Abstract
BACKGROUND:
Advanced knee osteoarthritis (KOA) impacts both knees, resulting in pain, deformity, and substantial restrictions in joint mobility.
OBJECTIVE:
This study aims to examine the effectiveness of combining arthroscopic debridement with functional exercise in treating advanced KOA.
METHODS:
A total of 296 patients diagnosed with advanced KOA were divided into two groups: the observation group ( 152) received arthroscopic debridement combined with functional exercise, while the control group ( 144) underwent arthroscopic debridement only. The study compared and observed the outcomes between the two groups.
RESULTS:
There were no significant differences in knee joint function, inflammation level, and oxidative stress between the two groups before treatment ( 0.05). Following treatment for six months, the observation group exhibited significantly lower visual analog scale (VAS) score, tissue inhibitors of metalloproteinase-1 (TIMP-1), tumor necrosis factor-alpha (TNF-), interleukin-1 (IL-1), matrix metalloproteinase-3 (MMP-3), and malondialdehyde (MDA) levels compared to the control group ( 0.05). Meanwhile, the observation group showed significantly higher levels of Lysholm score, hospital for special surgery (HSS) score, range of motion (ROM) of knee, peak torque (PT) and total work (TW) for knee extension and flexion, superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and glutathione (GSH) compared to the control group ( 0.05). Besides, the effective treatment rate in the observation group was notably higher than that in the control group (80.92% vs. 69.44%, 0.05).
CONCLUSION:
The combination of arthroscopic debridement with functional exercise is an effective treatment for advanced KOA. This approach not only enhances the function and strength of knee joint and reduces inflammatory response but also boosts the body’s antioxidant capacity. The treatment exhibits encouraging outcomes and warrants broad implementation.
Keywords: Arthroscopic debridement, Functional exercise, Advanced knee osteoarthritis, Therapeutic effects, Retrospective observation
1. Introduction
Knee osteoarthritis (KOA) is a chronic osteoarthritis characterized by articular cartilage degeneration and secondary bone hyperplasia, also known as proliferative arthritis and degenerative arthritis [1]. It is a prevalent orthopedic condition that significantly impacts the daily activities of individuals in the middle-aged and elderly population [2]. In China, the prevalence of osteoarthritis among individuals over 40 years old ranges from 10% to 17%, with the percentage rising to 50% among those over 60 years old and reaching 80% among individuals over 75 years old. This condition predominantly affects women more than men. The final disability rate associated with osteoarthritis is reported to be 53%, with a strong correlation to occupational factors, particularly in professions that involve prolonged squatting and kneeling [3]. Advanced KOA commonly affects both knee joints, leading to pain, deformity, and significant limitations in joint motor function. This can result in bed confinement and the inability to perform self-care tasks, profoundly impacting patients’ lives [4]. Currently, osteotomy or artificial joint replacement is commonly recommended for patients with advanced KOA, resulting in favorable outcomes [5]. Nonetheless, a significant percentage of individuals with advanced KOA either decline or are incapable of undergoing osteotomy or joint replacement procedures [6]. In clinical practice, addressing the treatment of patients in this particular group is a critical concern.
This study examined the clinical records of individuals diagnosed with advanced KOA who underwent arthroscopic debridement and/or participated in functional exercise sessions at our hospital from January 2017 to January 2022. By conducting follow-up assessments and statistical analysis, the study thoroughly assessed the treatment results, with the goal of evaluating the efficacy of combining arthroscopic debridement with functional exercises for the management of advanced KOA. Besides, we hypothesized that the combination of arthroscopic debridement and functional exercise will result in significantly greater improvements in pain relief and functional outcome for patients with advanced KOA compared to arthroscopic debridement alone. We anticipate that this synergistic approach will target both the structural damage within the joint through arthroscopic debridement and the functional deficits through tailored exercise programs, leading to enhanced long-term outcomes. By addressing both the mechanical pathology and the functional limitations simultaneously, we aim to provide more comprehensive and effective treatment for patients with advanced KOA.
