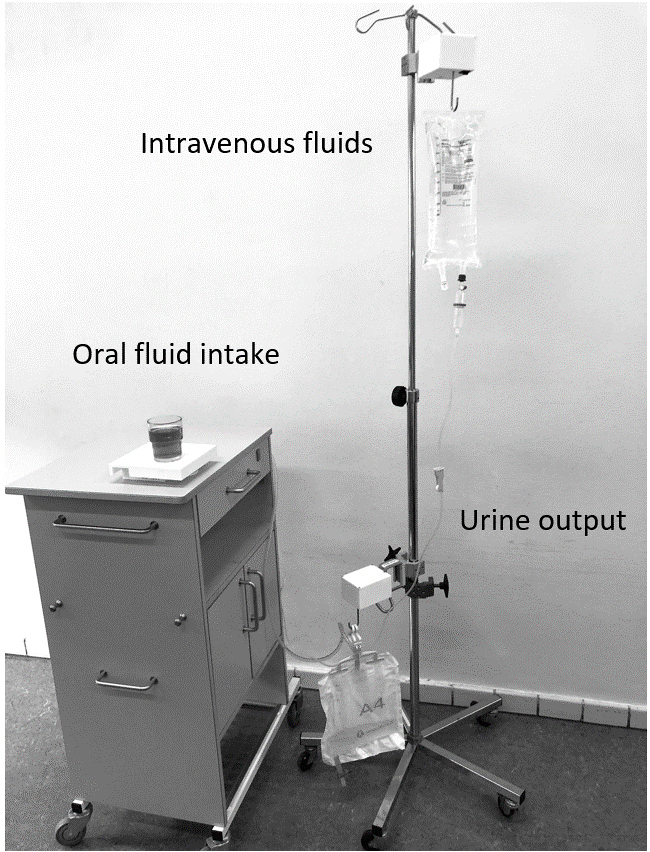
LICENSE consists of three devices measuring intravenous fluids, oral intake and urinary output.
Figure 2.
Graphic presentation of data in the LICENSE user interface.
Figure 3.
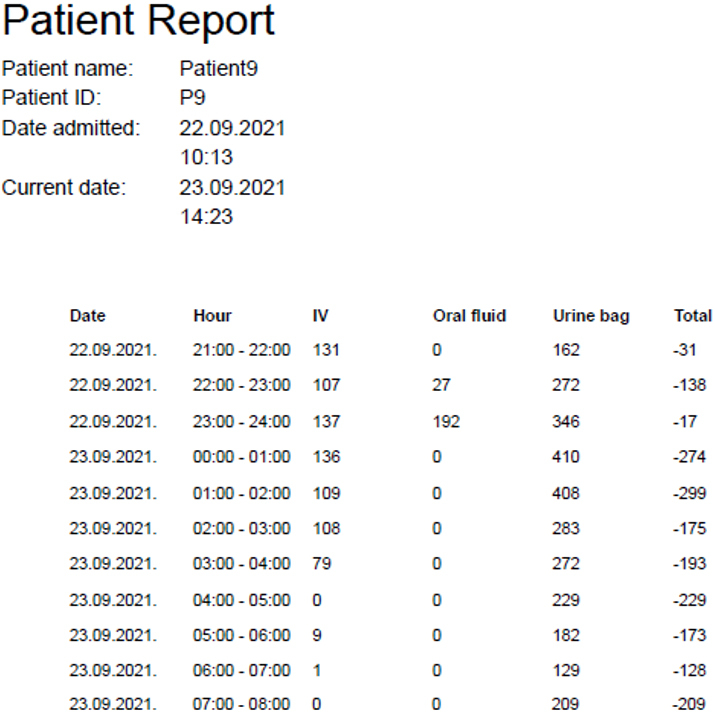
Numeric presentation of data in the LICENSE user interface.
After recruitment, a researcher registered the patient in the LICENSE user interface and ensured that all devices were powered on and ready to collect data. LICENSE was connected to the patient’s drip stand, and all fluids were measured while ensuring the urinary catheter bag was emptied. Patients and staff members were educated on how to use the LICENSE devices, and a written manual was made available in the staff office.
