Abstract
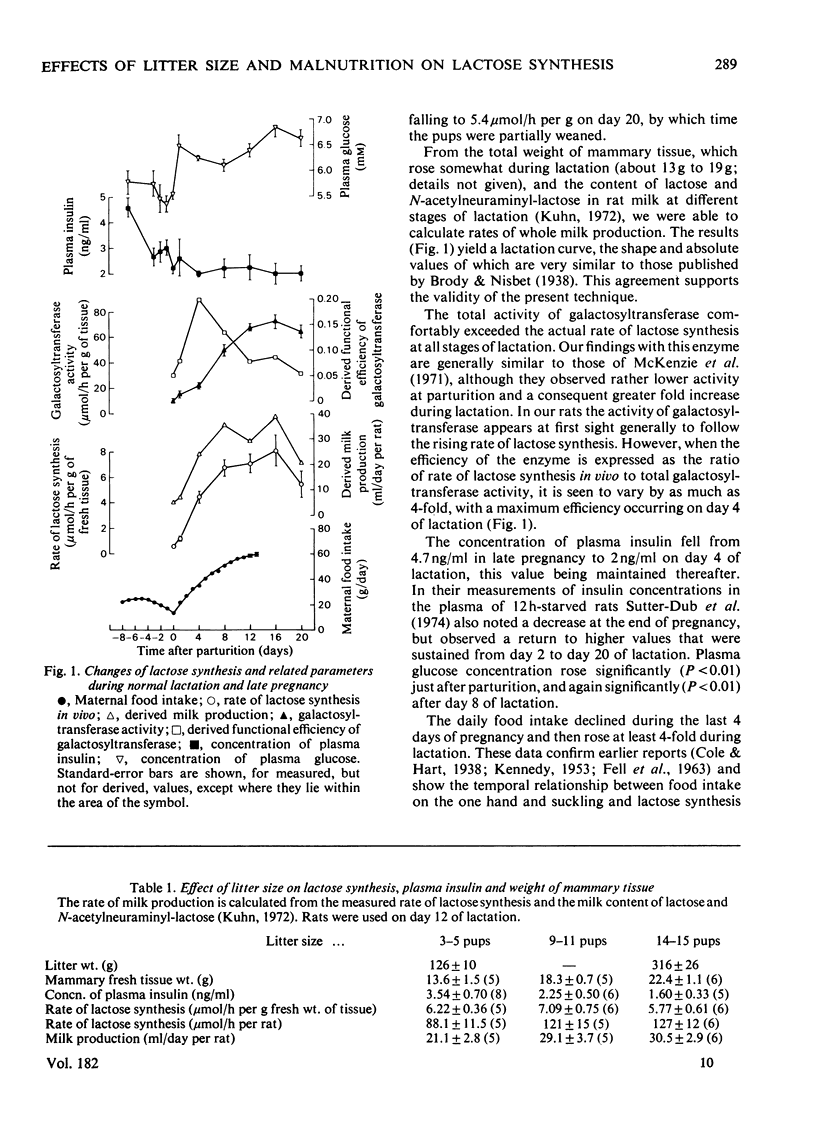
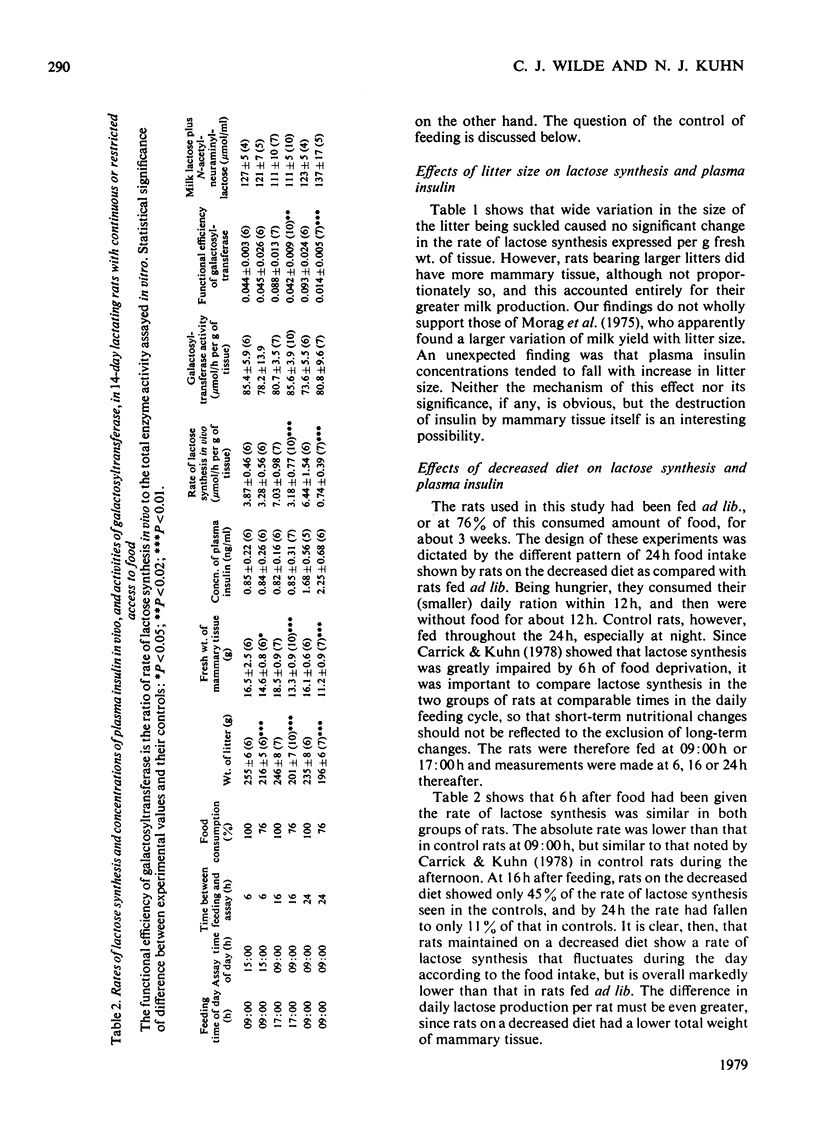
1. The rate of lactose synthesis per g of mammary tissue, measured in vivo by a radioisotopic technique, rose 13-fold between parturition and day 16 of lactation in the rat, but was unaffected by wide variation in litter size. 2. The increase reflected a greater tissue content of galactosyltransferase (EC 2.4.1.22), and was augmented by a rise in the total weight of mammary tissue. Superimposed on this were unpredictable changes in the functional efficiency of the enzyme. 3. Lactose synthesis in 14-day-lactating rats, permitted only 76% of the food intake of paired control rats over the previous 3 weeks, showed a pronounced diurnal variation at an overall rate markedly below that in control rats. 4. Such nutritional deficiency did not affect the tissue content of galactosyltransferase, but impaired its functional efficiency in a manner reversed by renewed feeding or by the preparation and incubation of acini in vitro. 5. Plasma insulin concentrations decreased at parturition and with increasing litter size, and remained relatively unchanged during lactation and malnutrition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Effect of insulin in vitro in pathways of glucose utilization, other than Embden-Meyerhof, in rat mammary gland. J Biol Chem. 1957 Feb;224(2):955–962. [PubMed] [Google Scholar]
- BALMAIN J. H., FOLLEY S. J., GLASCOCK R. F. Relative utilization of glucose and acetate carbon for lipogenesis by mammary gland slices, studies with tritium, 13C and 14C. Biochem J. 1954 Feb;56(2):234–239. [PMC free article] [PubMed] [Google Scholar]
- Carrick D. T., Kuhn N. J. Diurnal variation and response to food withdrawal of lactose synthesis in lactating rats. Biochem J. 1978 Jul 15;174(1):319–325. doi: 10.1042/bj1740319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coniglio J. G., Culp F. B. The effect of fasting on fatty acid synthesis in cell-free preparations of rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):419–421. doi: 10.1016/0005-2760(65)90052-4. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L. Dynamics of insulin release by perfused rat pancreases: effects of hypophysectomy, growth hormone, adrenocorticotropic hormone, and hydrocortisone. Endocrinology. 1973 Sep;93(3):602–609. doi: 10.1210/endo-93-3-602. [DOI] [PubMed] [Google Scholar]
- FELL B. F., SMITH K. A., CAMPBELL R. M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J Pathol Bacteriol. 1963 Jan;85:179–188. doi: 10.1002/path.1700850117. [DOI] [PubMed] [Google Scholar]
- Ford J. J., Melampy R. M. Gonadotropin levels in lactating rats: effect of ovariectomy. Endocrinology. 1973 Sep;93(3):540–547. doi: 10.1210/endo-93-3-540. [DOI] [PubMed] [Google Scholar]