2. Materials and methods
2.1. Patient selection
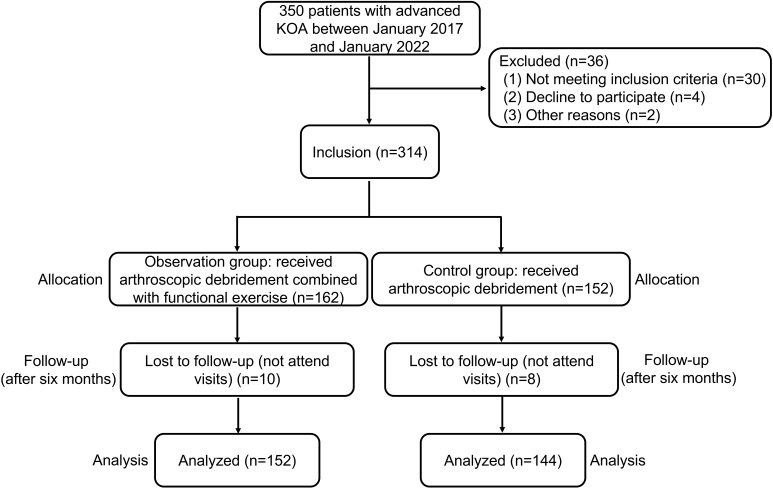
In this single-center, retrospective observational study, patients with advanced KOA who underwent arthroscopic debridement and/or engaged in functional exercise from January 2017 and January 2022 were identified. The final follow-up of patients was conducted in July 2022. Finally, a total of 296 patients with advanced KOA admitted to our hospital were selected as the study subjects. The inclusion criteria [7] were patients diagnosed with advanced bilateral KOA must meet specific criteria as outlined by the American College of Rheumatology in 1995. These criteria include (1) being over 40 years old, (2) experiencing knee pain for at least one month, (3) displaying osteophyte formation on x-rays, (4) having synovial fluid consistent with osteoarthritis upon examination, (5) experiencing morning stiffness lasting no more than 30 minutes, and (6) demonstrating bone friction during knee joint movement or examination. The diagnosis of KOA requires the presence of (2) (3), or (1) (2) (5) (6) or (2) (4) (5) (6). The severity of KOA is categorized into grades 0 to 4, determined by X-ray findings, or five grades if Kellgren and Lawrence’s method (K-L grade) is employed. Advanced KOA is distinguished by severe knee pain, reduced or absent joint space observed on X-rays, prominent osteophyte formation, structural irregularities, and marked sclerotic lesions, with grade 4 evaluations conducted by the same clinician. The exclusion criteria [8] were (1) patients with prosthetic revision or a confirmed knee joint infection, (2) patients with significant organic organ lesions, (3) patients with severe coagulation disorders, (4) patients experiencing mental confusion or cerebrovascular accidents impeding postoperative rehabilitation cooperation, (5) patients with muscle diseases like myasthenia gravis, progressive malnutrition, and periodic paralysis, and (6) patients frequently relying on adaptive walking aids. A flowchart of this study is presented in Fig. 1.
Figure 1.
Flowchart of the study design.
2.2. Treatment protocols
The control group was treated with arthroscopic debridement, and the observation group was treated with arthroscopic debridement combined with functional exercises. (1) Arthroscopic debridement involves a series of procedures performed under epidural anesthesia. The arthroscope is positioned at the anteromedial and lateral aspects of the knee joint using a standardized technique to remove hypertrophic inflammatory synovial tissue and loose fragments, address rough, softened, or exfoliated cartilage, eliminate osteophytes that impede joint mobility, trim any damaged meniscus, and conclude by thoroughly irrigating the joint cavity with 0.9% normal saline. The incision is then sutured, and the area is dressed with a thick bandage. Three days’ post-surgery, a 5 ml syringe is used to puncture the joint cavity through the outer aspect of the patella. If there is joint effusion, the fluid should be drained, followed by a slow injection of sodium hyaluronate (branded as Spite 20 mg/2 ml by Shandong Zhengda Freda Pharmaceutical Co., Ltd.) into the joint. The knee is passively mobilized to evenly distribute the medication throughout the joint. This treatment is administered weekly for a total of five injections.
(2) Functional exercise for knee joint involved various components. Firstly, isometric quadriceps contractions were initiated 24 hours’ post-knee arthroscopy, with the quadriceps muscles tightened in knee extension for 5 seconds then relaxed, repeated 30 times per day and progressing to 150 times per day. Ankle joint exercises for plantar flexion and dorsiflexion were also performed, starting at 60 repetitions per day and increasing to 90 daily, completed in three sets. Secondly, on the third postoperative day, straight-leg raises for the affected limb began, with repetitions starting at 30 times per day and increasing to 90, done in three times in a day. Thirdly, from the third day to one-week post-surgery, continuous passive motion therapy was used to aid in knee flexion and extension. Furthermore, starting from the second week, exercise intensity increased, including active knee flexion and extension, progressive weight-bearing strength training from 1/4 to 1/2 body weight, and gradually increasing exercise duration from 2 to 10 minutes per session, three times daily. By three weeks’ post-surgery, crutches were discontinued, range of motion (ROM) and muscle strength training continued, and resistance training for lower leg weight executed while sitting. Finally, after one month, patients incorporated long-term lower limb strength exercises such as walking, jogging, cycling, and swimming. The choice of functional exercises for advanced KOA is customized to meet the individual’s wishes and needs, under the guidance and supervision of a qualified physical therapist in our hospital to guarantee safety and efficacy.
2.3. Data collection
2.3.1. Physical factors
-
(1)
Measurement of knee ROM: the active ROM was assessed using a large plastic goniometer with 25-cm movable double arms, marked in 1-degree increments. This device has shown to be reliable when the patient maintains a consistent position throughout all measurements [9]. Evaluating knee flexion was conducted with the individual lying in a supine position by simultaneously flexing the hip and knee, while the foot on the measured side was extended as much as possible on the table (the individual should lie down on their back with the leg to be measured placed flat on the table. The foot should be positioned so that it is fully extended as far as the individual’s anatomical structures allow, with the toes pointing upwards). The other leg remained straight on the table. Furthermore, knee extension was measured with the patient supine on an examination couch, keeping the leg straight, while the examiner supported the leg’s weight as the patient performed the movement. The fully extended knee was considered the zero position, with the degrees of maximum flexion, maximum extension, and any extension deficit, if present, carefully noted. A negative ROM score for extension indicated the inability of the patient to reach the zero position. The range between maximum flexion and maximum extension was defined as the excursion range.