2.2.2. Standard manual procedure
To compare the measurements obtained by LICENSE with the standard manual procedure, manual measurements were performed in accordance with clinical guidelines, as typically done in daily clinical practice. Nursing staff served oral fluids, and their volumes were estimated using measuring lines on jugs or predefined volumes in different cups and glasses. Urinary bags were emptied once per shift, and urine volumes were measured using jugs with measuring lines. Intravenous fluids were documented based on the volume indicated on the manufacturer-labeled bag when emptied. The nursing staff documented the fluid measurements in the electronic patient record.
2.3. Data analysis
The primary outcome measure was the difference between the total fluid balance measured by LICENSE and the manual procedure. Total fluid balance was calculated for the entire individual observation period. In cases where the patient was discharged, or data collection was interrupted at an unscheduled time, the total fluid balance was calculated based on the last manual measurement, and LICENSE measurements were included until the same time.
Secondary outcomes included the agreement between LICENSE and the standard procedure for each device measuring intravenous fluids, oral fluids, and urine output, respectively. For all parameters, total fluid intake or output was calculated. For instance, the total urine output measured by LICENSE was compared to the total urine output measured by nursing staff. In cases of discrepancies, a detailed analysis of the fluid balance charts was conducted to explain the differences.
All differences were calculated by subtracting the measurements obtained by the standard method from the LICENSE measurements. Negative results indicated that manual measurements were larger than LICENSE measurements, while positive differences indicated that standard measurements were smaller.
2.3.1. Statistics
Considering the results of previous studies [8, 9], we conducted a power calculation for a one-sample proportion test, hypothesizing a divergence exceeding 500 ml in 35% of the fluid balance charts. The estimated sample size required was 51 participants. We considered a -value of less than 0.05 to be statistically significant.
For the statistical analyses, we utilized R software version 4.1.0. Descriptive statistics were presented as mean (SD) or median (IQR), depending on the normality of the data distribution, and proportions were also reported. To compare the results between the LICENSE measurements and the standard manual procedure, we employed paired -tests for normally distributed data. We used the Wilcoxon signed-rank test if the data did not meet the normality assumption. Furthermore, using a one-sample proportions test, we tested our primary hypothesis, which focused on the difference exceeding 500 ml in more than 35% of the fluid balance charts.
Agreement between manual charting and LICENSE measurements were illustrated by Bland-Altman plots, scatterplots and Pearson correlation.
3. Results
A total of 55 patients admitted to the Department of Urology were included in the study. These patients were observed for an average duration of 22.9 hours (SD 3.6). The mean age of the participants was 66.8 years (SD 12.5), and 74.5% were male. Among the participants, 22% were prescribed diuretics, and 65% received intravenous fluids or medication. The current fluid status was assessed in 25% of the patients, and 71% had fluid balance charting prescribed, which involved recording either intake only, both input and output, or hourly diuresis. The remaining 29% required postoperative fluid balance charting per local clinical guidelines. Additional patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Age, years, mean (sd), 55 | 66.8 (12.5) |
|---|---|
| Male, (%), 55 | 41 (74.5) |
| Diagnosis, (%), 52 | |
| Postobstructive diuresis | 11 (21) |
| Urinary retention | 1 (2) |
| Urosepsis/UTI | 10 (19) |
| Operation* | 16 (31) |
| Other (e.g. AKI) | 14 (27) |
| Fluid balance assessment (Yes), (%), 55 | 14 (25) |
| Prescribed fluid balance charting, (%), 55 | |
| No | 16 (29) |
| Yes, intake only | 5 (9) |
| Yes, both intake and output | 18 (33) |
| Yes, hourly diuresis | 14 (25) |
| Yes, output only | 2 (4) |
| Diuretics (Yes), (%), 54 | 12 (22) |
| Intravenous prescription (Yes), 55 | 36 (65) |
| Observation time, hours, mean (sd), 54 | 22.9 (3.6) |
*Operations include radical prostatectomy, nephroureterectomy, and heminephrectomy; AKI, acute kidney injury.