- Forsyth I. A. Reviews of the progress of dairy science. Section A. Physiology. Organ culture techniques and the study of hormone effects on the mammary gland. J Dairy Res. 1971 Oct;38(3):419–444. [PubMed] [Google Scholar]
- HILLS A. G., STADIE W. C. The effect of combined insulin upon the metabolism of the lactating mammary glands of the rat. J Biol Chem. 1952 Jan;194(1):25–31. [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H. Measurements of substrate uptake by mammary gland of the rat. Biochem J. 1972 Oct;129(5):1171–1173. doi: 10.1042/bj1291171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey E., Hervey G. R. The effects of progesterone on body weight and composition in the rat. J Endocrinol. 1967 Apr;37(4):361–381. doi: 10.1677/joe.0.0370361. [DOI] [PubMed] [Google Scholar]
- Hove K. Maintenance of lactose secretion during acute insulin deficiency in lactating goats. Acta Physiol Scand. 1978 Jun;103(2):173–179. doi: 10.1111/j.1748-1716.1978.tb06205.x. [DOI] [PubMed] [Google Scholar]
- Jones E. A., Cowie A. T. The effect of hypophysectomy and subsequent replacement therapy with sheep prolactin or bovine growth hormone on the lactose synthetase activity of rabbit mammary gland. Biochem J. 1972 Dec;130(4):997–1002. doi: 10.1042/bj1300997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMARESAN P., TURNER C. W. EFFECT OF GRADED LEVELS OF INSULIN ON LACTATION IN THE RAT. Proc Soc Exp Biol Med. 1965 Jun;119:415–416. doi: 10.3181/00379727-119-30198. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969 May;44(1):39–54. doi: 10.1677/joe.0.0440039. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan P., Turner C. W. Effect of insulin and alloxan on mammary gland growth in rats. J Dairy Sci. 1965 Oct;48(10):1378–1381. doi: 10.3168/jds.S0022-0302(65)88468-5. [DOI] [PubMed] [Google Scholar]
- Kyriakou S. Y., Kuhn N. J. Lactogenesis in the diabetic rat. J Endocrinol. 1973 Oct;59(1):199–200. doi: 10.1677/joe.0.0590199. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of alloxan diabetes on lactational performance and mammary tissue metabolism in the rat. Endocrinology. 1971 Apr;88(4):863–867. doi: 10.1210/endo-88-4-863. [DOI] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of insulin on isolated rat mammary cell metabolism: glucose utilization and metabolite patterns. Endocrinology. 1971 Nov;89(5):1263–1269. doi: 10.1210/endo-89-5-1263. [DOI] [PubMed] [Google Scholar]