-
(2)
Lysholm score: Physical function was assessed using the Lysholm knee scoring scale questionnaire [10], designed specifically for evaluating individuals with knee injuries. This questionnaire consists of eight items that address daily activities, pain, instability, and swelling. The total score ranges from 0 to 100, with higher scores denoting greater physical function.
-
(3)
Hospital for special surgery (HSS) score: The knee joint function was assessed utilizing the Hospital for Special Surgery (HSS) scoring system [11], which comprises a total of 100 points. This system encompasses evaluations for joint instability (10 points), flexion deformity (10 points), muscle strength (10 points), range of motion (18 points), overall function (22 points), and pain level (30 points). A higher score on the HSS scale indicates improved knee joint function.
-
(4)
Visual analog scale (VAS) score: The VAS assessment was employed, utilizing a 10 cm line ranging from 0 to 10 [12]. Participants were briefed that 0 denoted absence of pain, 5 indicated moderate pain, and 10 represented excruciating pain. A greater score on the scale signifies increased pain intensity. Subsequently, participants were tasked with marking on the line the score that most accurately reflected their pain level during both static periods and physical exertion.
2.3.2. Markers of strength testing
Strength testing was conducted using the Biodex System 3 Pro isokinetic dynamometer. Patients were positioned seated with their hips and knees at a 90-degree angle while their lower extremity was secured to the dynamometer arm. Patients were instructed to fully straighten and bend the knee as hard and fast as possible. Prior to the testing phase, patients underwent a familiarization process, which included three submaximal effort repetitions at 50% intensity and one maximal effort repetition. Subsequently, patients completed three consecutive maximum isokinetic extension and flexion trials at a speed of 90 degrees/second to assess peak knee extension and flexion strength. The mean isokinetic peak torque (PT) for knee extension (PT-e) and flexion (PT-f) was calculated based on the highest recorded PT from the three trials for each movement [13]. Also, the total work (TW, torque multiplied by angular displacement) measured in Joules of the extensor (TW-e) and flexor (TW-f) muscles of both knees were recorded for each velocity [14].
2.3.3. Sample preparation and analysis of serum proteins
All serum samples were obtained using a volume of 5 mL. Samples were promptly transferred to microcentrifuge tubes within 60 minutes of collection and centrifuged at 2000 rpm for 10 minutes. The resulting supernatant was then aliquoted into 200 L vials and stored at 80∘C until analysis. Serum markers were assessed using an automated analyzer (UniCel DXC-800, Beckman Coulter, Brea, CA, USA). Serum oxidative stress and antioxidant activity were evaluated using specific kits: superoxide dismutase (SOD) kit, glutathione (GSH) kit, total antioxidant capacity (T-AOC) kit, and malondialdehyde (MDA) kit from the Jiangcheng Institute of Biotechnology in Nanjing, China. Serum inflammatory and anti-inflammatory responses were assessed using the enzyme linked immunosorbent assay (ELISA) kits for tumor necrosis factor-alpha (TNF-, interleukin-1 (IL-1), matrix metalloproteinase-3 (MMP-3), and tissue inhibitors of metalloproteinase-1 (TIMP-1) from Jianglai Biotechnology, Shanghai, China.
2.3.4. Efficacy evaluation criteria
The efficacy evaluation criteria were applied to the 296 postoperative patients through follow-up via phone or visiting, utilizing Shahariarrer criteria for determination [15]. Results were categorized as follows: Good – complete alleviation of pain and tenderness, reduction in swelling within days, and restoration of normal joint function. Acceptable – significant pain reduction, absence of tenderness, essentially normal joint function, and no challenges with stair mobility. Poor outcomes were defined by the lack of improvement in pain, swelling, or motor function, or a worsening of these factors. Patients achieving good or acceptable results were categorized as valid, while those with poor outcomes were deemed invalid.
2.3.5. Statistical analysis
Data for continuous variables were presented as mean standard deviation (SD) and analyzed using Student’s -test. Categorical variables were expressed as percentages and compared using the Chi-squared test, or Fisher’s exact test when appropriate, for univariate analysis. Statistical analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). A two-sided p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Population characteristics
The clinical records were retrospectively analyzed and divided into two groups: the observation group ( 152) and the control group ( 144), based on different treatment methods. The control group comprised 44 male patients and 100 female patients, with a mean age of (69.6 7.9) years. The average duration of the disease was (10.9 3.8) years, mean body mass index (BMI) (30.09 6.58) Kg/m2, and included 24 cases of valgus deformity, 15 cases of varus deformity, and 12 cases of flexion contracture deformity. In addition, 42 cases involved the left knee, 50 cases involved the right knee, and 52 cases involved both knees. In the observation group, there were 58 male patients and 94 female patients, with a mean age of (70.8 7.6) years, average duration of the disease (10.5 3.2) years, mean BMI (29.87 6.04) Kg/m2, and 23 cases of valgus deformity, 18 cases of varus deformity, and 14 cases of flexion contracture deformity. In addition, 45 cases involved the left knee, 53 cases involved the right knee, and 54 cases involved both knees. Statistical analysis indicated no significant differences between the two groups concerning age, gender, disease duration, BMI, and comorbidities, as well as the side of involvement ( 0.05).