The mean total fluid balance measured by the LICENSE system was 459 ml (SD 1234.1 ml), while the manual documentation, according to the standard procedure, yielded a mean of 414.8 ml (SD 1146.5 ml). This resulted in a difference between the two methods of 44.2 ml (SD 891.9 ml). Differences ranged from 2230 ml to 2695 ml, and the absolute mean difference was 566.5 ml (IQR 189.2; 984.5). In 57.4% of patients (95% CI 43.2% to 70.8%), the difference between the methods exceeded 500 ml. Detailed results can be found in Table 2.
Table 2.
Fluid balance measurements
| Total fluid balance (LICENSE), ml, mean (sd), 54 | 459 (1234.1) |
|---|---|
| Total fluid balance (standard procedure), ml, mean (sd), 54 | 414.8 (1146.5) |
| Total differences between methods (license minus standard), 54 | |
| Total fluid balance, ml, mean (sd) | 44.2 (891.9)⋄ |
| Urine, ml, median (IQR) | 58.00 (271.8; 71.8) |
| Oral, ml, median (IQR) | 190.0 (828; 120.2) |
| Intravenous, ml, median (IQR) | 58.5 (0; 258.8) |
| Absolute difference in total fluid balance, ml, median (IQR) | 566.5 (189.2; 984.5) |
| Median divergences between methods in categorized total fluid balance, median (IQR), 53 | |
| 500 ml | 952 (625.2; 1269) |
| 0 to 500 ml | 121.8 (54.5; 283) |
| 0 to 500 ml | 171 (224.5; 148) |
| 500 ml | 824 (1128; 714) |
| Patients with an absolute difference between methods 500 ml, % (CI), 54 | |
| Total fluid balance | 57.4 (46.2; 100)** |
| Urinary output | 18.5 (9.2; 31.4) |
| Intravenous input | 11 (4.2; 22.6) |
| Oral intake | 42.6 (29.2; 56.8) |
| Mean bias between methods, ml (CI), 54 | |
| Urinary output | 68.7 (175.8; 38.4) |
| Intravenous input | 185.8 (76.6; 295) |
| Oral input | 298.7 (505.5; 91.8) |
| Pearson correlations coefficients | |
| Urinary output | 0.9667 |
| Intravenous input | 0.7958 |
| Oral input | 0.7842 |
| Intravenous fluids missing in the electronic fluid chart, % (CI), 35 | 77.1 (59.9; 89.6) |
⋄Non-significant mean difference in paired -test ( 0.7172); **p-value below 0.01 in one-sample proportion test testing the hypothesis that 35 % of fluid balance charts had a divergence of 500 ml ( 0.0003).
When comparing the LICENSE system to the standard manual procedure for each fluid balance parameter, we observed that in 18.5% of patients (95% CI 9.2% to 31.4%), the difference in urinary output measurements exceeded 500 ml. Regarding intravenous fluids documentation, differences of more than 500 ml were found in 11% of patients (95% CI 4.2% to 22.6%), and in oral fluid intake, the difference exceeded 500 ml in 42.6% of patients (95% CI 29.2% to 56.8%). We refer to Table 2 for a comprehensive overview of these findings.
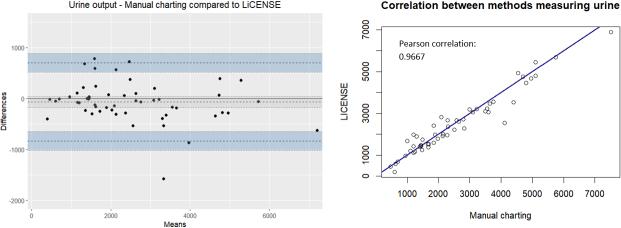
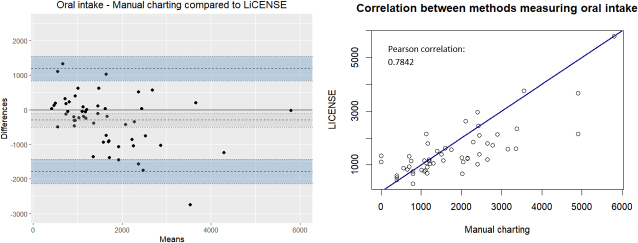
The differences in total fluid balance calculations displayed a normal distribution centred around zero (Fig. 4). However, urine output differences exhibited a negative skewness, and a mean difference of 68.7 ml (175.8 to 38.4). The Pearson correlation was 0.9667 (Fig. 5). Conversely, differences in intravenous fluids were positively skewed, with a mean bias of 185.8 ml (76.6 to 295.0) and a Pearson correlation of 0.7958 (Fig. 6). In the case of oral fluid measurements, differences were negatively skewed with a mean bias of 298.7 ml (505.5 to 91.8). The Pearson correlation was 0.7842 (Fig. 7).