- McKenzie L., Fitzgerald D. K., Ebner K. E. Lactose synthetase activities in rat and mouse mammary glands. Biochim Biophys Acta. 1971;230(3):526–530. doi: 10.1016/0304-4165(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Miller T. B., Jr, Hazen R., Larner J. An absolute requirement for insulin in the control of hepatic glycogenesis by glucose. Biochem Biophys Res Commun. 1973 Jul 17;53(2):466–474. doi: 10.1016/0006-291x(73)90685-2. [DOI] [PubMed] [Google Scholar]
- Morag M., Popliker F., Yagil R. Effect of litter size on milk yield in the rat. Lab Anim. 1975 Jan;9(1):43–47. doi: 10.1258/002367775780994844. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ariyanayagam A. D., Kuhn N. J. Progesterone and the metabolic control of the lactose biosynthetic pathway during lactogenesis in the rat. Biochem J. 1973 Dec;136(4):1105–1116. doi: 10.1042/bj1361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K., Yokoyama A. Body weight and food consumption of lactating rats: effects of ovariectomy and of arrest and resumption of suckling. J Endocrinol. 1967 Jul;38(3):251–261. doi: 10.1677/joe.0.0380251. [DOI] [PubMed] [Google Scholar]
- Pepe G. J., Rothchild I. A comparative study of serum progesterone levels in pregnancy and in various types of pseudopregnancy in the rat. Endocrinology. 1974 Jul;95(1):275–279. doi: 10.1210/endo-95-1-275. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Control of glucose metabolism in isolated acini of the lactating mammary gland of the rat. The ability of glycerol to mimic some of the effects of insulin. Biochem J. 1977 Dec 15;168(3):465–474. doi: 10.1042/bj1680465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Effects of acetoacetate administration on glucose metabolism in mammary gland of fed lactating rats. Biochem J. 1977 Jun 15;164(3):749–752. doi: 10.1042/bj1640749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud S. C. Importance of insulin for mammary growth. Indian J Exp Biol. 1971 Jul;9(3):307–311. [PubMed] [Google Scholar]
- Sutter-Dub M. T., Leclercq R., Sutter B. C., Jacquot R. Plasma glucose, progesterone and immunoreactive insulin levels in the lactating rat. Horm Metab Res. 1974 Jul;6(4):297–300. doi: 10.1055/s-0028-1093852. [DOI] [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of alloxan-diabetes and treatment with anti-insulin serum on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1968 Sep;109(3):407–417. doi: 10.1042/bj1090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis, galactosyltransferase activity and plasma insulin concentration throughout lactation in the rat [proceedings]. Biochem Soc Trans. 1977;5(4):1040–1041. doi: 10.1042/bst0051040. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., McKeown S. R., Ilic V. Interactions of glucose, acetoacetate and insulin in mammary-gland slices of lactating rats. Biochem J. 1975 Aug;150(2):145–152. doi: 10.1042/bj1500145. [DOI] [PMC free article] [PubMed] [Google Scholar]