3.2. Comparison to the levels of physical factors between the two groups before and after treatment
There was no significant difference in the levels of physical factors (Lysholm score, HSS score, ROM, VAS score) between the two groups before treatment ( 0.05). After treatment for six months, the values of the indexes including Lysholm score, HSS score, ROM in the observation group were significantly higher than those in the control group ( 0.05), while VAS score in the observation group was lower than that in the control group ( 0.05). The results are detailed in Table 4.
3.3. Comparison to the levels of strength testing markers between the two groups before and after treatment
Table 1.
Comparison of the levels of physical factors between the two groups before and after treatment ( s)
| Groups | Lysholm score | HSS score | ROM (∘) | VAS score | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation group ( 152) | 49.84 5.65 | 88.86 6.65 | 36.64 6.78 | 85.17 7.52 | 83.32 6.47 | 111.14 10.21 | 8.22 1.12 | 2.54 0.51 |
| Control group ( 144) | 50.32 7.86 | 83.65 5.78 | 36.66 5.75 | 80.86 5.55 | 84.54 7.71 | 104.32 10.37 | 8.19 0.98 | 3.02 0.45 |
| value | 0.606 | 7.177 | 0.027 | 5.586 | 1.478 | 5.700 | 0.245 | 8.568 |
| value | 0.545 | 0.000 | 0.978 | 0.000 | 0.141 | 0.000 | 0.807 | 0.000 |
Table 2.
Comparison of the levels of strength testing markers between the two groups before and after treatment ( s)
| Groups | PT-f (Nm) | PT-e (Nm) | TW-f (J) | TW-e (J) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation group ( 152) | 33.65 5.55 | 63.54 6.73 | 44.67 7.98 | 91.28 8.09 | 321.93 42.46 | 635.14 54.42 | 385.62 50.69 | 643.17 40.52 |
| Control group ( 144) | 34.43 6.76 | 59.25 7.52 | 45.79 8.21 | 84.67 7.01 | 326.99 52.48 | 578.54 44.39 | 395.61 60.62 | 604.86 40.55 |
| value | 1.079 | 5.177 | 1.190 | 7.495 | 0.914 | 9.774 | 1.541 | 8.127 |
| value | 0.281 | 0.000 | 0.235 | 0.000 | 0.361 | 0.000 | 0.124 | 0.000 |
Table 3.
Comparison to the levels of inflammatory factors between the two groups before and after treatment ( s, pg/mL)
| Groups | MMP-3 | TIMP-1 | TNF- | IL-1 | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation group ( 152) | 233.27 25.43 | 120.64 17.12 | 734.52 54.08 | 658.53 48.32 | 69.09 18.65 | 35.14 7.87 | 187.65 35.87 | 83.09 10.07 |
| Control group ( 144) | 229.51 26.44 | 146.39 26.77 | 730.73 58.35 | 686.09 37.54 | 68.54 20.71 | 42.09 9.09 | 179.61 45.98 | 94.76 10.53 |
| value | 1.247 | 9.912 | 0.580 | 5.459 | 0.240 | 7.043 | 1.682 | 9.746 |
| value | 0.213 | 0.000 | 0.562 | 0.000 | 0.810 | 0.000 | 0.094 | 0.000 |
Table 4.
Comparison to the levels of oxidative stress indicators between the two groups before and after treatment ( s)
| Groups | MDA (nmol/mL) | SOD (U/mL) | T-AOC (IU/mL) | GSH (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation group ( 152) | 16.69 5.12 | 8.65 1.04 | 67.51 10.05 | 124.07 23.35 | 1.44 0.29 | 3.55 0.78 | 1243.64 289.54 | 1756.87 235.09 |
| Control group ( 144) | 16.51 6.08 | 10.43 1.85 | 69.06 12.45 | 108.76 29.53 | 1.48 0.31 | 2.83 0.66 | 1265.74 332.83 | 1565.43 132.54 |
| value | 0.276 | 10.173 | 1.181 | 4.961 | 1.147 | 8.550 | 0.610 | 8.566 |
| value | 0.783 | 0.000 | 0.238 | 0.000 | 0.252 | 0.000 | 0.542 | 0.000 |
In the pre-treatment phase, there was no notable disparity in the levels of strength testing markers (PT-f, PT-e, TW-f, TW-e) between the two groups ( 0.05). Following six months of treatment, the observation group exhibited significantly elevated values in the aforementioned indexes compared to the control group ( 0.05). Detailed results are presented in Table 4.
3.4. Comparison to the levels of inflammatory factors between the two groups before and after treatment
There was no significant difference observed in the levels of inflammatory factors (MMP-3, TIMP-1, TNF-, IL-1) between the two groups prior to treatment ( 0.05). Post six months of treatment, the values of TIMP-1, TNF-, and IL-1, as well as MMP-3 in the observation group were significantly lower compared to the control group ( 0.05). Detailed results are presented in Table 4.