Figure 4.
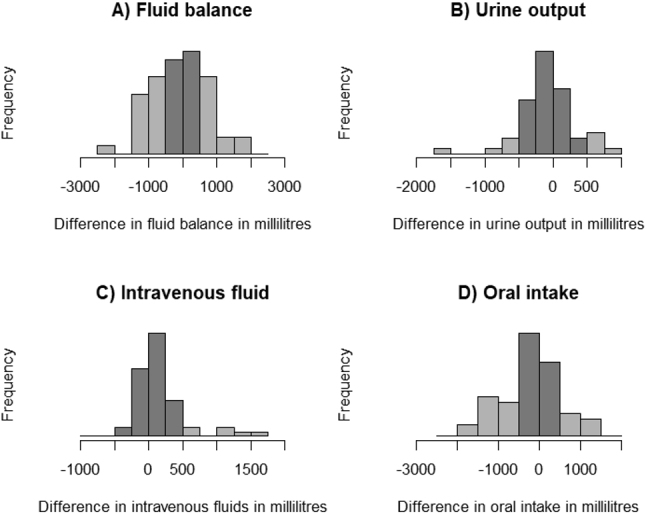
Histograms illustrating the difference between the standard manual method and LICENSE measurements (darker grey areas within 500 ml range). Differences are calculated as LICENSE minus standard.
Figure 5.
The agreement between manually charted urine output and LICENSE measurements illustrated by a Bland-Altman plot (left) and a scatterplot with Pearson correlation (right).
Figure 6.
The agreement between manually charted intravenous fluids and LICENSE measurements illustrated by a Bland-Altman plot (left) and a scatterplot with Pearson correlation (right).
Figure 7.
The agreement between manually charted oral fluids and LICENSE measurements illustrated by a Bland-Altman plot (left) and a scatterplot with Pearson correlation (right).
4. Discussion
The average discrepancy of 44.2 ml between LICENSE measurements and the standard procedure may initially seem convincing. However, the mean value was calculated based on a wide range of positive and negative differences that offset each other, illustrated by a mean absolute difference of 566.5 ml. More than half the patients (57.4%) exhibited a difference exceeding 500 ml, primarily caused by incomplete or inaccurate manual documentation. Nevertheless, we also identified sources of error related to the oral LICENSE device. As a result, we cannot solely attribute the differences between the methods to inaccurate manual documentation and assert that LICENSE provides precise measurements in contrast to a manual method prone to errors. Consequently, drawing meaningful conclusions regarding the primary outcome, which was the difference in total fluid balance measured by LICENSE and the standard manual method, becomes impossible. Consequently, we shifted our focus to the secondary outcomes and examined various potential reasons for the observed disparities. Nevertheless, we have acquired valuable knowledge concerning common sources of error in manual documentation and the feasibility of the LICENSE system, which will guide improvements.
4.1. Urine output measurements
When comparing urine output measurements between LICENSE and the standard manual method, we observed both negative and positive differences, with 18.5% of patients exhibiting differences exceeding 500 ml and a mean bias of 68.7 ml. A closer examination of individual patients’ fluid balance charts revealed inconsistencies that may have resulted from inaccurate manual estimation and rounding of numbers when reading measuring lines on urine jugs. A mean difference of 10.8 ml (9.6 to 11.9 ml) was found when comparing reading measuring lines to a reference weight [13]. This finding is supported by a study by Minor et al. [20], which reported that nurses significantly overestimated hourly urine output and demonstrated that automation improved accuracy. Positive differences in urine output measurements may be attributed to missing documentation of manual readings. Another source of divergence occurred when the catheter bag was removed from the LICENSE hook and not properly repositioned afterwards.