3.5. Comparison to the levels of oxidative stress indicators between the two groups before and after treatment
In the pre-treatment phase, there was no significant difference observed in the levels of oxidative stress indicators (MDA, SOD, T-AOC, GSH) between the two groups ( 0.05). After six months of treatment, the observation group exhibited significantly lower value of MDA compared to the control group ( 0.05). Additionally, the observation group showed higher levels of SOD and GSH, as well as T-AOC than the control group ( 0.05). Further details can be found in Table 4.
3.6. Comparison to the treatment effects between the two groups
After six months of treatment, the effective rate in the observation group substantially surpassed that of the control group (80.92% vs. 69.44%). Statistical analysis indicated a significant difference between the two groups ( 0.05). Detailed findings are presented in Table 5.
Table 5.
Comparison to the treatment effects between these two groups (, %)
| Groups | Good | Acceptable | Poor | Valid |
|---|---|---|---|---|
| Observation group ( 152) | 84 (54.56) | 39 (25.66) | 29 (19.08) | 123 (80.92) |
| Control group ( 144) | 65 (45.14) | 35 (24.31) | 44 (30.56) | 100 (69.44) |
| value | 4.643 | |||
| value | 0.031 |
4. Discussion
KOA is a significant global health issue, impacting over 500 million people worldwide and carrying personal and societal repercussions [16]. The condition is marked by progressive knee joint pain, swelling, and stiffness. In severe instances, it may lead to joint deformity, ultimately resulting in the impairment of everyday functionality and work capacity [17]. Due to the aging of the population, the prevalence and impact of the disease are expected to significantly increase [18]. Advanced KOA not only causes pain and restricts movement but also leads to impaired physical function and overall limitations in mobility [19, 20]. Individuals with advanced KOA often experience varying levels of reduced functionality [21, 22, 23]. The objectives of symptomatic conservative therapies are to alleviate pain and uphold or enhance functionality [24]. Treatment approaches, including medications, local intra-articular injections, physical modalities, exercises, self-management programs, and surgeries, are aimed at alleviating symptoms and preserving functionality.
Exercise therapy typically emphasizes strength and flexibility training for the thigh muscles surrounding the knee [25]. Several clinical trials have demonstrated the effects of functional exercise in KOA patients [26, 27], but further experimentation is required to confirm the effect of this method for the advanced KOA. The objective of this work was to investigate into the application value of functional exercise in the treatment of patients with advanced KOA. In this study, a retrospective analysis was conducted on the clinical case data of 296 patients diagnosed with advanced KOA at our hospital. The patients were categorized into two groups based on the treatment methods administered. The control group included patients treated solely with arthroscopic debridement, while the observation group consisted of patients who underwent both arthroscopic debridement and functional exercise. The results in present study demonstrated that the treatment effect in the observation group was markedly superior to that in the control group.
Knee joint function was evaluated through the Lysholm knee scoring scale, where higher scores indicate improved recovery of knee function [28]. Additionally, patients were evaluated using the VAS to quantify pain levels, with scores directly correlating to the intensity of pain [29]. HSS score provided a comprehensive assessment of knee function changes, offering a precise measure of joint functionality recovery [30]. Furthermore, enhancing ROM during knee flexion, hip, and pelvic movements has been identified as crucial for improving walking speed post-stretching exercises [31]. In current study, after six months of treatment, the indices such as the Lysholm score, HSS score, and ROM in the observation group showed a significant increase compared to those in the control group ( 0.05). Additionally, VAS score in the observation group was significantly lower than that in the control group ( 0.05). The results showed that functional exercise combined with arthroscopic debridement was highly effective in enhancing knee joint function and relieving pain in advanced KOA patients.
Muscles and tendons possess the ability to store and release elastic strain energy, thus playing a crucial role in preventing joint injuries [32]. Specifically, the quadriceps muscle is vital for absorbing impact on the knee joint, particularly through its eccentric action [33]. Consequently, deficits in quadriceps strength can compromise its function in load absorption, leading to pain and physical impairment in individuals with KOA [34]. Quadriceps muscle weakness has been identified as a contributing factor to the onset of symptoms in patients with radiographic KOA [35]. A previous study demonstrated a significant correlation between knee extensor torque and symptoms in patients with KOA [36]. In the present study, the average post-treatment PT and TW of the extension and flexion muscles exhibited a significant increase in the observation group compared to the control group among patients with advanced KOA ( 0.05). These findings suggested that functional exercise combined with arthroscopic debridement could enhance the function and strength of knee extension and flexion muscles in individuals with advanced KOA.
Osteoarthritis is a chronic joint disease that progresses slowly and manifests through symptoms such as pain, stiffness, limited movement, swelling, and disability. The condition is defined by the degradation of articular cartilage, the development of osteophytes at joint edges, and persistent nonspecific inflammation of the synovium [37]. This inflammatory process initiates both anabolic and catabolic events, leading to the production of chemokines, cytokines, matrix metalloproteinases, and tissue inhibitors of metalloproteinase [38]. Therefore, such molecules are also detectable in the synovial fluid of patients with advanced KOA. Heard et al. examined the protein expression levels of MMPs and TIMPs in synovial fluid derived from normal joints, early osteoarthritis joints, and advanced osteoarthritis joints [39]. The study revealed a notable increase in MMP-3 expression in advanced osteoarthritis samples compared to early osteoarthritis samples, with a considerably higher level in advanced osteoarthritis samples compared to normal samples. Moreover, the expression levels of TIMP-1 were found to be higher in advanced osteoarthritis samples when compared to those in early osteoarthritis samples. Also, increasing evidence suggests that the local production of pro-inflammatory cytokines such as IL-1, TNF-, IL-6, IL-15, IL-17, and IL-18 plays a significant role in the pathogenesis of osteoarthritis [40]. In the current study, it was observed that the values of TIMP-1, TNF-, and IL-1, as well as MMP-3 in the observation group were significantly lower compared to the control group after treatment for six months, and it demonstrated that functional exercise combined with arthroscopic debridement could relieve the inflammatory response, a potent contributor to chronic pain.
The underlying mechanism of cartilage matrix degradation in KOA is not well understood, with reactive oxygen species being implicated as the primary causative factors [41]. Besides, the degeneration of cartilage in KOA results from an imbalance in cellular activity triggered by multiple factors, wherein oxidative stress significantly contributes to the disease pathogenesis [42]. The evolution of KOA can be correlated with oxidative stress markers or antioxidant status [43]. Moreover, it was reported that oxidative stress including antioxidative indicators (SOD, GSH, and T-AOC) and biomarker of oxidative damage (MDA) may be linked to functional KOA pain, and lowering it could provide relief from pain and be a better alternative treatment method [44, 45]. These factors reveal the importance of maintaining a redox balance for the joints and the whole body’s health, emphasizing the importance of an individualized therapeutic approach based on antioxidant effects [43]. In the present study, it showed that the observation group displayed a significantly lower level of MDA in comparison to the control group ( 0.05). Moreover, the observation group demonstrated higher concentrations of SOD, GSH, and T-AOC than the control group ( 0.05). These results suggested that functional exercise combined with arthroscopic debridement could alleviate oxidative stress, thereby diminishing functional pain of body for advanced KOA patients.
These findings have significant implications for clinical practice and can offer valuable insights for enhancing treatment approaches for patients with advanced KOA. Incorporating functional exercise as a complementary intervention to arthroscopic debridement can potentially become a standard practice in the clinical management of advanced KOA. This integrated approach addresses both the structural damage and functional deficits associated with the condition, leading to better overall outcomes for patients. Healthcare providers may consider implementing structured exercise programs as part of the multidisciplinary treatment plan for patients undergoing arthroscopic debridement, aiming to enhance pain relief, functional improvement, and long-term joint health.
The results of this study underscore the importance of personalized and comprehensive care for patients with advanced KOA. By tailoring treatment approaches to individual needs and leveraging the synergistic effects of surgical intervention and rehabilitative exercises, clinicians can optimize patient outcomes and quality of life. Moving forward, the integration of functional exercise with arthroscopic debridement could become a key component in the holistic management of advanced KOA, emphasizing a proactive and patient-centered approach to care.
5. Conclusion
In conclusion, the results of our research advance the understanding of the validity of the functional exercise in patients with advanced KOA. The integration of functional exercise with arthroscopic debridement significantly relieves pain levels and improves functional outcomes in patients with advanced KOA compared to those treated with arthroscopic debridement alone. This combined approach holds promise for enhancing the overall prognosis of such patients. We recommend future research that increases our understanding of reproducibility and responsiveness of the functional exercises in patients with advanced KOA, in order to facilitate the integration of this measure into clinical practice. Overall, these study findings provide evidence for the effectiveness of combining functional exercise with arthroscopic debridement in the treatment of advanced KOA and pave the way for further advancements in clinical practice. By integrating these results into treatment guidelines and practice protocols, healthcare professionals can enhance the quality of care and outcomes for patients with this challenging condition.
5.1. Study limitations
Nevertheless, the current study is associated with certain limitations. Firstly, this study is a follow-up retrospective observation with a low evidence grade. Therefore, randomized, double-blind, and multicenter clinical trials with a large sample size are necessary for further comprehensive investigation. Secondly, the absence of thorough records on patients’ daily lives and potential factors influencing the conclusions limits the study’s control. Utilizing a more detailed questionnaire for patient follow-up could address this limitation. Another constraint was the lack of imaging assessment for evaluating KOA progression. However, considering that in late-stage KOA, clinical improvement holds more value than radiographic disease progression, this limitation may be deemed acceptable. Notably, the sample’s homogeneity in this study is noteworthy as only patients with grade 4 KOA (according to X-ray or K-L grade) and sufficient symptoms were included.
Author contributions
SH: Methodology, Investigation and Writing-Original draft. QW, DC and PZ: Designed the figures, Methodology and Investigation. DC: Conceptualization, Methodology and Reviewing the draft. All authors read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Ethical approval
The study was approved by the Human Research Ethics Committee of Longyan First Affiliated Hospital of Fujian Medical University (approval number: LYREC2022-02-01) and conducted according to the guidelines of the Declaration of Helsinki.
Funding
None to report.
Informed consent
All participants provided written informed consent prior to data collection.