4.2. Intravenous fluid measurements
The histogram and Bland-Altman plot depicting differences in intravenous fluid measurements (Figs 4 and 6) demonstrated positive skewness. Upon scrutinizing individual charts, we discovered that missing documentation of intravenous fluids, particularly intravenous antibiotics, in the electronic fluid balance chart was the underlying cause. Notably, 77.1% of patients received intravenous fluids or medication according to both LICENSE and the electronic medication list, but this information was not reflected in the electronic fluid chart. Consequently, fluid intake was underestimated in the electronic fluid chart, consistent with a study reporting that 66.9% of errors in fluid balance calculations were due to the omission of intravenous drugs [21]. Another study reported a discrepancy between intravenous volumes documented on paper charts and those recorded electronically, with 26.1% lacking electronic recording [22].
Inaccurate manual documentation, resulting from documenting intravenous fluid volumes based on the volume indicated by the manufacturer on the fluid bag, emerged as another reason for divergence. Firstly, some fluids remained in the infusion lines when infusions ended; secondly, the volume specifications provided by manufacturers are not always accurate. A study comparing fluid weights with nurses’ documentation identified a significant difference; however, it did not specify whether nurses documented higher or lower values [23]. Another study of 3-liter bags reported an average overfill of 3.8%, corresponding to a mean overfill of 115.3 ml [24].
Some patients received intravenous infusions when they were recruited to the project, and the remaining volume at enrollment was estimated; however, estimating volumes from intravenous fluid bags is imprecise.
4.3. Oral fluid measurements
Significant and common differences in oral fluid measurements were observed, with 42.6% of patients exhibiting differences exceeding 500 ml and a negative skewness with a mean bias of 298.7 ml (Fig. 7). Various factors may contribute to these large differences. As reported in previous studies [8, 10, 25], calculation errors may have played a role in the current study. Also, missing manual oral fluid documentation in the electronic fluid chart would lead to positive differences. Negative differences may arise from nursing staff overestimating fluid intake due to inaccurate readings or erroneous assumptions, such as assuming a full jug (1000 ml) without precise knowledge of the actual volume served. Studies addressing inaccurate estimations of oral fluids found that only 27% [26] and 50% [27] of nurses’ estimations fell within a 10% error margin. Furthermore, most volumes were overestimated [13, 26, 27], which could explain some differences, particularly those falling between 0 and 500 ml. Through subsequent tests, we identified sources of error associated with the design of the oral LICENSE device. If staff removed empty glasses or cups from the oral device without replacing them, LICENSE interpreted the weight change as fluid intake. Consequently, we instructed staff to leave cups and glasses on the device or replace them with a similar fluid container. Furthermore, LICENSE only transferred data every 30 seconds. As a result, when drinks were served, and the patient immediately consumed them, LICENSE failed to accurately register the total amount of fluids served, leading to negative differences.
4.4. Implications
This study illustrated that omissions in manual fluid balance charting play a significant role in discrepancies related to urine output, intravenous fluids, and oral fluids. Several factors have been identified as potential causes for missed nursing care, including communication issues, shift types, available nursing resources, and workload [28]. In the context of fluid balance charting, causes of errors and omissions include increased workload and time constraints due to staff shortages [9, 29, 30, 31], poor communication among staff members [29, 30], and frequent change of caregivers [32]. Thus, automating fluid balance charting may improve charting quality. However, LICENSE needs further revision.
Further, this study demonstrated the necessity of evaluating medical devices in real-life settings. The oral LICENSE device performed satisfactorily in a controlled validation study [13]; however, use in real life revealed possible mishandling, resulting in faulty data. When a revised LICENSE prototype has been developed, preventing the risk of errors identified in the current version, the usability and feasibility need testing. Initially, in a study including observations or video surveillance to ensure the new LICENSE prototype version is indeed improved. Subsequently, a larger-scale study validating the effectiveness of the revised prototype. Another essential step for LICENSE to reach the European market is CE marking, as this is mandatory to ensure that medical devices comply with standards in the European Union [33].