Acknowledgments
The authors would like to thank the study participants.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1]. Geng R, Li J, Yu C, Zhang C, Chen F, Chen J, Ni H, Wang J, Kang K, Wei Z, Xu Y, Jin T. Knee osteoarthritis: Current status and research progress in treatment (Review). Exp Ther Med. 2023; 26(4): 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Kao MH, Tsai YF. Illness experiences in middle-aged adults with early-stage knee osteoarthritis: findings from a qualitative study. J Adv Nurs. 2014; 70(7): 1564-1572. [DOI] [PubMed] [Google Scholar]
- [3]. Sun X, Zhen X, Hu X, Li Y, Gu S, Gu Y, Dong H. Osteoarthritis in the middle-aged and elderly in China: prevalence and influencing factors. Int J Environ Res Public Health. 2019; 16(23): 4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Quatman CE, Hettrich CM, Schmitt LC, Spindler KP. The clinical utility and diagnostic performance of magnetic resonance imaging for identification of early and advanced knee osteoarthritis: a systematic review. Am J Sports Med. 2011. Jul; 39(7): 1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Wang L, Wang Q, Li Q, Song F. A comparative study of total knee arthroplasty and unicondylar knee arthroplasty in the treatment of knee osteoarthritis. Contrast Media Mol Imaging. 2022; 2022: 7795801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Dong C, Zhao C, Wang F. Clinical benefit of high tibial osteotomy combined with the intervention of platelet-rich plasma for severe knee osteoarthritis. J Orthop Surg Res. 2022; 17(1): 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Xu Y, Wu K, Liu Y, Geng H, Zhang H, Liu S, Qu H, Xing G. The effect of extracorporeal shock wave therapy on the treatment of moderate to severe knee osteoarthritis and cartilage lesion. Medicine (Baltimore). 2019; 98(20): e15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Zhao Z, Yang T, Qin C, Zhao M, Zhao F, Li B, Liu J. Exploring the potential of the sit-to-stand test for self-assessment of physical condition in advanced knee osteoarthritis patients using computer vision. Front Public Health. 2024; 12: 1348236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Huang MH, Yang RC, Lee CL, Chen TW, Wang MC. Preliminary results of integrated therapy for patients with knee osteoarthritis. Arthritis Rheum. 2005; 53(6): 812-820. [DOI] [PubMed] [Google Scholar]
- [10]. Peccin MS, Ciconelli R, Cohen M. Questionario especıfico para sintomas do joelho. Acta Ortop Bras. 2006; 14: 268-272. [Google Scholar]
- [11]. Burnett W, Kontulainen S, McLennan C, Hazel D, Talmo C, Hunter D, Wilson D, Johnston J. Patella bone density is lower in knee osteoarthritis patients experiencing moderate-to-severe pain at rest. J Musculoskelet Neuronal Interact. 2016; 16(1): 33-39. [PMC free article] [PubMed] [Google Scholar]
- [12]. Dixon JS, Bird HA. Reproducibility along a 10 cm vertical visual analogue scale. Ann Rheum Dis. 1981; 40(1): 87-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Pinto RF, Birmingham TB, Philpott HT, Primeau CA, Leitch KM, Arsenault DA, Appleton CT. Changes and associations between gait biomechanics and knee inflammation after aspiration and glucocorticoid injection for knee osteoarthritis. Arthritis Care Res (Hoboken). 2023; 75(8): 1764-1772. [DOI] [PubMed] [Google Scholar]
- [14]. Kömürcü E, Yüksel HY, Ersöz M, Aktekin CN, Hapa O, Çelebi L, Akbal A, Biçimoğlu A. Effect of surgical closing in total knee arthroplasty at flexion or extension: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2014; 22(12): 3067-3073. [DOI] [PubMed] [Google Scholar]
- [15]. Shahariarre H. Degenerative arthritis of the knee. O’comer RL. Text-book arthroscopic surgery philadephia: JB Lippineotal. 1984; 266-267, 269-272. [Google Scholar]
- [16]. Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a lancet commission. Lancet. 2020; 396: 1711-1712. [DOI] [PubMed] [Google Scholar]
- [17]. Ochiai N, Ohtori S, Sasho T, Nakagawa K, Takahashi K, Takahashi N, Murata R, Takahashi K, Moriya H, Wada Y, Saisu T. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthritis Cartilage. 2007; 15(9): 1093-1096. [DOI] [PubMed] [Google Scholar]
- [18]. Perruccio AV, Power JD, Badley EM. Revisiting arthritis prevalence projections–it’s more than just the aging of the population. J Rheumatol. 2006; 33(9): 1856-1862. [PubMed] [Google Scholar]
- [19]. Dunlop DD, Song J, Semanik PA, Sharma L, Chang RW. Physical activity levels and functional performance in the osteoarthritis initiative: a graded relationship. Arthritis Rheum. 2011; 63(1): 127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003; 48(12): 3359-3370. [DOI] [PubMed] [Google Scholar]
- [21]. Clynes MA, Jameson KA, Edwards MH, Cooper C, Dennison EM. Impact of osteoarthritis on activities of daily living: does joint site matter? Aging Clin Exp Res. 2019; 31(8): 1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. King LK, Kendzerska T, Waugh EJ, Hawker GA. Impact of osteoarthritis on difficulty walking: A population-based study. Arthritis Care Res (Hoboken). 2018; 70(1): 71-79. [DOI] [PubMed] [Google Scholar]
- [23]. Hall M, Bennell KL, Wrigley TV, Metcalf BR, Campbell PK, Kasza J, Paterson KL, Hunter DJ, Hinman RS. The knee adduction moment and knee osteoarthritis symptoms: relationships according to radiographic disease severity. Osteoarthritis Cartilage. 2017; 25(1): 34-41. [DOI] [PubMed] [Google Scholar]
- [24]. Blomqvist P, Feltelius N, Ekbom A, Klareskog L. Rheumatoid arthritis in Sweden. Drug prescriptions, costs, and adverse drug reactions. J Rheumatol. 2000; 27(5): 1171-1177. [PubMed] [Google Scholar]
- [25]. Lun V, Marsh A, Bray R, Lindsay D, Wiley P. Efficacy of hip strengthening exercises compared with leg strengthening exercises on knee pain, function, and quality of life in patients with knee osteoarthritis. Clin J Sport Med. 2015; 25(6): 509-517. [DOI] [PubMed] [Google Scholar]
- [26]. Vassão PG, Parisi J, Penha TFC, Balão AB, Renno ACM, Avila MA. Association of photobiomodulation therapy (PBMT) and exercises programs in pain and functional capacity of patients with knee osteoarthritis (KOA): a systematic review of randomized trials. Lasers Med Sci. 2021; 36(7): 1341-1353. [DOI] [PubMed] [Google Scholar]
- [27]. Mazloum V, Rabiei P, Rahnama N, Sabzehparvar E. The comparison of the effectiveness of conventional therapeutic exercises and Pilates on pain and function in patients with knee osteoarthritis. Complement Ther Clin Pract. 2018; 31: 343-348. [DOI] [PubMed] [Google Scholar]
- [28]. Liu Y, Chen R, Zhang Y, Wang Q, Ren JL, Wang CX, Xu YK. Clinical value of ankle flexion and extension exercises combined with a psychological intervention in knee osteoarthritis. World J Psychiatry. 2023; 13(10): 743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. García-Coronado JM, Martínez-Olvera L, Elizondo-Omaña RE, Acosta-Olivo CA, Vilchez-Cavazos F, Simental-Mendía LE, Simental-Mendía M. Effect of collagen supplementation on osteoarthritis symptoms: a meta-analysis of randomized placebo-controlled trials. Int Orthop. 2019; 43(3): 531-538. [DOI] [PubMed] [Google Scholar]
- [30]. Wang XF, Ma ZH, Teng XR. Isokinetic strength test of muscle strength and motor function in total knee arthroplasty. Orthop Surg. 2020; 12(3): 878-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Magnusson SP, Simonsen EB, Aagaard P, Sørensen H, Kjaer M. A mechanism for altered flexibility in human skeletal muscle. J Physiol. 1996; 497(Pt 1): 291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Zhu Q, Li J, Fang M, Gong L, Sun W, Zhou N. Effect of Chinese massage (Tui Na) on isokinetic muscle strength in patients with knee osteoarthritis. J Tradit Chin Med. 2016; 36(3): 314-320. [DOI] [PubMed] [Google Scholar]
- [33]. Hinman RS, Bennell KL, Metcalf BR, Crossley KM. Delayed onset of quadriceps activity and altered knee joint kinematics during stair stepping in individuals with knee osteoarthritis. Arch Phys Med Rehabil. 2002; 83(8): 1080-1086. [DOI] [PubMed] [Google Scholar]
- [34]. Hortobágyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004; 51(4): 562-569. [DOI] [PubMed] [Google Scholar]
- [35]. Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, Nevitt M. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009; 61(9): 1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Serrão PR, Gramani-Say K, Lessi GC, Mattiello SM. Knee extensor torque of men with early degrees of osteoarthritis is associated with pain, stiffness and function. Rev Bras Fisioter. 2012; 16(4): 289-294. [DOI] [PubMed] [Google Scholar]
- [37]. Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008; 16(Suppl 3): S1-S3. [DOI] [PubMed] [Google Scholar]
- [38]. Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, Rod E, Čukelj F, Vrdoljak T, Vidović D, Starešinić M, Sabalić S, Dobričić B, Petrović T, Antičević D, Borić I, Košir R, Zmrzljak UP, Primorac D. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021; 22(17): 9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012; 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014; 2014: 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Ostalowska A, Birkner E, Wiecha M, Kasperczyk S, Kasperczyk A, Kapolka D, Zon-Giebel A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage. 2006; 14(2): 139-145. [DOI] [PubMed] [Google Scholar]
- [42]. Liu L, Luo P, Yang M, Wang J, Hou W, Xu P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front Mol Biosci. 2022; 9: 1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Tudorachi NB, Totu EE, Fifere A, Ardeleanu V, Mocanu V, Mircea C, Isildak I, Smilkov K, Cărăuşu EM. The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants (Basel). 2021; 10(6): 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Strath LJ, Jones CD, Philip George A, Lukens SL, Morrison SA, Soleymani T, Locher JL, Gower BA, Sorge RE. The effect of low-carbohydrate and low-fat diets on pain in individuals with knee osteoarthritis. Pain Med. 2020; 21(1): 150-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Li S, Xu M, Niu Q, Xu S, Ding Y, Yan Y, Guo S, Li F. Efficacy of procyanidins against in vivo cellular oxidative damage: A systematic review and meta-analysis. PLoS One. 2015; 10(10): e0139455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.