4.5. Limitations
This study is subject to several limitations. Firstly, due to uncertainties primarily associated with the oral LICENSE device, we were unable to draw meaningful conclusions regarding our primary outcome. However, the study provided valuable insights for improving the digital technology and identifying sources of errors in manual fluid balance charting. Additionally, while data supported some causes of errors, others relied on clinical experience and speculation. A more comprehensive understanding of error sources would require additional observations or video surveillance. The threshold of 500 ml is not supported by evidence and may vary depending on the clinical context, patient characteristics, comorbidities, and illness severity [34].
5. Conclusion
The LICENSE system shows promise in enhancing the quality of fluid balance charting. However, further adjustments are necessary to improve its accuracy and usability, particularly concerning the oral device. We anticipate that the implementation of such improvements will have a positive impact on the work environment of nursing staff by saving time. Nonetheless, the effects on the work environment should be investigated in future studies.
Acknowledgments
The authors have no acknowledgements.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- [1]. Socha-Dietrich K. Empowering the health workforce. Strategies to make the most of the digital revolution.: OECD; 2020.
- [2]. Özgür Y, Akın S. The effects of fluid balance disorders on mortality in patients hospitalized for acute disease in the internal medicine clinic. J Acute Med. 2021; 11(2): 49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from sepsis: Results from a large international audit. Crit Care Med. 2017; 45(3): 386-94. [DOI] [PubMed] [Google Scholar]
- [4]. Hamrick I, Norton D, Birstler J, Chen G, Cruz L, Hanrahan L. Association between dehydration and falls. Mayo Clin Proc Innov Qual Outcomes. 2020; 4(3): 259-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Shahidi Delshad E, Sanadgol H, Bakhshandeh H, Saberian M, Alavi SM. Fluid balance has effects on the length of hospital stay after coronary artery bypass grafting surgery. Iran J Kidney Dis. 2020; 14(1): 36-43. [PubMed] [Google Scholar]
- [6]. Chan HYL, Cheng A, Cheung SSS, Pang WW, Ma WY, Mok LC, et al. Association between dehydration on admission and postoperative complications in older persons undergoing orthopaedic surgery. J Clin Nurs. 2018; 27(19-20): 3679-86. [DOI] [PubMed] [Google Scholar]
- [7]. Wuethrich PY, Burkhard FC, Thalmann GN, Stueber F, Studer UE. Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: A randomized clinical trial. Anesthesiology. 2014; 120(2): 365-77. [DOI] [PubMed] [Google Scholar]
- [8]. Diacon A, Bell J. Investigating the recording and accuracy of fluid balance monitoring in critically ill patients. Southern African Journal of Critical Care. 2014; 30(2). [Google Scholar]
- [9]. Madu A, Asogan H, Raoof A. Education and training as key drivers for improving the quality of fluid balance charts: Findings from a quality improvement project. BMJ Open Quality. 2021; 10(3): e001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Leinum LR, Krogsgaard M, Tantholdt-Hansen S, Baandrup AO, Gögenur I, Azawi N. Quality of fluid balance charting and interventions to improve it: A systematic review. BMJ Open Quality. 2023; 12(4): e002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Eklund A, Slettengren M, van der Linden J. Performance and user evaluation of a novel capacitance-based automatic urinometer compared with a manual standard urinometer after elective cardiac surgery. Crit Care. 2015; 19(1): 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Hersch M, Einav S, Izbicki G. Accuracy and ease of use of a novel electronic urine output monitoring device compared with standard manual urinometer in the intensive care unit. J Crit Care. 2009; 24(4): 629e13-17.. [DOI] [PubMed] [Google Scholar]
- [13]. Leinum LRBA, Gögenur I, Krogsgaard M, Azawi N. Digitizing fluid balance monitoring may offer a solution for optimizing patient care. Technology and Health Care. 2024; 32(2): 1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Rogers EM. Diffusion of Innovations. 5th ed: Simon & Schuster Ltd; 2003.
- [15]. Hartley J, Knell L. Innovation, exnovation and intelligent failure. Public Money & Management. 2022; 42(1): 40-8. [Google Scholar]
- [16]. Meissner D, Kotsemir M. Conceptualizing the innovation process towards the ‘active innovation paradigm’ – trends and outlook. Journal of Innovation and Entrepreneurship. 2016; 5(1): 14. [Google Scholar]
- [17]. Flessa S, Huebner C. Innovations in Health Care-A Conceptual Framework. Int J Environ Res Public Health. 2021; 18(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Organisations EAoRaT. The TRL Scale as a Research & Innovation Policy Tool, EARTO Recommendations. 2014. 30 April 2014.
- [19]. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013; 310(20): 2191. [DOI] [PubMed] [Google Scholar]
- [20]. Minor J, Smith A, Deutsch F, Kellum JA. Automated versus manual urine output monitoring in the intensive care unit. Sci Rep. 2021; 11(1): 17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Szmuda T, Waszak PM, Rydz C, Springer J, Budynko L, Szydlo A, et al. The challenges of hypervolemic therapy in patients after subarachnoid haemorrhage. Neurol Neurochir Pol. 2014; 48(5): 328-36. [DOI] [PubMed] [Google Scholar]
- [22]. Perotti S, Ritchie A. The impact of hybridisation on the accuracy of fluid balance documentation: A retrospective cross-sectional analysis of intravenous fluid order and administration documentation using a partly-computerized medical record in an australian tertiary teaching hospital. Stud Health Technol Inform. 2019; 264: 1751-2. [DOI] [PubMed] [Google Scholar]
- [23]. Haghighi S, Nourbakhsh S, Hashemi A, Haghighi S, Hashemi A, Alizadehasl A. Comparing the accuracy of the flowmeter in calculating the amount of fluid intake with conventional prescription of fluids by nurses in cardiovascular disease patients. Iranian Heart Journal. 2015; 16(1): 34-7. [Google Scholar]
- [24]. Nikolopoulos I, Phillips G. Reliability of fluid monitoring during operative hysteroscopy. Gynecological Surgery. 2016; 13(1): 23-6. [Google Scholar]
- [25]. Perren A. Fluid balance in critically ill patients should we really rely on it? Minerva Anestesiologica. 2011; 77(8): 802-11. [PubMed] [Google Scholar]
- [26]. Tattersall C. Nursing staff’s ability to gauge fluid intake. Nurs Times. 2016; 112(45/46): 19-22. [Google Scholar]
- [27]. Michelsen CF, Søndergaard Svendsen MB, Bagger ML, Konradsen H. A study on accuracy and precision of fluid volume measurements by nurses, patients and healthy persons in a clinical setting. Nursing Open. 2022; 9(2): 1303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Blackman I, Henderson J, Willis E, Hamilton P, Toffoli L, Verrall C, et al. Factors influencing why nursing care is missed. J Clin Nurs. 2015; 24(1-2): 47-56. [DOI] [PubMed] [Google Scholar]
- [29]. Reid J, Robb E, Stone D, Bowen P, Baker R, Irving S, et al. Improving the monitoring and assessment of fluid balance. Nurs Times. 2004; 100(20): 36-9. [PubMed] [Google Scholar]
- [30]. Asfour HI. Fluid balance monitoring accuracy in intensive care units. IOSR J Nur Heal Sci. 2016; 5(4VI): 53-62. [Google Scholar]
- [31]. Wehrle CJ, Walker M, Worthey A, Jones CE, Lewis F, Arora TK. Barriers to accurate fluid measurement in perioperative patients: A mixed methods approach. J Surg Res. 2021; 260: 95-103. [DOI] [PubMed] [Google Scholar]
- [32]. Yang S-H, Mu P-F, Wu H-L, Curia M. Fluid balance monitoring in congestive heart failure patients in hospital: A best practice implementation project. JBI Database System Rev Implement Rep. 2019; 17(10): 2202-11. [DOI] [PubMed] [Google Scholar]
- [33]. Badnjevic A. Evidence-based maintenance of medical devices: Current shortage and pathway towards solution. Technology and Health Care. 2023; 31: 293-305. [DOI] [PubMed] [Google Scholar]
- [34]. McGee WT, Raghunathan K. Physiologic goal-directed therapy in the perioperative period: the volume prescription for high-risk patients. J Cardiothorac Vasc Anesth. 2013; 27(6): 1079-86. [DOI] [PubMed] [Google Scholar]